Introduction
The superior sinus venosus defect is an uncommon CHD located at the junction of the superior vena cava and the right atrium. Atrial septal defects arise from incomplete septation of the atrial chambers due to abnormal development or resorption of the septum primum and septum secundum, resulting in a communication between the left and right atria. Sinus venosus defects, on the other hand, are characterised by deficient incorporation of the sinus venosus into the right atrium and are located outside the true interatrial septum. They are typically associated with partial anomalous pulmonary venous drainage of the right upper and/or right middle pulmonary veins. The defect arises due to deficient folding of the atrial wall, which forms the posterior wall of the superior vena cava and the anterior wall of the right upper pulmonary vein, leading to abnormal drainage of the right upper pulmonary vein into the right atrium instead of the left atrium. Reference Garg, Tyagi and Radha1–Reference Rosenthal, Qureshi and Sivakumar23 In recent years, transcatheter correction of superior sinus venosus defect has emerged as a promising alternative to the standard surgical correction, offering the potential for less invasive management with comparable outcomes. Reference Riahi, Velasco Forte and Byrne5,Reference Pascual-Tejerina, Sánchez-Recalde, Cantador, López, Gómez-Ciriza and Gutiérrez-Larraya6,Reference Haddad, Bonnet, Gewillig and Malekzadeh-Milani12
Surgical correction is the gold-standard treatment, employing baffles to achieve closure of the inter atrial shunt and divert pulmonary venous blood to the left atrium, or caval division (Warden) procedure and anastomosis between the superior vena cava and the right atrial appendage. Reference El-Andari, Moolla and John24,Reference Sojak, Sagat, Balazova and Siman25 These surgical methods, while effective, carry inherent risks associated with open-heart surgery, with long-term morbidity risks such as vascular stenosis and arrhythmia. Reference Sandoval, Rosenthal and Arias18,Reference Rosenthal, Qureshi and Sivakumar23
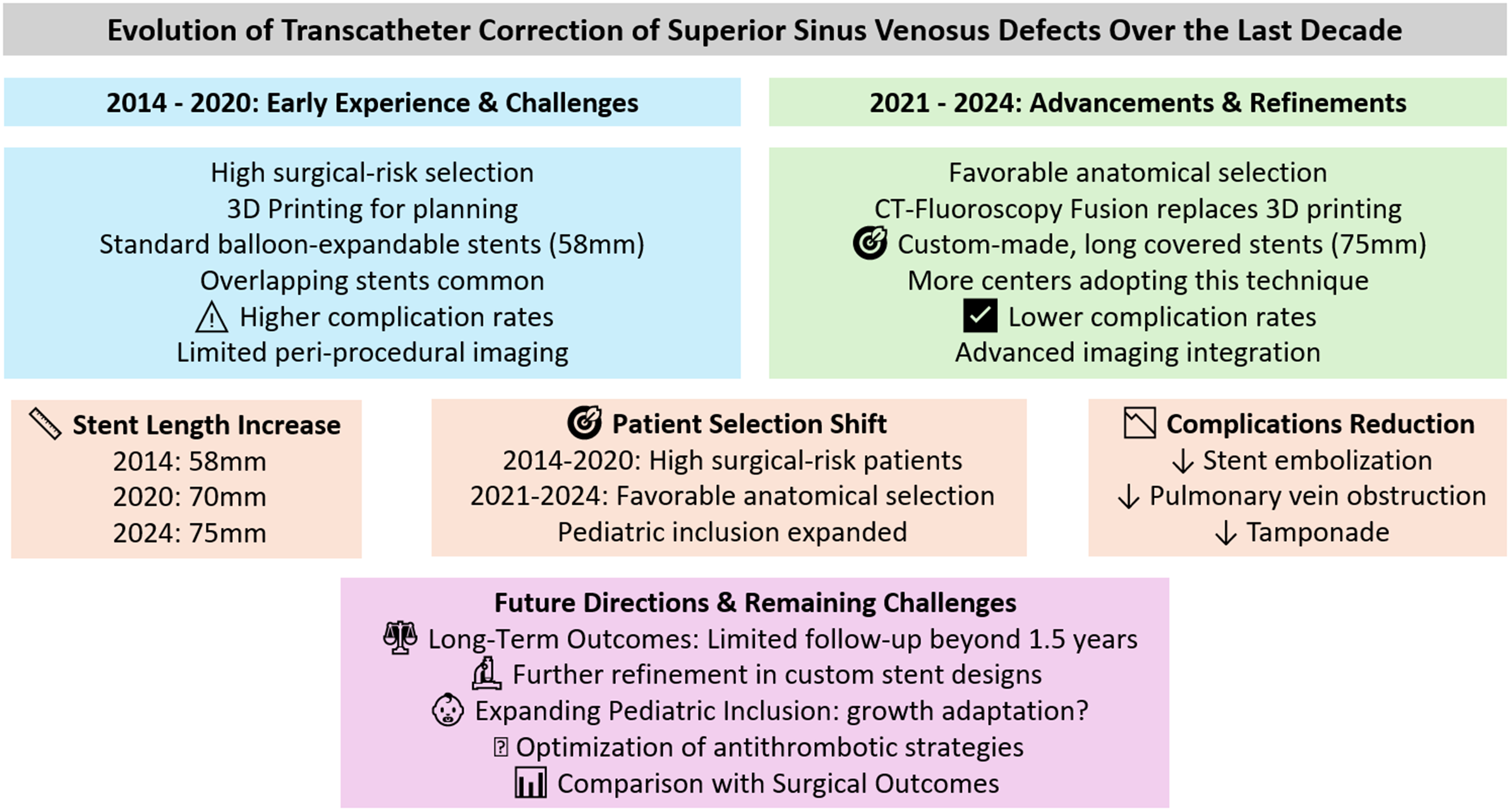
The concept of transcatheter correction was first reported in detail in 2014, focusing on implanting a long, covered stent within the superior vena cava to redirect the anomalous pulmonary venous return to the left atrium while closing the atrial defect. Reference Garg, Tyagi and Radha1 The early experience highlighted challenges, including stent instability with risk of embolisation, recognising that precise pre-procedural planning can mitigate these risks. Reference Hascoët, Roussin and Batteux26–Reference Baruteau, Hascoet and Malekzadeh-Milani28
The evolution of transcatheter correction of superior sinus venosus defect has followed a steep learning curve. Reference Sivakumar21–Reference Rosenthal, Qureshi and Sivakumar23 As more centres have adopted this technique and accumulated experience, the procedure has become more structured, driven by advancements in imaging, Reference Riahi, Velasco Forte and Byrne5 improvements in stent design with custom-made stents, Reference Rosenthal, Qureshi and Jones11 and procedural techniques contributing to enhanced outcomes. Reference Batteux, Ciobotaru, Bouvaist, Kempny, Fraisse and Hascoet19,Reference Hejazi, Hijazi, Saloos, Ibrahim, Mann and Boudjemline15
This analysis aims to provide a comprehensive review of the evolution of transcatheter correction of superior sinus venosus defect over the first decade of its practice. By analysing the outcomes and procedural modifications reported in the literature, this review highlights significant advancements and discusses how these developments have contributed to the improved procedural safety, efficacy, and feasibility.
Methods
Search Strategy and Study Selection: A systematic search of the literature was conducted to identify studies focusing on the percutaneous treatment of superior sinus venosus defect. The databases searched included MEDLINE and Google Scholar. The search terms used were “sinus venosus,” “Sinus Venosus ASD,” “SVD,” “SSVD,” “Sinus Venosus defect,” “Sinus Venous septal defects,” and “SVASD,” combined with “Percutaneous,” “device,” “interventional,” “intervention(al),” and “transcatheter.”
Inclusion and Exclusion Criteria: The initial search yielded 344 results. Studies were included if they reported on the use of transcatheter techniques for the correction of superior sinus venosus defect and provided sufficient data on patient outcomes, procedural details, and follow-up. Case reports, case series, and cohort studies were considered. Studies were excluded if they focused solely on surgical correction or if they lacked adequate outcome data. Articles that were reviews or editorials without original data were also excluded. A total of 23 studies Reference Garg, Tyagi and Radha1–Reference Rosenthal, Qureshi and Sivakumar23 were identified and included in the final analysis. The selection process involved screening titles and abstracts for relevance, followed by a full-text review to confirm eligibility.
Data Extraction and Quality Assessment: Data from the included studies were extracted using a standardised form, capturing information on study design, patient characteristics, procedural details, outcomes, and follow-up. For cohort studies, quality was assessed using the Newcastle-Ottawa Scale, which evaluates studies based on three broad criteria: selection, comparability, and outcome. Scores range from 0 to 9, with higher scores indicating higher quality. Case reports and case series were assessed using the Joanna Briggs Institute checklist. To ensure data accuracy and avoid duplication, patient data reported in multiple publications were meticulously cross-referenced. Overlapping patient cohorts were identified and subtracted as precisely as possible to prevent redundancy and inaccuracies. Due to possible unidentified or misinterpret redundancy the cumulative descriptive results should be considered as an estimate of the precise number and percentage. In cases where patients were included in more than one cohort, the earlier year of publication was assigned. While we used the year of publication to indicate the chronological sequence of data, this approach may present a limitation, particularly in studies that include patients spanning multiple years.
Statistical Analysis: A descriptive synthesis of all included studies was performed. Descriptive and statistics were used to analyse aggregated study characteristics, patient demographics, procedural techniques, and outcomes. A descriptive trend analysis over consecutive time intervals (years) and a comparison between earlier (2014–2020) and later studies (2021–2024) were conducted to observe changes in procedural techniques, materials, outcomes, and procedural success rates over time. Stent lengths were analysed by plotting individual data points with median trends and interquartile ranges, visualising the changes over time. Additionally, a spider graph was generated to compare the distribution of various procedural outcomes and techniques across different study periods, enabling a multi-variable comparison in a single plot. Procedural factors were scaled to a common range (1 to 10) to facilitate comparison. For each factor, raw frequencies or percentages were collected from the earlier and later studies. All statistical analyses were conducted using Python (libraries: Pandas, Matplotlib, and Seaborn).
Ethical Considerations This analysis was conducted in accordance with PRISMA guidelines. As the study involved the analysis of published data, ethical approval was not required.
Results
Quality assessment (appendix 1)
The included studies present a broad spectrum of evidence supporting the evolving practice of percutaneous treatment for superior sinus venosus defect. The 8 cohort studies, assessed using NOS, generally exhibit strong methodological quality, particularly in patient selection and outcome measurement. These studies provide robust evidence supporting the safety and efficacy of transcatheter interventions. However, some limitations were noted in the follow-up duration.
The 15 case reports and series were evaluated using the Joanna Briggs Institute checklist. These studies offer detailed procedural descriptions, exploring the innovative approaches employed in complex cases.
Overall, the combination of 8 cohort studies and 15 case series or reports offers a comprehensive view, but the lack of control groups and long-term follow-up restrict the generalisability.
Description of the studies
The dataset comprises a total of circa 395 subjects across 23 studies evaluating the interventional management of superior sinus venosus defect. These studies were published over a period from 2014 to 2024, with a mean publication year of 2020.8 and a standard deviation of 2.8 years. Age ranged from 4 to 79 years, with a median age of 42.8 years with weights ranging from 17 kg to 145 kg and a median weight of 69 kg.
Pre-interventional phase
Patient selection criteria
Favourable anatomy was the most common criterion for patient selection, accounting for 56% of patients across 11 studies. This focus on anatomical suitability became more prominent in later studies (87% versus 50%). Conversely, 20% of patients across 7 studies were selected primarily due to high surgical risk, a criterion more frequently applied in earlier studies. A combined criterion of favourable anatomy and relative contraindications for surgery was used for 22% of patients across 4 studies.
Exclusion criteria based on imaging findings
For 53% of excluded patients, no specific exclusion criteria were mentioned. When specified, unsuitable anatomy (general consideration) was the exclusion reason for 34% of patients across 4 studies, while more specific anatomical issues, such as a deeper caudal extension of the atrial communication, accounted for the exclusion of 19% of patients across 4 later studies. The detailed specification of such exclusion criteria became more common in recent studies.
Exclusion criteria based on peri-interventional findings
Peri-interventional exclusion criteria were reported with varying levels of detail. For 74% of excluded patients, no specific peri-interventional exclusion criteria were mentioned. The remaining 26% of patients across 9 studies were excluded due to pulmonary vein occlusion observed during balloon test occlusion of the superior vena cava.
Proportion of patients excluded and planned for surgery
In the subset of studies that reported detailed exclusion criteria, 158 patients were evaluated, of which approximately 29% were recommended for surgery. Exclusions were primarily due to the risk of pulmonary vein occlusion during superior vena cava balloon interrogation (51%) or a comment of “unsuitable anatomy” (29%). A smaller proportion of patients were excluded for other anatomical challenges, such as native stenosis of the pulmonary veins or inappropriate right upper pulmonary veins alignment. The exclusion rate was higher in earlier studies (37%), where broader criteria were applied, but lower in later studies (21%).
Pre-interventional imaging
Cardiac CT was the most frequently utilised imaging modality, employed in around 79% of the total patient population and was consistently applied across both earlier and later studies. Cardiovascular magnetic resonance (CMR) was used in 9 studies, though the exact percentage of patients having CMR remains unclear due to inconsistent reporting.
3D printing was used in 9 studies, covering around 17% of the total patients. The use of 3D printed models was more prevalent in earlier studies (40% versus 16%). Virtual Reality Models were employed in 6 studies, involving 17% of total patients. The application of virtual reality was noted in both earlier and later studies, but more frequently in later study.
Peri-interventional phase
Imaging modalities
Transesophageal echocardiography (70% of patients) and conventional angiography (100% of patients) were consistently the cornerstone imaging techniques, universally applied across all studies. In addition to these core modalities, several studies incorporated advanced imaging techniques. CT-fluoroscopy fusion was employed in 15% of patients to enhance procedural accuracy by combining detailed anatomical data from CT with the real-time capabilities of fluoroscopy (28% in later studies versus 9% in earlier studies). transesophageal echocardiography-fluoroscopy fusion was introduced in some later studies. Additionally, 3D-rotational angiography was used in 2 studies.
Vascular access
Right femoral vein was the most utilised vascular access point, employed across all studies. The right internal jugular vein was also frequently used (67% of patients). The left femoral vein was accessed less frequently. Arterial access, including the left femoral artery and other arterial sites, was reportedly used in approximately 10 studies, mostly in earlier studies.
Pulmonary vein access and imaging strategies
Various strategies for pulmonary vein access were employed during superior vena cava balloon test occlusion. Transseptal puncture to gain access was utilised in approximately 36% of the patients of the later studies and 17% of the earlier studies. Retrograde arterial access was reported in 6 studies, encompassing about 12% of the patients.
A combination of conventional angiography and transesophageal echocardiography was the most frequently used imaging technique for pulmonary vein imaging, reported in 14 studies, 84% of the patients. This combination provided detailed visualisation of the pulmonary veins during balloon test occlusion and allowed for continuous monitoring of the pulmonary vein-left atrium pressure gradient. Rotational angiography was mentioned in only 1 study. In 7 studies, which included approximately 28% of the patients, the specific imaging technique for pulmonary vein assessment during balloon test occlusion was not detailed, indicating a potential gap in the reporting of procedural details.
Continuous monitoring of the pulmonary vein-left atrium pressure gradient during balloon test occlusion was specified in 14 studies, covering about 70% of the patients. This practice was typically performed in real-time during the intervention. In the other studies, details on whether pulmonary vein pressure was monitored continuously during the procedure were not provided, which may indicate either a lack of standardised monitoring or, most probably, incomplete reporting.
Balloon test occlusion of the superior vena cava
Compliant balloons were primarily used in 49%, non-compliant balloons in 44%, and semi-compliant balloons in 7% of patients. A combination of both compliant and non-compliant balloons was reported in isolated cases, highlighting a tailored approach based on specific patient needs. There was a trend towards the increased use of non-compliant balloons in the later studies (circa 45% versus 34% of patients).
Regarding the imaging modalities used for selecting balloon size during superior vena cava test occlusion, there was a noticeable shift towards the use of cardiac CT data, either alone or in combination with transesophageal echocardiography, instead of conventional angiograms. Angiography alone was used when other imaging modalities were unavailable or when the anatomy was less complex. Rotational angiography, an advanced technique, was used in only one study.
Stent implantation
Balloon-expandable stents were the primary choice in 96% of cases. The most common type was the Cheatham-Platinum Stent (NuMED Inc.), used in 71% of cases as a standalone option. The Optimus-CVS PTFE-Covered XXL (AndraTec**GmbH), was used in 11% of cases, often as a double stent technique. The G-ARMOR™ (NuMED Inc.) accounted for 3% of cases, while the BeGraft (Bentley) was less frequently used, noted in about <1% of cases. The type of stent used across the different years is plotted in Figure 1.
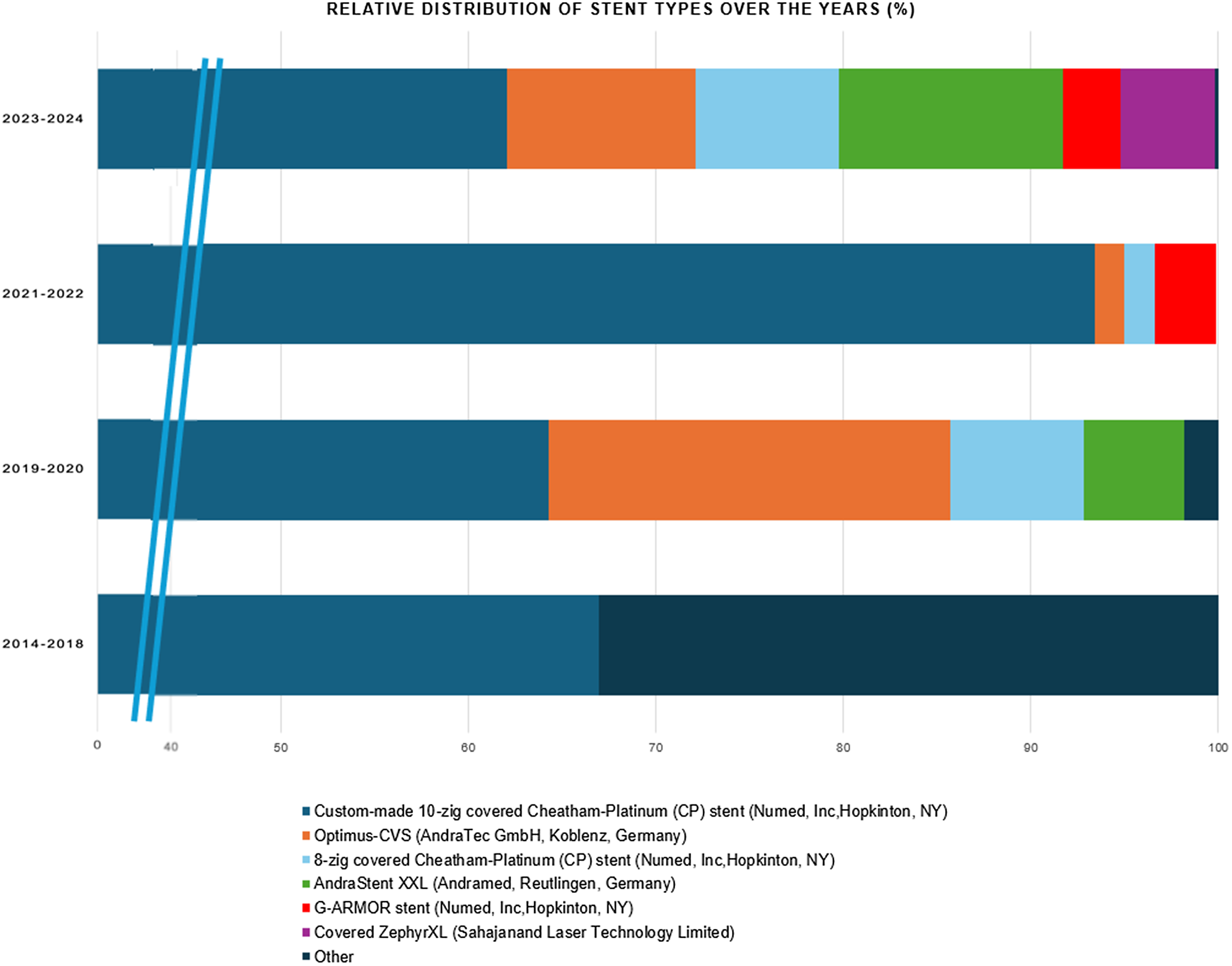
Figure 1. Distribution of stent types over the years. This bar graph illustrates the percentage distribution of different stent types across various year groups. The predominant stent, represented in blue, is the custom-made 10-zig covered Cheatham-platinum (CP) stent. Other stent types, including the optimus-CVS (orange), 8-zig covered CP stent (light blue), andraStent XXL (green), G-ARMOR stent (red), and covered zephyrXL (purple), are less frequently used but start to appear more prominently in the later years. The x-axis has been condensed between 0–40% to increase the visibility of these newer stent types.
The median total individual stent length exhibited an increasing trend across the years. In 2018, it was 55 mm (interquartile range: 50–60 mm), followed by an increase to 60 mm in 2019 (interquartile range: 60–60 mm). In 2020, the median rose to 70 mm (IQR: 60–75 mm), where it remained stable in 2021 (interquartile range: 60–80 mm). In 2022, it further increased to 75 mm (IQR: 60–80 mm), and by 2023 and 2024, the median consistently reached 75 mm with interquartile range of 75–100 mm and 65–75 mm, respectively. This overall upward trend in stent lengths over the years is accompanied by an increasing interquartile range, reflecting a broader variation in stent lengths used (Figure 2).
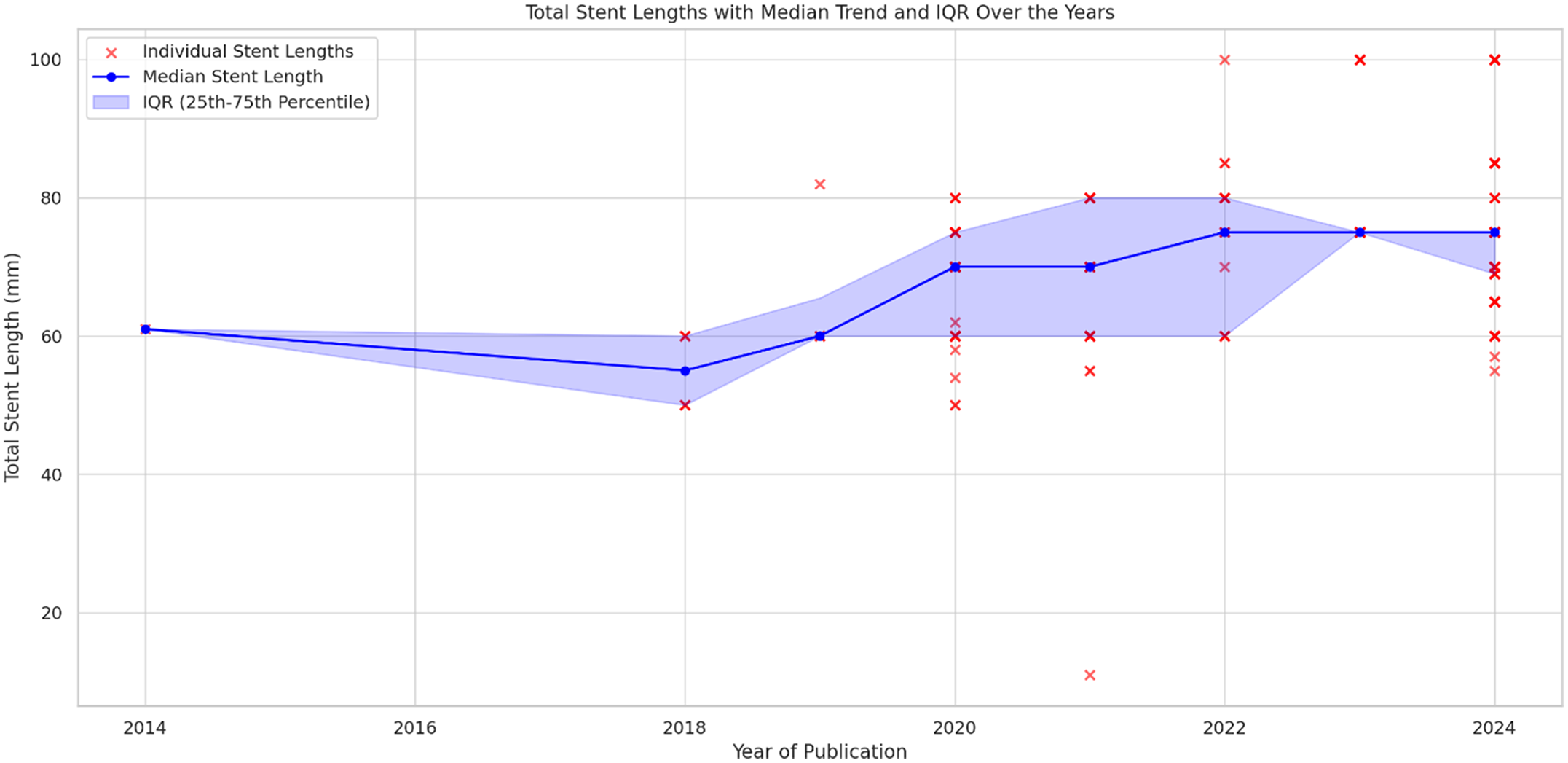
Figure 2. Stent lengths with median trend and IQR over the years. This scatter plot and line graph combination illustrates individual stent lengths (red dots) plotted alongside the median stent length (blue line) for each year. Additionally, the shaded blue region represents the interquartile range (IQR). A gradual increase in stent lengths is observed, with the median stent length rising from around 58 mm in 2018 to approximately 75 mm by 2024. The variability of stent lengths, as indicated by the widening IQR, becomes more pronounced, reflecting an increase in the range of stent lengths used over time. The graph reveals a clear upward trend in stent lengths, with greater variability in the stent selection process in recent years.
Self-expandable stents were utilised in only 4% of cases, reserved for specific situations requiring more flexibility. Among these, the Zenith Flex AAA Endovascular Graft Extension Cuffs (Cook Medical) was used in 1 case. Other self-expandable stents included the Endurant Self-expanding Stent, used in 2% of cases, and the Nano Self-expanding Stent, employed in 1 case.
Single covered stent placement was employed in 50% of patients and remains the most common approach across the studies, especially in recent years. In more recent studies, there is a marked trend towards the use of single, custom-made, longer stents (80% of patients). Overlapping covered stents were more commonly used as a reactive strategy due to residual shunt or since a single covered stent in the desired length was not available. The use of anchoring bare-metal stents is not consistently reported and seems to relate to a case-based strategy. They were primarily used reactively in instances where the initial single stent placement was unstable or when additional anchoring was required.
The veno-venous loop technique, involving the creation of a stable rail between the femoral vein and the internal jugular vein, was explicitly described in 12 studies, accounting for 68% of the total patient population. This suggests that while the technique was available, it was not uniformly applied, likely depending on specific procedural needs or operator preference.
Suture holding techniques were utilised in 13% of patients. This approach was generally applied in cases requiring additional stability, especially in more complex anatomies or when there was concern over stent migration or instability. While this technique is not as common, it has been more frequently reported in the context of newer studies.
Use of atrial septal occluders
In the earlier studies, atrial septal occluders were used in 4% of patients possibly due to less refined stenting techniques or a greater need for additional closure mechanisms. However, in more recent studies, the use of occluders decreased to 2%.
Pulmonary vein strategy
Balloon inflation as pulmonary vein protection strategy during stent deployment was utilised in 28% of patients across 18 studies, in the other patients a bailout balloon was available but not inflated. This last strategy was the most used in the recent studies.
Post-interventional phase
Peri-interventional complications
Procedure abortion occurred in approximately 7% of cases, normally due to the risk of pulmonary vein occlusion. However, this frequency declined in more recent studies to 2%. Stent embolisation was noted in 10 patients, all with surgical retrieval. The frequency of stent embolisation was higher in earlier studies. Tamponade was reported in 2 cases, Reference Hansen, Duong and Jivanji10,Reference Rosenthal, Qureshi and Jones11 necessitating surgical drainage in all instances, proportionally more frequent in earlier studies. Pulmonary vein obstruction leading to lobectomy was described in 1 case. Reference Rosenthal, Qureshi and Jones11 However, this complication has not been seen in more recent studies. Thrombotic complications were rare, occurring in 6 of patients. Reference Rosenthal, Qureshi and Jones11,Reference Hejazi, Hijazi, Saloos, Ibrahim, Mann and Boudjemline15,Reference Rosenthal, Qureshi and Sivakumar23 These issues typically involved small distal stent clots resolved with anticoagulation. There was no significant trend over time in the occurrence of thrombotic events.
Access site complications were reported in 10 patients, Reference Riahi, Velasco Forte and Byrne5,Reference Hansen, Duong and Jivanji10,Reference Rosenthal, Qureshi and Jones11,Reference Hejazi, Hijazi, Saloos, Ibrahim, Mann and Boudjemline15,Reference Rosenthal, Qureshi and Sivakumar23 including minor hematomas and more severe issues like femoral artery pseudoaneurysm. The was one case of major access site bleeding. Transient arm neuropraxia was mentioned infrequently, affecting 4 of patients.(Reference Hansen, Duong and Jivanji10,Reference Rosenthal, Qureshi and Jones11,Reference Rosenthal, Qureshi and Sivakumar23)
Arrhythmias Reference Hansen, Duong and Jivanji10,Reference Rosenthal, Qureshi and Jones11,Reference Vettukattil, Subramanian, Barthur and Mahimarangaiah16,Reference Sandoval, Rosenthal and Arias18,Reference Rosenthal, Qureshi and Sivakumar23 occurred in 7 patients, with atrial fibrillation (3 patients) and sinus node dysfunction (2 patients) being the most common types. Sinus node dysfunction required pacemaker implantation three days after the intervention in one case. Reference Rosenthal, Qureshi and Sivakumar23 The incidence of arrhythmias did not significantly decrease over time.
Antithrombotic strategy
The most common post-interventional antithrombotic strategy was a combination of dual antiplatelet therapy (DAT) for 2 months followed by ASA for 6 months. In high-risk cases, this regimen was modified by extending dual antiplatelet therapy for 2 months followed by aspirin for 6 months, and in some instances, aspirin was prescribed lifelong if a residual shunt was present. Aspirin alone was used in 4% of patients for 12 months, while another strategy combining aspirin for 6 months with anticoagulation for 6 months was also used in 4% of patients. Some studies employed an extended dual antiplatelet therapy regimen for 6 to 12 months, coupled with aspirin for 12 months. In 40% of patients, the specifics of the antithrombotic strategy were not provided.
Follow-up
The overall follow-up time across all studies was approximately 1.6 years, with a range from 1 day to 12.4 years.
Residual leaks were observed in 6% of patients during follow-up. Trace residual leaks immediately after the procedure were generally insignificant and did not require intervention. However, significant shunts, which necessitated further intervention (additional stent implantation or septal occluder device) within 6 months, were observed in 8 patients. Reference Butera, Sturla, Pluchinotta, Caimi and Carminati7,Reference Abdullah, Alsalkhi and Khalid8,Reference Brancato, Stephenson and Rosenthal17,Reference Sagar, Sivakumar, Thejaswi and Rajendran22,Reference Rosenthal, Qureshi and Sivakumar23 No procedure-related mortality was reported.
Paediatric patients
Ten studies specifically reported on the treatment of paediatric patients (under 18 years of age) with transcatheter correction of superior sinus venosus defect. A total of at least 51 paediatric patients were treated across these studies, with 37 of these patients reported in the study by Rosenthal et al. (2024). Reference Rosenthal, Qureshi and Sivakumar23 However, this last study did not analyse the paediatric patients separately from the adult cohort, and thus, specific details on the paediatric population were not provided. In the other nine studies, 14 paediatric patients were included, ranging from 6 to 17 years old. Reference Butera, Sturla, Pluchinotta, Caimi and Carminati7–Reference Hansen, Duong and Jivanji10,Reference Schleiger, Nordmeyer, Kramer, Berger, Egorova, Ahmed, Moreno-Ruíz, Monosilio and Mohan14,Reference Brancato, Stephenson and Rosenthal17,Reference Kusa, Skierska and Olczak20–Reference Sagar, Sivakumar, Thejaswi and Rajendran22 These patients were selected for transcatheter intervention based on a variety of factors, including the complex anatomy that posed high surgical risks, comorbid conditions such as pulmonary hypertension, and parental preference to avoid open-heart surgery. Reference Abdullah, Alsalkhi and Khalid8,Reference Hansen, Duong and Jivanji10
The stents used in these paediatric interventions were primarily CP stents. Reference Butera, Sturla, Pluchinotta, Caimi and Carminati7–Reference Hansen, Duong and Jivanji10,Reference Schleiger, Nordmeyer, Kramer, Berger, Egorova, Ahmed, Moreno-Ruíz, Monosilio and Mohan14,Reference Brancato, Stephenson and Rosenthal17,Reference Kusa, Skierska and Olczak20–Reference Rosenthal, Qureshi and Sivakumar23 In some instances, additional stents were implanted to address instability or residual shunts. CP stent length ranged 39-60 mm normally balloon-in-balloon (BIB) balloons with diameters ranged 12–18 mm. Reference Butera, Sturla, Pluchinotta, Caimi and Carminati7,Reference Abdullah, Alsalkhi and Khalid8,Reference Kusa, Skierska and Olczak20 Vascular access was femoral vein in all cases. Reference Butera, Sturla, Pluchinotta, Caimi and Carminati7–Reference Hansen, Duong and Jivanji10,Reference Schleiger, Nordmeyer, Kramer, Berger, Egorova, Ahmed, Moreno-Ruíz, Monosilio and Mohan14,Reference Brancato, Stephenson and Rosenthal17,Reference Kusa, Skierska and Olczak20–Reference Sagar, Sivakumar, Thejaswi and Rajendran22
The outcomes for these patients were favourable. All studies reported successful defect closure with no major residual shunting or stent migration. No significant complications related to vascular access were reported. The long-term follow-up data, where available, indicated stable stent placement. Reference Butera, Sturla, Pluchinotta, Caimi and Carminati7,Reference Sivakumar21,Reference Sagar, Sivakumar, Thejaswi and Rajendran22
Discussion
Pre-interventional phase
Over time, the criteria for patient selection became more refined. There was a clear shift from selecting patients with a high risk for a surgical repair towards a focus on favourable anatomy in later studies. Ideal anatomy can be defined as a defect that does not have a caudal extension towards the oval fossa and drainage of right upper pulmonary vein is relatively posterior and close to the cavoatrial junction. Reference Sivakumar, Qureshi, Pavithran, Vaidyanathan and Rajendran9,Reference Sivakumar27 Exclusion criteria also became more detailed and consistently applied in recent studies. Reference Butera, Sturla, Pluchinotta, Caimi and Carminati7,Reference Morgan and Zablah13,Reference Batteux, Ciobotaru, Bouvaist, Kempny, Fraisse and Hascoet19 In the subset of earlier studies that reported detailed exclusion criteria Reference Sivakumar, Qureshi, Pavithran, Vaidyanathan and Rajendran9–Reference Hansen, Duong and Jivanji10,Reference Brancato, Stephenson and Rosenthal17–Reference Batteux, Ciobotaru, Bouvaist, Kempny, Fraisse and Hascoet19 37% of the selected patients were excluded from transcatheter superior sinus venosus defect correction and recommended for surgery. The secondary (peri-procedural) exclusion rate was lower in later studies (21%), indicating more effective patient referral and preliminary screening. Advanced imaging modalities such as 3D printing were standardly utilised in earlier studies to ensure procedural success, Reference Pascual-Tejerina, Sánchez-Recalde, Cantador, López, Gómez-Ciriza and Gutiérrez-Larraya6,Reference Sivakumar, Qureshi, Pavithran, Vaidyanathan and Rajendran9 their use decreased slightly in later studies Reference Riahi, Velasco Forte and Byrne5,Reference Abdullah, Alsalkhi and Khalid8,Reference Schleiger, Nordmeyer, Kramer, Berger, Egorova, Ahmed, Moreno-Ruíz, Monosilio and Mohan14,Reference Sagar, Sivakumar, Thejaswi and Rajendran22,Reference Rosenthal, Qureshi and Sivakumar23 with was a shift towards a more selective use of these advanced imaging modalities. Reference Vettukattil, Subramanian, Barthur and Mahimarangaiah16,Reference Kusa, Skierska and Olczak20 This evolution suggests that extensive pre-planning, while still important, became less universally necessary as procedural techniques and operator experience developed, allowing for a more streamlined approach in less complex cases. Reference Sivakumar, Qureshi, Pavithran, Vaidyanathan and Rajendran9,Reference Sandoval, Rosenthal and Arias18,Reference Rosenthal, Qureshi and Sivakumar23
Peri-interventional phase
Imaging modalities
The consistency in using transesophageal echocardiography across both earlier and later studies underscores the fundamental importance of this imaging technique, which was used in almost in all patients. A clear trend emerged in later studies, showing a significant increase in the use of advanced imaging techniques, including CT-fluoroscopy fusion Reference Rosenthal, Qureshi and Jones11,Reference Hejazi, Hijazi, Saloos, Ibrahim, Mann and Boudjemline15,Reference Sagar, Sivakumar, Thejaswi and Rajendran22 and transesophageal echocardiography-fluoroscopy fusion. Reference Sivakumar21,Reference Rosenthal, Qureshi and Sivakumar23 Overall, there is a trend towards integrating advanced imaging techniques in more complex cases during the intervention to enhance interventional decision making and techniques, tailored to the specific needs of each patient. Reference Butera, Sturla, Pluchinotta, Caimi and Carminati7,Reference Hansen, Duong and Jivanji10,Reference Sandoval, Rosenthal and Arias18,Reference Kusa, Skierska and Olczak20,Reference Rosenthal, Qureshi and Sivakumar23 Nevertheless, experienced operators demonstrated excellent results using core imaging only. Reference Sivakumar, Qureshi, Pavithran, Vaidyanathan and Rajendran9,Reference Hejazi, Hijazi, Saloos, Ibrahim, Mann and Boudjemline15,Reference Sivakumar21
Vascular access
The data illustrate a consistent use of the right femoral vein and right internal jugular vein. Reference Garg, Tyagi and Radha1–Reference Velasco Forte, Byrne and Valverde4,Reference Pascual-Tejerina, Sánchez-Recalde, Cantador, López, Gómez-Ciriza and Gutiérrez-Larraya6,Reference Butera, Sturla, Pluchinotta, Caimi and Carminati7 Arterial access, including the left femoral artery and other arterial sites, was utilised in a smaller subset of patients. However, there is a slight decrease in the use of arterial access in the later studies, which suggests a growing confidence with this procedure and a different access site to the pulmonary veins. Reference Sagar, Sivakumar, Thejaswi and Rajendran22,Reference Rosenthal, Qureshi and Sivakumar23 The vascular access varied depending on patient’s characteristic and relates to an evolving practice aimed at enhancing procedural precision and outcomes in more complex cases. Reference Sivakumar, Qureshi, Pavithran, Vaidyanathan and Rajendran9,Reference Kusa, Skierska and Olczak20,Reference Rosenthal, Qureshi and Sivakumar23
Pulmonary vein access and imaging strategies
There was a noticeable shift in the preferred methods for pulmonary vein access. In the later studies, transseptal access became more prevalent, Reference Hansen, Duong and Jivanji10,Reference Vettukattil, Subramanian, Barthur and Mahimarangaiah16,Reference Rosenthal, Qureshi and Sivakumar23 while earlier studies relied more on access across the superior sinus venosus defect into the pulmonary veins from femoral vein access. Reference Riahi, Velasco Forte and Byrne5,Reference Abdullah, Alsalkhi and Khalid8 Regarding techniques for imaging the pulmonary veins, only one study Reference Velasco Forte, Byrne and Valverde4 relied on advanced imaging techniques (rotational angiography or 3D transesophageal echocardiography). Specifically, the combination of conventional angiography with 2D transesophageal echocardiography was the standard approach. Later studies consistently referred to continuous monitoring of pulmonary vein pressure, Reference Sivakumar, Qureshi, Pavithran, Vaidyanathan and Rajendran9–Reference Rosenthal, Qureshi and Sivakumar23 to decrease risk.
Balloon occlusion of the superior vena cava
There was a trend towards the increased use of non-compliant balloons in the later studies, Reference Schleiger, Nordmeyer, Kramer, Berger, Egorova, Ahmed, Moreno-Ruíz, Monosilio and Mohan14,Reference Brancato, Stephenson and Rosenthal17–Reference Batteux, Ciobotaru, Bouvaist, Kempny, Fraisse and Hascoet19,Reference Rosenthal, Qureshi and Sivakumar23 but more interesting is the trend towards the increasing use of a combination of compliant and non-compliant balloons. Reference Batteux, Ciobotaru, Bouvaist, Kempny, Fraisse and Hascoet19,Reference Rosenthal, Qureshi and Sivakumar23 Specifically, if there is any sign pulmonary vein occlusion during compliant balloon inflation, the balloon is exchanged for a non-compliant or semi-compliant balloon to reduce bulging of the balloon in the pulmonary vein as cause of obstruction. Reference Sivakumar, Qureshi, Pavithran, Vaidyanathan and Rajendran9,Reference Rosenthal, Qureshi and Sivakumar23,Reference Hascoët, Roussin and Batteux26,Reference Sivakumar27
Stent implantation
Balloon-expandable stents, particularly the Cheatham-platinum (CP) stent, dominated the field. The use of self-expandable stents remained relatively rare and was typically reserved for isolated cases. Reference Abdullah, Alsalkhi and Khalid8,Reference Haddad, Bonnet, Gewillig and Malekzadeh-Milani12 Regarding the technique of implantation there was a shift towards single, custom-made stents. In the early phases of superior sinus venosus defect treatment, overlapping covered stents were more commonly used. This was probably due to the limitations in stent design and customisation at the time combined with a limited experience. Reference Gertz, Strife, Shah, Parris and Grizzard3,Reference Abdullah, Alsalkhi and Khalid8,Reference Vettukattil, Subramanian, Barthur and Mahimarangaiah16 This change also reflects significant advancements in imaging technologies, which allow for more precise measurements and matching with the specific anatomical needs. Reference Hansen, Duong and Jivanji10,Reference Schleiger, Nordmeyer, Kramer, Berger, Egorova, Ahmed, Moreno-Ruíz, Monosilio and Mohan14,Reference Batteux, Ciobotaru, Bouvaist, Kempny, Fraisse and Hascoet19,Reference Sivakumar21–Reference Rosenthal, Qureshi and Sivakumar23 The result has been a more stable and secure stent placement, reducing the need for overlapping stents and atrial septal occluder to treat residual shunt. Reference Butera, Sturla, Pluchinotta, Caimi and Carminati7,Reference Haddad, Bonnet, Gewillig and Malekzadeh-Milani12,Reference Hejazi, Hijazi, Saloos, Ibrahim, Mann and Boudjemline15,Reference Kusa, Skierska and Olczak20,Reference Rosenthal, Qureshi and Sivakumar23
The use of suture holding techniques Reference Haddad, Bonnet, Gewillig and Malekzadeh-Milani12,Reference Hejazi, Hijazi, Saloos, Ibrahim, Mann and Boudjemline15 has emerged in recent years. Although not as widely used this technique is frequently applied in specific scenarios where added stability is crucial. Reference Butera, Sturla, Pluchinotta, Caimi and Carminati7,Reference Morgan and Zablah13,Reference Brancato, Stephenson and Rosenthal17 This method has gained attention in complex cases where there is a high risk of stent migration or where anatomical features demand extra security. Reference Hejazi, Hijazi, Saloos, Ibrahim, Mann and Boudjemline15,Reference Sivakumar21,Reference Rosenthal, Qureshi and Sivakumar23
Pulmonary vein strategy
In older studies, balloon inflation during stent deployment was commonly used as a preventive measure. Reference Riahi, Velasco Forte and Byrne5,Reference Haddad, Bonnet, Gewillig and Malekzadeh-Milani12,Reference Sandoval, Rosenthal and Arias18 More recent studies show an increasing focus on the use of bailout balloons as a reactive measure in cases of mild right upper pulmonary vein obstruction without the prophylactic use of a protective balloon inflation. Reference Hansen, Duong and Jivanji10,Reference Brancato, Stephenson and Rosenthal17,Reference Rosenthal, Qureshi and Sivakumar23 This trend suggests an increasing procedural confidence and a thoughtful balance of both proactive and reactive strategies in ensuring pulmonary vein protection. Reference Butera, Sturla, Pluchinotta, Caimi and Carminati7,Reference Schleiger, Nordmeyer, Kramer, Berger, Egorova, Ahmed, Moreno-Ruíz, Monosilio and Mohan14,Reference Batteux, Ciobotaru, Bouvaist, Kempny, Fraisse and Hascoet19,Reference Sagar, Sivakumar, Thejaswi and Rajendran22
Operator learning curve and procedural refinement
Across the decade of published experience, there is a discernible trend that reflects an operator learning curve influencing procedural efficiency and safety. Although procedural metrics such as fluoroscopy time or procedure duration were inconsistently reported, the observed reduction in complication rates, the shift towards more standardised imaging and implantation techniques, and the growing adoption of single custom-made stents are indirect indicators of increased operator proficiency. Early complications such as stent embolisation, procedural abortion, or residual leaks were more frequent during initial experiences and diminished in later cohorts, suggesting that procedural success and safety improved in parallel with increased operator familiarity and technical refinement. While the current analysis does not allow for direct stratification by operator experience, the overall trends support the notion that accumulating institutional and operator expertise has played an important role in enhancing outcomes.
Post-interventional phase
Periinterventional complications
When considering trends over time a significant decrease in several key complications is evident. Reference Pascual-Tejerina, Sánchez-Recalde, Cantador, López, Gómez-Ciriza and Gutiérrez-Larraya6,Reference Sivakumar, Qureshi, Pavithran, Vaidyanathan and Rajendran9,Reference Hejazi, Hijazi, Saloos, Ibrahim, Mann and Boudjemline15 Procedure abortion rates fell to 2% in more recent studies. Reference Abdullah, Alsalkhi and Khalid8,Reference Schleiger, Nordmeyer, Kramer, Berger, Egorova, Ahmed, Moreno-Ruíz, Monosilio and Mohan14,Reference Rosenthal, Qureshi and Sivakumar23 As experience with the procedure grew, the incidence of complications also diminished. Reference Sivakumar, Qureshi, Pavithran, Vaidyanathan and Rajendran9,Reference Schleiger, Nordmeyer, Kramer, Berger, Egorova, Ahmed, Moreno-Ruíz, Monosilio and Mohan14,Reference Rosenthal, Qureshi and Sivakumar23 For instance, stent embolisation, which was observed in 10 patients across the studies and required surgical retrieval, became less common in later studies. Reference Sivakumar, Qureshi, Pavithran, Vaidyanathan and Rajendran9–Reference Rosenthal, Qureshi and Jones11,Reference Rosenthal, Qureshi and Sivakumar23 Similarly, cardiac tamponade, which necessitated surgical drainage in all reported cases, has decreased in frequency. Reference Hansen, Duong and Jivanji10,Reference Rosenthal, Qureshi and Jones11,Reference Rosenthal, Qureshi and Sivakumar23 The decline in pulmonary vein obstruction, which in 1 case led to lobectomy, Reference Rosenthal, Qureshi and Jones11 further illustrates the improved outcomes associated with enhanced pre-procedural planning.
Thrombotic complications remained rare Reference Rosenthal, Qureshi and Jones11,Reference Hejazi, Hijazi, Saloos, Ibrahim, Mann and Boudjemline15,Reference Rosenthal, Qureshi and Sivakumar23 under the established antithrombotic strategies. Arrhythmias were reported in 7 patients, Reference Hansen, Duong and Jivanji10,Reference Rosenthal, Qureshi and Jones11,Reference Vettukattil, Subramanian, Barthur and Mahimarangaiah16,Reference Sandoval, Rosenthal and Arias18,Reference Rosenthal, Qureshi and Sivakumar23 with sinus node dysfunction leading to pacemaker implantation in one case. Reference Sandoval, Rosenthal and Arias18 While the incidence of arrhythmias has not significantly decreased over time, the recognition of the risk associated with excessive stretching in the atrium in the region of the sinus node has been discussed, especially since it is possible that the sinus node or its arteries are more subendocardially and cranially located than usual. Reference Sandoval, Rosenthal and Arias18 However, systematic rhythm monitoring, such as routine post-procedural Holter ECG or long-term rhythm surveillance, was not consistently reported across studies, which limits comprehensive evaluation of the true arrhythmic burden over time.
Antithrombotic strategy
Earlier studies primarily used aspirin alone or combined it with short-term anticoagulation, with less frequent use of extended dual antiplatelet therapy. Reference Pascual-Tejerina, Sánchez-Recalde, Cantador, López, Gómez-Ciriza and Gutiérrez-Larraya6,Reference Abdullah, Alsalkhi and Khalid8,Reference Batteux, Ciobotaru, Bouvaist, Kempny, Fraisse and Hascoet19 More recent studies show a clear trend towards utilising dual antiplatelet therapy followed by aspririn, reflecting a growing consensus on this approach’s effectiveness in preventing thrombotic complications post-interventionally. Reference Butera, Sturla, Pluchinotta, Caimi and Carminati7,Reference Schleiger, Nordmeyer, Kramer, Berger, Egorova, Ahmed, Moreno-Ruíz, Monosilio and Mohan14,Reference Sivakumar21 The extension of antithrombotic therapy in high-risk cases further underscores the refinement of these strategies in line with patient risk profiles. Reference Haddad, Bonnet, Gewillig and Malekzadeh-Milani12,Reference Hejazi, Hijazi, Saloos, Ibrahim, Mann and Boudjemline15,Reference Rosenthal, Qureshi and Sivakumar23
Follow-up
In the earlier studies, outcomes were more frequently marked by complications such as residual leaks leading to reintervention with additional stent placement or atrial septal defects device implantation. Reference Riahi, Velasco Forte and Byrne5,Reference Haddad, Bonnet, Gewillig and Malekzadeh-Milani12,Reference Sandoval, Rosenthal and Arias18,Reference Mejia, Leahy, Zablah and Morgan29 This was largely attributable to the initial learning curve associated with the procedure and the limitations in available techniques and stent designs at that time. Reference Abdullah, Alsalkhi and Khalid8,Reference Schleiger, Nordmeyer, Kramer, Berger, Egorova, Ahmed, Moreno-Ruíz, Monosilio and Mohan14,Reference Kusa, Skierska and Olczak20
The precision offered by new peri-interventional imaging and adopted custom-made, longer stents designed for single deployment modalities allow for better pre-procedural planning and more accurate stent deployment, which in turn seems to lead to a reduction in the incidence of residual leaks compared to earlier reports. However, the lack of standardised follow-up protocols across studies—such as variations in imaging modalities, timing of assessments, and clinical endpoints—introduces significant variability in outcome reporting. This inconsistency limits the ability to uniformly assess long-term efficacy and the durability of complete defect closure across different cohorts.
Conclusion
The evolution of practice in the treatment of sinus venosus defect over the past decade has shown significant advancements in patient selection, imaging techniques, and procedural strategies (Figure 3). There was a marked shift from selecting patients based primarily on high surgical risk to focusing more on favourable anatomical considerations, which was reflected in a lower secondary exclusion rate in later studies. The use of advanced imaging modalities like 3D printing was initially common but has decreased slightly as the field matured, with a notable increase in the use of CT-fluoroscopy fusion.
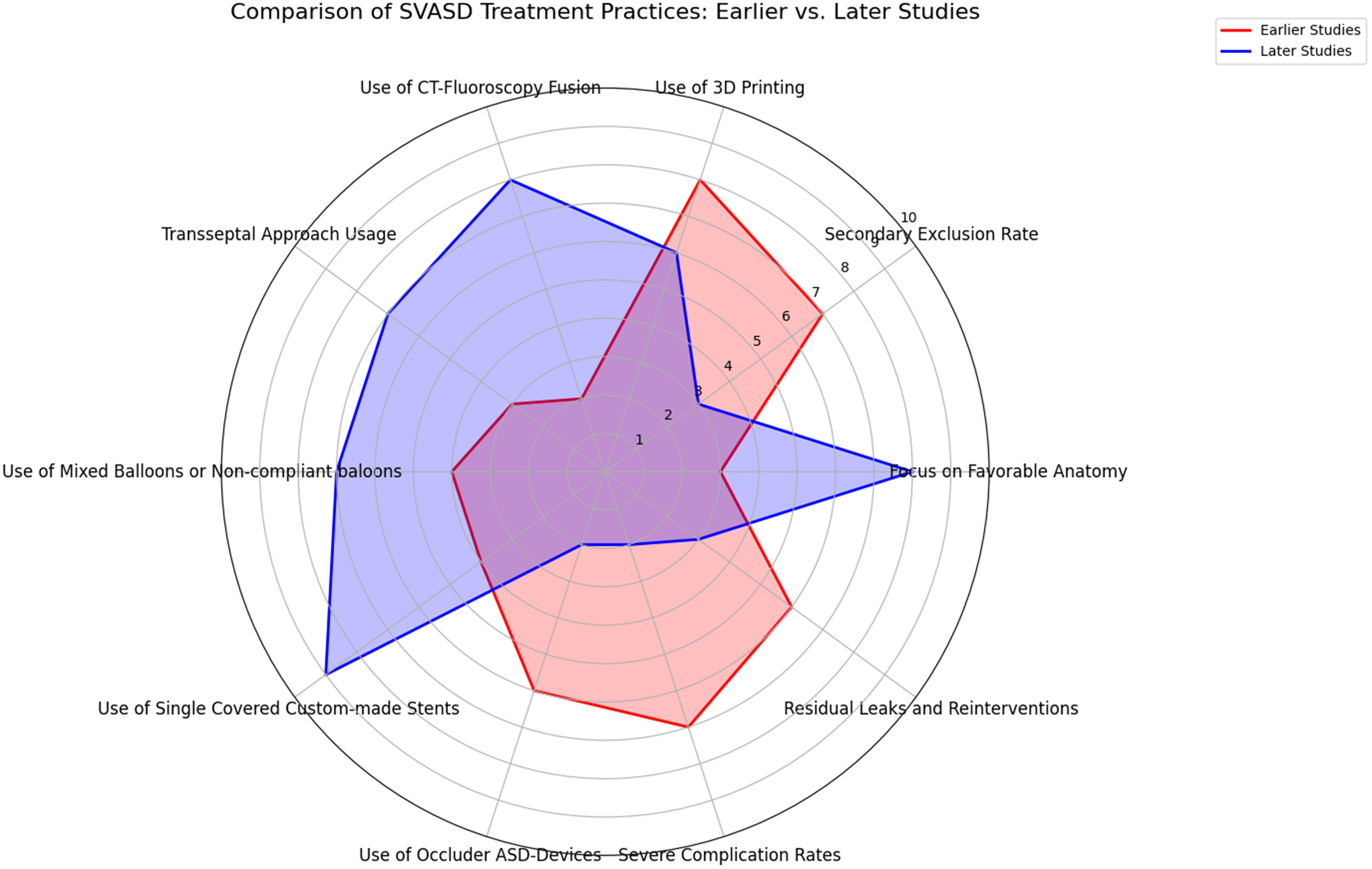
Figure 3. Spider chart comparing key aspects of SSVD treatment practices between earlier and later studies. This radar chart illustrates the evolution of superior vena cava syndrome treatment practices by comparing key metrics between earlier (2014–2020) and later studies (2021–2024). favorable anatomy: later studies show an increased focus on selecting patients based on favorable anatomy. Secondary exclusion rate: a lower secondary exclusion rate in later studies indicates improved preliminary screening and patient referral. Advanced imaging: while 3D printing was more common in earlier studies, later studies show increased use of CT-fluoroscopy fusion. Transseptal approach to the pulmonary veins: the transseptal approach became more prevalent in later studies. Non-compliant or mixed balloons: later studies show a more targeted use of compliant and non-compliant balloons. Single custom-made stents: a shift towards using single, long, custom-made covered stents is evident in later studies. Complication rates: later studies report fewer severe complications, such as stent embolisation and tamponade, and lower rates of residual leaks and reinterventions.
Procedural strategies have also evolved with a growing preference for a combination of compliant and non-compliant or non-compliant balloons to assess more precisely the risk of pulmonary vein obstruction. Moreover, there was a significant shift towards the use of single, custom-made stents, replacing the earlier reliance on overlapping stents and reducing the need for septal occluder devices.
The reduction in severe complications such as stent embolisation, tamponade, and pulmonary vein obstruction supports an ongoing refinement of sinus venosus defect correction techniques. The integration of advanced peri-interventional imaging and the adoption of custom-made stents have improved pre-procedural planning and accuracy in stent deployment, contributing to a reduction in residual leaks and the need for reinterventions.
In conclusion, the procedure’s refinement has allowed for broader patient inclusion, including selected paediatric cases. This expansion of the patient population, coupled with continuous technological improvements, suggests that transcatheter correction of sinus venosus defect is becoming an increasingly viable alternative to traditional surgical approaches. Continued exploration through larger, more rigorous cohort studies will be essential to validate these findings and enhance the long-term understanding of this emerging treatment modality.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S1047951125100681.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article. Supplementary data that support the findings of this study are available from AC upon reasonable request.
Author contribution
GM and AC conceptualised study. AC and GM drafted the manuscript. All authors made substantial contributions to the design of the work and revised it critically for important intellectual content and gave their final approval of the version to be published.
Statement of authorship
All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Financial support
None.
Competing interests
Gareth J Morgan is consultant for NuMED Inc.
Ethical standard
This analysis was conducted in accordance with PRISMA guidelines. As the study involved the analysis of published data, ethical approval was not required.