Introduction
During the COVID-19 pandemic, care home residents in England were disproportionately affected, experiencing high rates of severe illness and mortality [1]. Throughout the pandemic, older age, frailty, and underlying health conditions were consistently the main individual-level risk factors for severe COVID-19 outcomes, including hospitalization and death [Reference Hollinghurst2]. In 2021, the median age of residents in care homes for adults over 65 in England was 86 years and 5 months [3]. Prior to the rollout of the COVID-19 vaccination programme on 8 December 2020, and during the emergence of SARS-CoV-2 Variants of Concern (VOCs), the heightened vulnerability of older care home residents led to extensive and prolonged restrictions on visits, communal activities, and staff movement, on a scale not previously seen. Vulnerability to both severe COVID-19 outcomes and the unintended effects of public health and social measures [4] varied considerably between different types of care homes. Residents of care homes for younger adults primarily require care due to learning disabilities, autism, or other neurodevelopmental conditions. Although a proportion of these residents were at increased risk of severe outcomes due to underlying health conditions, many were not.
Care homes in England are registered with the Care Quality Commission (CQC) according to specialism, with distinct registration for caring for older adults over 65 years and younger adults under 65, and the option to register for more than one category of care [5]. Small care homes (SCHs), defined in line with CQC criteria as those registered with 10 or fewer beds, generally have communal facilities such as kitchens and living areas. They typically provide homes for residents under 65 years with learning and/or physical disabilities. These residents may actively engage in external leisure activities, education, training, and employment. From a transmission perspective, SCHs are often comparable to settings registered with the CQC as supported living or extra care housing and may differ only in that accommodation is contracted separately from care [6]. During the COVID-19 pandemic, supported living and extra care housing settings were advised to follow care home guidance whenever residents shared substantial communal facilities and most received CQC-regulated personal care [7], in contrast to settings providing only social support, such as cooking, cleaning, or shopping.
Mathematical modelling used to inform care home policy during the COVID-19 pandemic primarily focused on larger care homes with over 30 beds, overlooking SCHs with household-like characteristics. However, outbreaks in SCHs can be expected to show a high early attack rate due to dense social networks among a small number of residents. These residents often have minimal or no physical disability, unlike those typically living in larger care homes. As a result, a highly transmissible virus like SARS-CoV-2 would be expected to spread rapidly within an SCH, leading to high incidence in the first few days and limited opportunity for hidden or prolonged transmission chains.
In this study, we aimed to analyse COVID-19 outbreak trajectories and attack rates in relation to care home type with a view to informing evidence-based and differentiated approaches to future outbreaks.
Methods
Setting
The study aimed to include all care homes in England and their resident populations, during two key periods of the COVID-19 pandemic: Wave 2 (10 December 2020 to 1 March 2021) and the Omicron wave (15 December 2021 to 21 February 2022). These periods were chosen to capture the dynamics of COVID-19 transmission during two phases of the pandemic for which high-quality diagnostic data were available, with particular focus on the emergence of the highly transmissible Omicron variant.
Study population
The analysis included care homes with two or more residents testing positive for SARS-CoV-2 within a 14-day period during the specified study windows. Residents were eligible if they had a positive SARS-CoV-2 test that could be linked to a CQC registered care home, enabling linkage to CQC registration data.
Data sources
The UKHSA Epidemiology Cell (EpiCell) line list for positive COVID-19 tests [8] is a person-level dataset for laboratory-confirmed cases, derived from the Second-Generation Surveillance System (SGSS) and subsequently deduplicated and cleaned by EpiCell. SGSS is the UKHSA’s laboratory surveillance system for notifiable infectious diseases and antimicrobial resistance in England. In collaboration with the Geospatial Team, EpiCell then identified care homes via Unique Property Reference Number linkage. The resulting dataset includes each care home’s address, a specimen date, and date of death (if within 28 days of a positive test).
The CQC register [9] provides up-to-date information on active care home locations in England, including bed capacity and registration type. The Capacity Tracker [10] is a web-based application that enables care homes to share a core subset of data, including available capacity, in real time. Between May 2020 and 31 March 2022, submissions were incentivized via the Adult Social Care Infection Control and Testing Fund [11], and monthly reporting is now mandatory under the Health and Care Act 2022.
Care home size, registration type, and outbreak characteristics were ascertained using CQC-registered postcodes from the CQC Register for Active Locations [12] and matched to the UKHSA EpiCell line list for positive SARS-CoV-2 tests to perform descriptive analysis. Care home residents were identified using linkage to CQC data; homes designated for older people were those marked as serving ‘Older People’ and/or ‘dementia’ in the CQC register. All others were classified as homes for younger adults. While some younger adults may reside in homes registered for older people, the extent of misclassification was considered minimal.
Bias
To avoid counting ongoing outbreaks, care homes with any cases in the 28 days prior to each study period (10 December 2020 to 1 March 2021 and 15 December 2021 to 21 February 2022) were excluded. Records marked as ‘Staff’ in the EpiCell dataset were also excluded. Cases that could not be linked to a CQC postcode and care homes with missing capacity data were also excluded.
Care home resident denominators for calculating transmission patterns over a 50-day period were derived from occupancy data in the Capacity Tracker. Care home occupancy was chosen as the most appropriate denominator for estimating relative transmission patterns between homes of different sizes because average care home occupancy in England is 80%. This approach minimized potential bias related to large care homes with many unoccupied beds. However, for comparison and to inform policymaking contexts where current occupancy may not be known, we also calculated transmission patterns using registered bed capacity.
Study size
The sample size was determined based on the availability of data from care homes during the study periods. All care home residents who tested positive for COVID-19 between 10 December 2020 and 1 March 2021, or 15 December 2021 and 21 February 2022, and could be matched to a CQC-registered care home were included.
Exposure variables included care home size, defined by number of residents and categorized as small care homes (SCHs; ≤10 beds), mid-sized homes (11–49 beds), and large homes (≥50 beds), and registration type, distinguishing homes for older people from those for younger adults; separate analyses were conducted using bed count. Outcome variables comprised the attack rate, defined as the proportion of residents testing positive for SARS-CoV-2, at outbreak detection and subsequent time points, along with key outbreak characteristics.
Statistical methods
Descriptive analyses were conducted to characterize outbreaks by care home size, using the proportion of residents positive for COVID-19 at outbreak detection and subsequent time points. No formal statistical comparisons were made. A sensitivity analysis was performed using registered bed numbers rather than resident counts.
Results
Demographics of the care home resident population
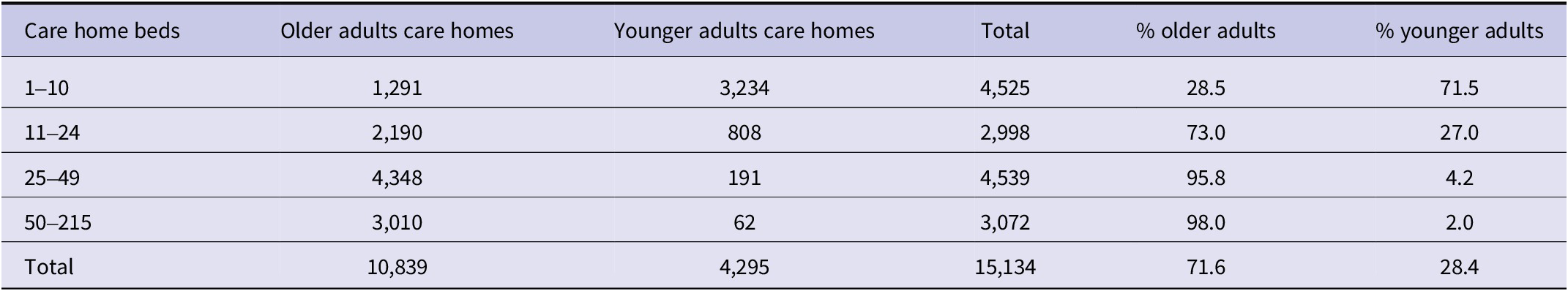
In March 2022, England had 15,134 CQC-registered care homes with 457,183 beds (Table 1). Of the 4,525 SCHs, 3,234 (71.5%) catered to younger adults and 1,291 (28.5%) to older adults, totalling 27,585 beds. Nearly half of all younger adult beds were in SCHs, compared with 2.1% of older adult beds.
Table 1. Care home type, size, and distribution by registration type, March 2022

Note: Percentages refer to the proportion of care homes in each size category.
Most care homes for older adults were mid-to-large-sized: 4,348 (40.1%) had 25–49 beds, and 3,010 (27.8%) had 50 or more beds, while only 62 (1.4%) of care homes for younger adults had 50 or more beds. These patterns reflect a structural divergence in care provision: younger adults are predominantly cared for in smaller homes, whereas older adults are largely cared for in mid-to-large-sized homes.
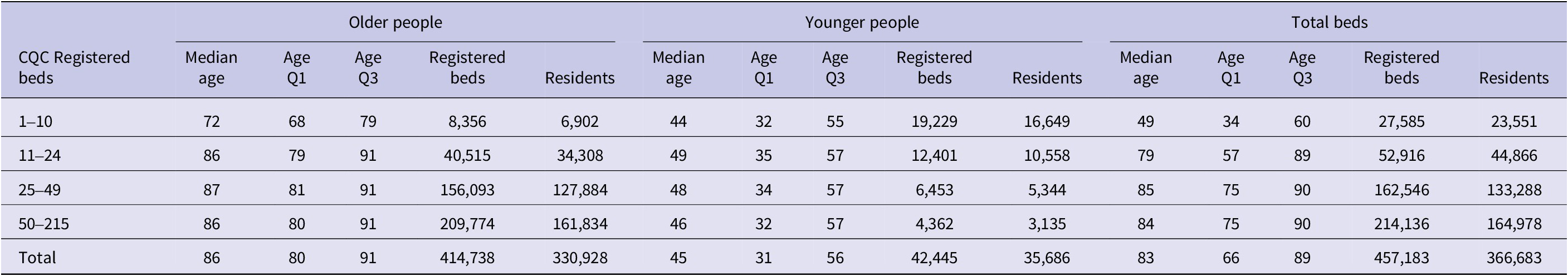
Table 2 shows resident demographics by home size and type. Across all homes, the median age of residents who tested positive for COVID-19 was 83 years (IQR 66–89). In SCHs, residents were younger, with a median age of 49 years (IQR 34–60). In mid-to-large care homes for older adults, median ages ranged from 86 to 87 years (IQR 79–91). These differences highlight the demographic heterogeneity of the care home sector and the varying needs of residents by age and home type.
Table 2. Distribution of care homes England by size, type, and median age, March 2022

Note: Median age and quartiles (Q1–Q3) of residents by care home size and type. Registered beds and residents are counts as of March 2022.
Cases: Wave 2 and Omicron wave
During Wave 2 (10 December 2020–1 March 2021), 40,266 positive COVID-19 tests from the UKHSA EpiCell dataset were successfully matched to 5,655 unique CQC-registered care home postcodes. For the Omicron wave (15 December 2021–21 February 2022), 53,108 positive tests were matched to 6,973 care homes. An additional 316 care homes were excluded due to missing occupancy data in the Capacity Tracker, preventing accurate calculation of attack rates.
COVID-19 attack rate: Omicron wave
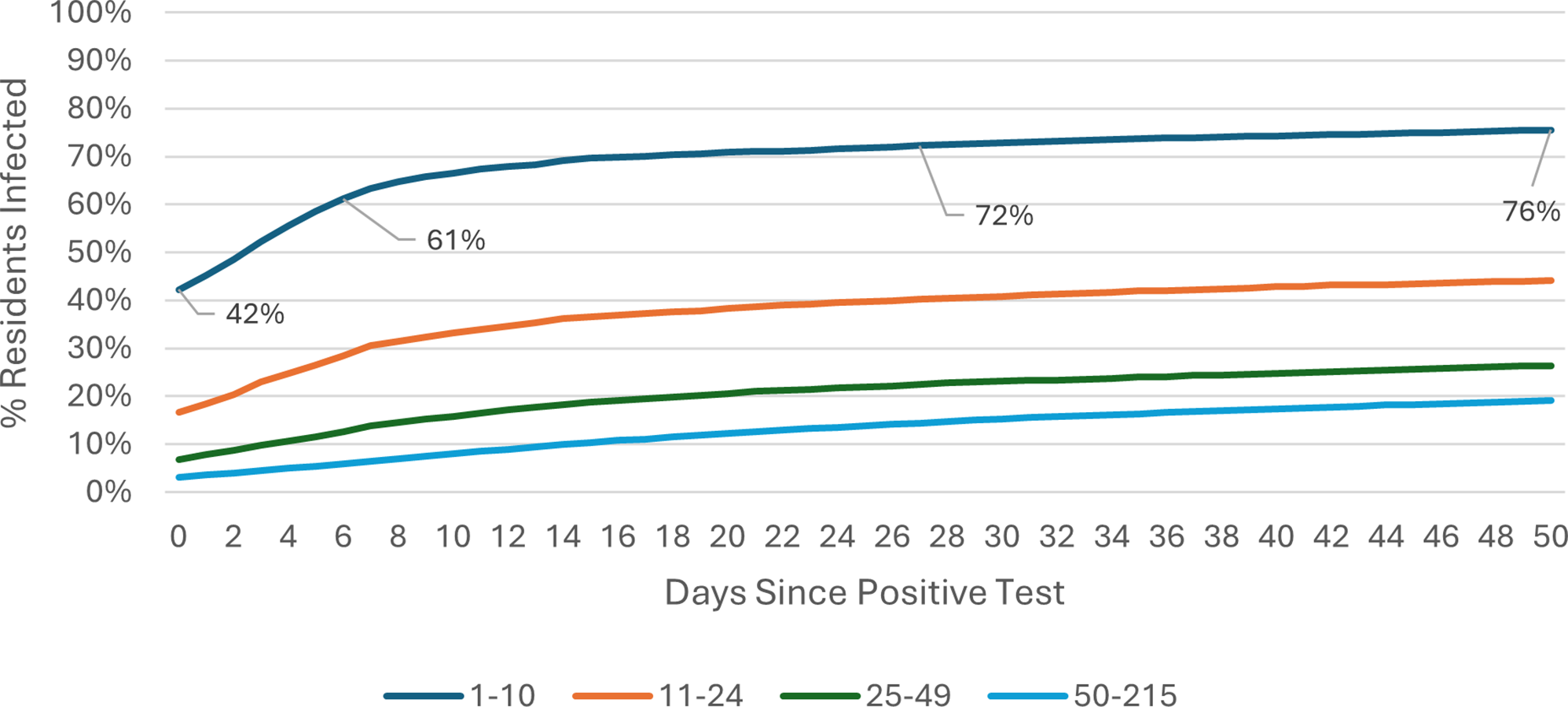
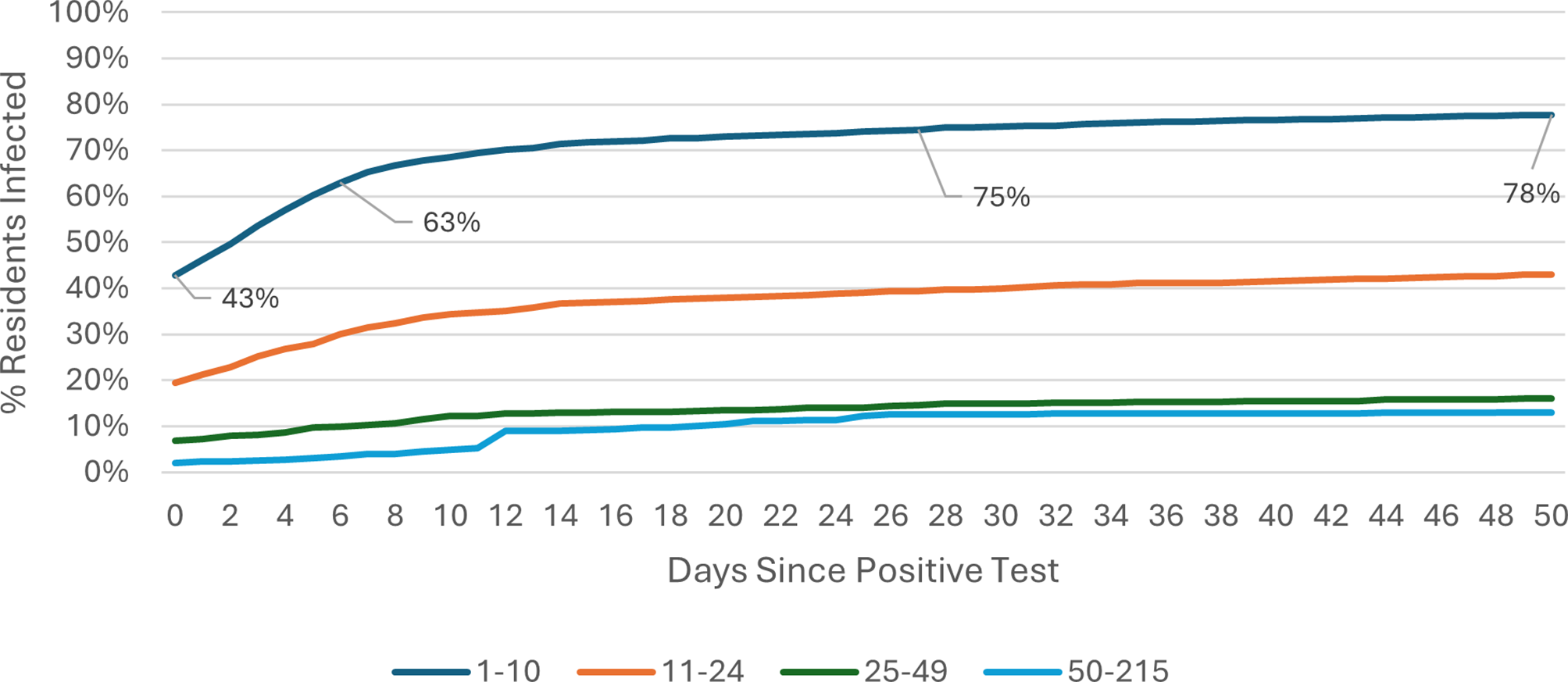
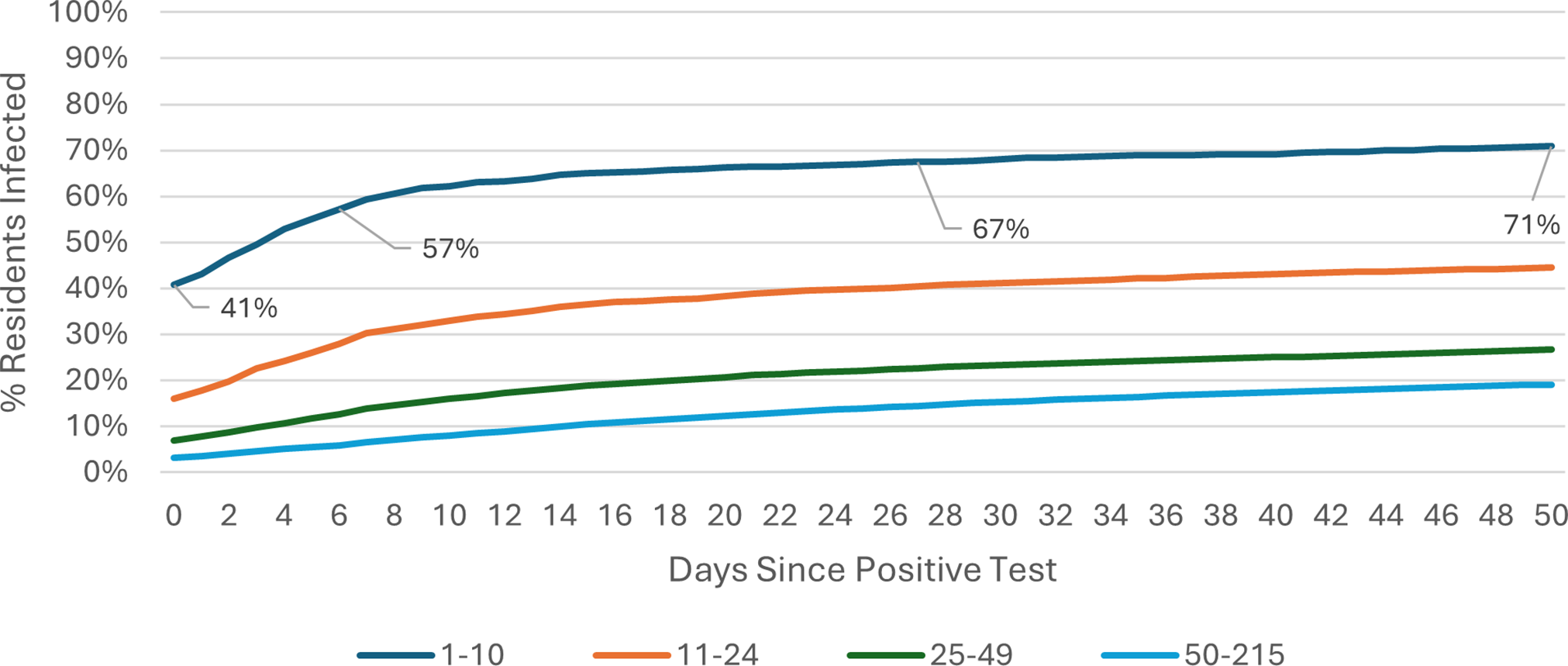
The COVID-19 attack rate over the 50-day Omicron wave period was calculated using care home occupancy data as the denominator. Outbreak trajectories varied notably by care home size and registration type. Across all care homes (Figure 1), outbreaks in SCHs progressed rapidly, with 42% of residents already positive at the time of detection, rising to 61% by day 7, whereas care homes with 25–49 beds showed slower progression, increasing from 3% to 6% over the same period. In care homes for younger adults (Figure 2), SCHs showed a steeper trajectory, with positivity rising from 43% at day 0 to 63% by day 7. Among SCHs registered for older adults (Figure 3), progression was slightly slower, increasing from 41% to 57%, while in care homes for older adults with 50+ beds, positivity remained substantially lower, rising from 3% to 6% over the same timeframe.

Figure 1. Outbreak trajectories during the Omicron wave in all care homes, by care home occupancy (15 December 2021–21 February 2022).

Figure 2. Outbreak trajectories during the Omicron wave in care homes for younger adults, by care home occupancy (15 December 2021–21 February 2022).

Figure 3. Outbreak trajectories during the Omicron wave in care homes for older adults, by care home occupancy (15 December 2021–21 February 2022).
Outbreak trajectories during Wave 2 followed a similar pattern but at lower levels prior to the emergence of the more transmissible Omicron variant [Reference Bálint, Vörös-Horváth and Széchenyi13]. These results are also shown in Supplementary Table 1, calculated using care home occupancy as the denominator. A parallel analysis for both Wave 2 and Omicron, using CQC-registered bed numbers as the denominator, is presented in Supplementary Table 2.
Discussion
The transmission dynamics of COVID-19 outbreaks in SCHs of all types differed markedly from patterns seen in larger care homes. SCHs consistently showed high attack rates at first detection and by day 7, reaching a plateau earlier than larger care homes. Overall attack rates by the end of the outbreak were also higher in SCHs, particularly in homes for younger adults. This marked difference has important implications for policymakers, informing which outbreak measures are likely to be effective in controlling transmission according to care home size.
Beyond care home size, infection prevention and control (IPC) measures and other non-pharmaceutical interventions (NPIs) likely influenced outbreak patterns. CQC’s COVID-19 Insight reports in 2020 [14] indicated generally strong assurance around IPC across home types, although implementation and intensity varied. Staff were required to wear masks, and cohorting was commonly used to limit transmission. However, adherence may have been more challenging in smaller care homes for younger adults due to communal living, social interaction needs, and, in some cases, cognitive or behavioural factors.
These contextual challenges were compounded by differences in staffing structures. Larger care homes typically employ staff with formal IPC training and dedicated infection control roles but experience higher staff movement between homes, a recognized transmission risk. In contrast, SCHs, particularly those for younger adults, rely on small, multi-role teams, sometimes including live-in staff who provide both personal and domestic support, supporting continuity of care but often with limited formal IPC training. At the start of the COVID-19 pandemic, national data on IPC training levels and compliance in England were lacking. While basic IPC training was mandatory for personal care providers, it was not included in the skills framework for learning disability care, where staff frequently work flexibly across home and community environments [15]. Taken together, these structural, behavioural, and staffing factors likely underpinned the rapid outbreak progression observed in SCHs.
Relationship to previous literature
The COVID-19 pandemic generated extensive research on outbreaks in care homes for older adults. One of the largest studies, VIVALDI [Reference Shrotri16], followed residents and staff across care homes in England with a median resident age of 86, bed capacity of 40–63, and occupancy of 82.5%. The study found that two doses of COVID-19 vaccine were 61.7% effective against infection and 96.4% effective against death during the first year. Although immunity waned over time, a booster largely restored protection; by the Omicron wave, effectiveness against infection had declined due to both waning and immune escape, while protection against severe outcomes remained largely preserved.
Vaccine rollout initially prioritized older adult residents in larger care homes, ensuring early access to first and second doses during the winter of 2020–2021. In contrast, residents of SCHs for younger adults were vaccinated later and generally had lower uptake. This delayed and less comprehensive immunity contributed to rapid outbreak progression in SCHs, highlighting the need for proportionate, tailored outbreak control measures that balance infection prevention with resident wellbeing.
Subsequent studies reinforce the protective effect of vaccination in care homes. Giddings et al. [Reference Giddings17] examined outbreak severity and duration across English care homes between November 2020 and June 2021, reporting that following COVID-19 vaccine introduction, outbreak duration and resident attack rates were substantially reduced, with typical attack rates in larger homes ranging from 13% to 27% by day 28. These population-level benefits complement the individual-level vaccine effectiveness reported by the VIVALDI study [Reference Shrotri16], which found that vaccination markedly reduced infection, hospitalization, and death among care home residents and lowered infection risk among staff, contributing to reduced transmission within care homes. Earlier investigations, including Ladhani et al. [Reference Ladhani18], focused on larger London care homes with 43–100 residents in April 2020, reporting resident attack rates of 70–75% before vaccination and widespread outbreak control measures were implemented.
An international review of respiratory outbreaks in elderly care homes [Reference Utsumi19] similarly found high attack rates, often exceeding 50%, and prolonged transmission in large facilities during seasonal pathogen circulation. These findings contrast with the lower overall attack rates observed in larger English care homes in our study (13–27%) and underscore the distinctive outbreak dynamics of smaller homes, where rapid within-home transmission led to early plateaus. Outbreaks of this type are underrepresented in published literature, which tend to describe prolonged or complex events in larger care homes. Our study helps to address this evidence gap by providing national-level data on SCH outbreak trajectories to inform proportionate, tailored infection-control strategies.
Strengths and limitations
In interpreting our findings, it is important to note that COVID-19 outbreaks in care homes for older adults typically had multiple genomic lineages, indicating multiple introductions of the virus from the community [Reference Tang20]. We did not have genomic data on these outbreaks; this is important context both for interpreting our study and informing policy decisions.
Our study used a complete national dataset for England, prospectively collected during the COVID-19 pandemic. COVID-19 test results were matched to CQC-registered care homes using postcodes, excluding approximately 15% of homes as only those with unique postcodes could be linked. It is unclear whether this disproportionately affected smaller or larger care homes. This missingness is likely Missing Not At Random (MNAR), since exclusion probability is related to underlying characteristics; for example, larger care homes often share postal addresses, particularly when multiple facilities are co-located. Such systematic missingness cannot be fully addressed using standard statistical methods, as it is associated with unobserved structural factors [Reference Dong and Peng21].
We also lacked data on resident health status, environmental factors, social and community activities, and new admissions. The absence of these variables may contribute to unmeasured sources of bias, further compounding the challenges posed by MNAR data.
Implications for clinical practice, policy, and future research
Guidance should consider the implications of swift early transmission and faster progression to eventual outbreak size, which occurs in SCHs, noting the wider evidence cited above on multiple introductions in larger care homes. SCHs for younger adults, by design, emulate shared households, with communal facilities that may facilitate rapid resident-to-resident transmission.
Given these patterns and the limited scope for hidden chains of COVID-19 transmission, our data provide empirical evidence to support the management of SCHs in a similar way to households in the wider community. This will require strategies to protect particularly vulnerable residents from cases of active infection during outbreaks of COVID-19, including supporting residents to participate in protective measures.
During the COVID-19 pandemic, some settings other than care homes (e.g., supported living, extra care settings) were managed in the same way as homes of any size. Importantly, given the strikingly different patterns of transmission in smaller vs. larger care homes, future outbreak management should consider the likelihood that prolonged control measures in SCHs are unlikely to reduce transmission or eventual outbreak size. This distinction is crucial for assessing trade-offs for active younger populations in care homes, whose wellbeing may be substantially impacted by the loss of external visits for leisure, education, and other family or community activities. This was recognized in December 2022 guidance published by the Department of Health and Social Care DHSC [22] to provide care homes with autonomy to initiate COVID-19 outbreak management risk assessments to inform decisions about which outbreak measures best fit their individual settings. Testing guidance was streamlined for SCHs to reduce the number of tests staff and residents needed to take in the event of an outbreak.
While specific to COVID-19, these findings may have implications for differential management of other infectious hazards in care homes, including periods of closure to admissions and visiting. This should be explored in future research and evaluation. Despite the limitations in this dataset, the opportunity to analyse patterns of transmission and outbreak trajectories across all care homes highlights the value of timely, accessible, and systematically collected data to inform tailored advice in future outbreaks. A comprehensive observatory enabling characterization by care home size and type could strengthen national preparedness for future infectious hazards while supporting evidence-based interventions that protect residents and safeguard their quality of life.
Supplementary material
The supplementary material for this article can be found at http://doi.org/10.1017/S0950268825100757.
Data availability statement
A limited dataset analysed during the current study is available from the corresponding author on reasonable request, subject to data protection.
Acknowledgements
The authors would like to thank all data contributors and colleagues at the UK Health Security Agency for their support during this study.
Author contribution
Conceptualization: C.C.; J.C.; E.O.; R.H.; S.W.; Writing - review & editing: C.C.; J.C.; A.F.; W.M.; M.R.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical standard
Routine data were used in the process of service evaluation and optimization of UKHSA advice. Ethical review was therefore not required in accordance with Health Research Authority (HRA) guidance, confirmed through use of the HRA tool [23]. All data were collected within statutory approvals granted to UKHSA for infectious disease surveillance and control, with stringent measures in place to safeguard data security and confidentiality in accordance with the Data Protection Act 2018 and Caldicott principles.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.