Being diagnosed and living with cancer is psychologically stressful and has been linked to depression, anxiety, pain and suicidal behaviour. Reference Stenager, Stenager, Erlangsen and Wasserman1–Reference Zabora, BrintzenhofeSzoc, Curbow, Hooker and Piantadosi4 Findings suggest that physical conditions are linked to an elevated risk of suicide, even with no presence of comorbid mental disorders. Reference Stenager, Stenager, Erlangsen and Wasserman1,Reference Erlangsen, Stenager, Conwell, Andersen, Hawton and Benros5 Suicide rates have been found to vary with respect to the site of cancer, and high risks have been reported for patients with lung, prostate, colorectal, liver, head and neck and pancreatic cancers. Reference Storm, Christensen and Jensen2,Reference Choi and Park6,Reference Fang, Fall, Mittleman, Sparen, Ye and Adami7 Furthermore, risk may also be elevated for individuals recently diagnosed, or those with high-tumour stage, cancers. Reference Storm, Christensen and Jensen2,Reference Yousaf, Christensen, Engholm and Storm8 The existing evidence has either been limited to select cancer sites Reference Calati, Filipponi, Mansi, Casu, Peviani and Gentile9 or does not reflect recent periods. Reference Fang, Fall, Mittleman, Sparen, Ye and Adami7,Reference Henson, Brock, Charnock, Wickramasinghe, Will and Pitman10 Also, there is a scarcity of evidence accounting for potential confounding related to income level, chronic disorders and psychiatric disorders, including depression, which have all been shown to impact suicidality. Reference Henson, Brock, Charnock, Wickramasinghe, Will and Pitman10,Reference Fazel and Runeson11 The number of individuals diagnosed with cancer has increased in recent decades, partially due to the growing number of older adults, increased screening and better diagnostic tools. Worldwide, more than 19 million persons were diagnosed with a new cancer in 2020. Reference Sung, Ferlay, Siegel, Laversanne, Soerjomataram and Jemal12
Aim
The aim of this nationwide, population-based study was to examine whether individuals first-time diagnosed with cancer within the past 5 years had higher rates of suicide when compared with similar individuals with no cancer diagnosis. In addition, associations with respect to time since diagnosis and stage and site of cancer were examined.
Method
Study design and setting
A cohort study design was applied to longitudinal, retrospective data on all individuals living in Denmark between 1 January 2000 and 31 December 2021. Data derived from the Civil Registration System, Reference Pedersen13 which lists all persons living in the country, together with their unique personal identifier assigned at birth or upon first immigration. Reference Erlangsen and Fedyszyn14 Using this identifier, individual-level linkage of data across national administrative registers was facilitated. Information on cancer diagnoses from the Cancer Registry Reference Gjerstorff15 was linked to data on physical and mental comorbidities and causes of death from the National Patient Register (since 1977), Reference Lynge, Sandegaard and Rebolj16 the Psychiatric Central Research Register (since 1969) Reference Mors, Perto and Mortensen17 and the Cause of Death Registry, Reference Helweg-Larsen18 respectively.
Study population
The study population consisted of all individuals aged 15 years or older who were recorded as living in Denmark at some point between 1 January 2000 and 31 December 2021. Individuals turning 15 years old or those immigrating into the country during follow-up were included based on the date of the respective event. Individuals were censored at time of death, emigration, diagnosis of a second primary cancer, fifth year following first cancer diagnosis, and end of follow-up, which ever occurred first.
Exposure
Individuals diagnosed with a malignant cancer were identified in the Cancer Registry, which includes a complete, tumour-level coverage of all diagnoses of malignant neoplasms since 1943. Reference Gjerstorff15 In addition to diagnoses recorded according to the tenth revision of the International Classification of Diseases (ICD-10), 19 the registry contains information on stage recorded according to tumour, nodes and metastases (TNM) classification whenever applicable Reference Engholm, Lundberg, Konig, Olafsdottir, Johannesen and Pettersson20 and graded as stages 1–4, where stage 1 indicates localised presence and subsequent stages denote the level of invasiveness up to stage 4, which denotes distant metastatic spread. The register was updated until 31 December 2020, and cancer diagnoses given during 2021 were identified in the National Patient Registry. The following cancer sites were identified: head and neck, oral cavity, larynx, pharynx, oesophagus, thyroid, stomach, colon, rectum, liver, pancreas, lung, breast, endometrium, uterus, ovary, cervix, prostate, testis, bladder, kidney and central nervous system; also included were mesothelioma, sarcoma, melanoma, Hodgkin’s lymphoma, non-Hodgkin’s lymphoma, multiple myeloma and leukaemia (Supplementary Table 1 available at https://doi.org/10.1192/bjp.2025.10363). Any examined cancer was indicative of an individual who was diagnosed for the first time with a cancer included in the cancer sites listed above. Non-melanoma skin cancer was not included due to its low mortality. People were considered as exposed from the date of first diagnosis. We opted to consider individuals as exposed only during the first 5 years following first diagnosis, because other studies have found incidence rates of suicide to be highest in the period immediately following cancer diagnosis. Reference Du, Shi, Yu, Liu, Jin and Yan21 This was confirmed by preliminary analyses where risks decreased after 5 years (not shown). Individuals receiving a diagnosis of a second cancer within the first 5 years were censored. Stage and time since first diagnosis of cancer were captured by separate covariates.
Measures
Analyses were adjusted for the following covariates: calendar period (2000–2009, 2010–2021); gender (male/female); age group (15–44, 45–54, 55–64, 65–84, ≥85 years); civil status (never married, married/cohabiting, divorced, widowed); educational level (elementary school, secondary school or higher education, including missing); household income level (yearly household income level per adult: lowest quartile, low middle, high middle, highest quartile, missing); Charlson comorbidity index (0, ≥1); psychiatric disorder prior to any cancer diagnosis (yes, no); and suicide attempt prior to any cancer diagnosis (yes, no). The Charlson comorbidity index is a predefined score estimating the individual risk of mortality based on the number and severity of comorbid health conditions. Reference Charlson, Pompei, Ales and MacKenzie22 For this study, cancer was removed from the index because this was the examined exposure. Individuals who had been diagnosed with a psychiatric disorder during a hospital contact since 1969, and prior to the diagnosis of cancer as recorded in the Psychiatric Central Research Register, were considered as such. Suicide attempt was defined as a record of hospital contact, in either the National Patient Register since 1977 or the Psychiatric Central Research Register since 1969 with a diagnosis (ICD-10: X60–84), or where the reason of contact was listed as being ‘suicide attempt’.
Outcome
The studied outcome was death by suicide, as identified in the Cause of Death Register through ICD codes (ICD-10: X60–84, Y87.0) or where the manner of death was listed as ‘suicide’. Reference Helweg-Larsen18
Statistical analysis
Incidence rates were calculated per 100 000 person-years (Supplementary Table 4). Rates of suicide for individuals diagnosed with a cancer were compared to those for individuals not diagnosed with cancer, and are expressed as adjusted incidence rate ratio (aIRR). Using persons without any site of cancer as a reference group, it was possible to compare the rates of different cancer sites. We used Poisson regression analyses to estimate aIRRs with 95% CI and P-values as a marker of statistical significance. In separate models, aIRRs were calculated by time since first diagnosis (no cancer, <6 months, 6–12 months, 1–2 years, 2–3 years, 3–4 years, 4–5 years), stage (no cancer, stage 1, stage 2, stage 3, stage 4) and site of cancer (listed above, and using no cancer as reference).
All covariates were included as time varying and were updated either on the exact date of change or on a yearly basis. Because preliminary analyses did not reveal interaction effects with respect to gender, we opted to analyse males and females jointly, except for breast and uterus (including endometrial, cervical and ovarian) cancer, which were examined only for females, and for prostate and testis cancer, which were examined only for males. To reduce bias from competing risks of all-cause mortality related to cancer prognosis, in the sensitivity analysis we assessed rates among first-time diagnosed during the first year of exposure only. Furthermore, risk relative to time before diagnosis was examined using cumulative incidence curves, taking into account competing risks from all-cause mortality. A comparison group was formed by matching each exposed individual with two persons of the same gender and birth year and who were alive on the date of first diagnosis and unexposed at the time of matching.
Data management and analyses were carried out at Statistics Denmark using SAS (Cary, NC, USA). 23 The project was approved by the Danish Data Protection Agency (Region H: P-2020-305).
Results
A total of 6 987 998 individuals (50% females) were followed over 98 319 168 person-years (50% females) during 2000–2021. Of these, 707 513 individuals (10% of total, 53% females) were diagnosed with a first primary cancer (age at first diagnosis, median: 69 years; interquartile range 59–77 years) and observed over 1 734 167 person-years. During the 5 years of follow-up, 601 individuals with a recent cancer died of suicide, corresponding to a suicide rate of 34.7 per 100 000 person-years, while the cancer-free population had a suicide rate of 13.3 (Table 1). Approximately 23% of those with cancer who died from suicide had other comorbid health conditions as per the Charlson comorbidity index, while this proportion was 12% among cancer-free individuals (Supplementary Table 4).
Table 1 Descriptive statistics of study population, including every person above 15 years of age living in Denmark between 2000 and 2021, obtained using national register data. The study outcome was death by suicide. For descriptive statistics on all variables, see Supplementary Table 4
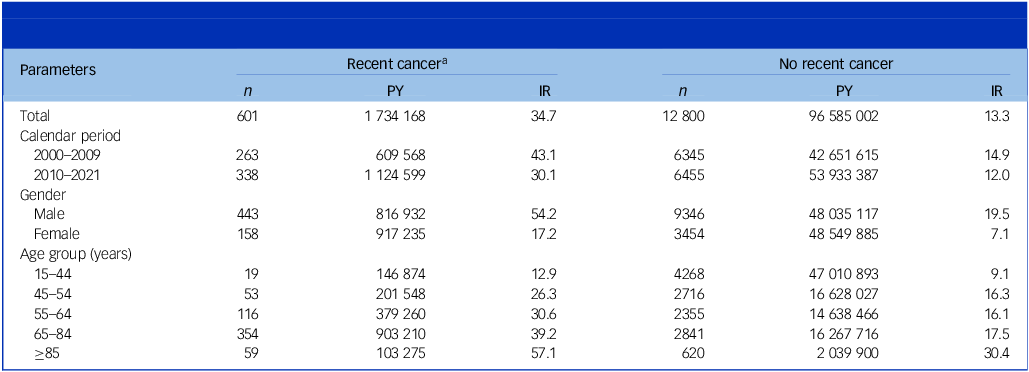
PY, person-years; IR, incidence rate per 100 000 PY.
a Diagnosed within the past 5 years with any of the cancers specified in Supplementary Table 1.
Individuals with any of the examined cancer sites had an aIRR of 2.2 (95% CI: 2.1–2.3) (Fig. 1 and Supplementary Table 2) compared with cancer-free individuals.
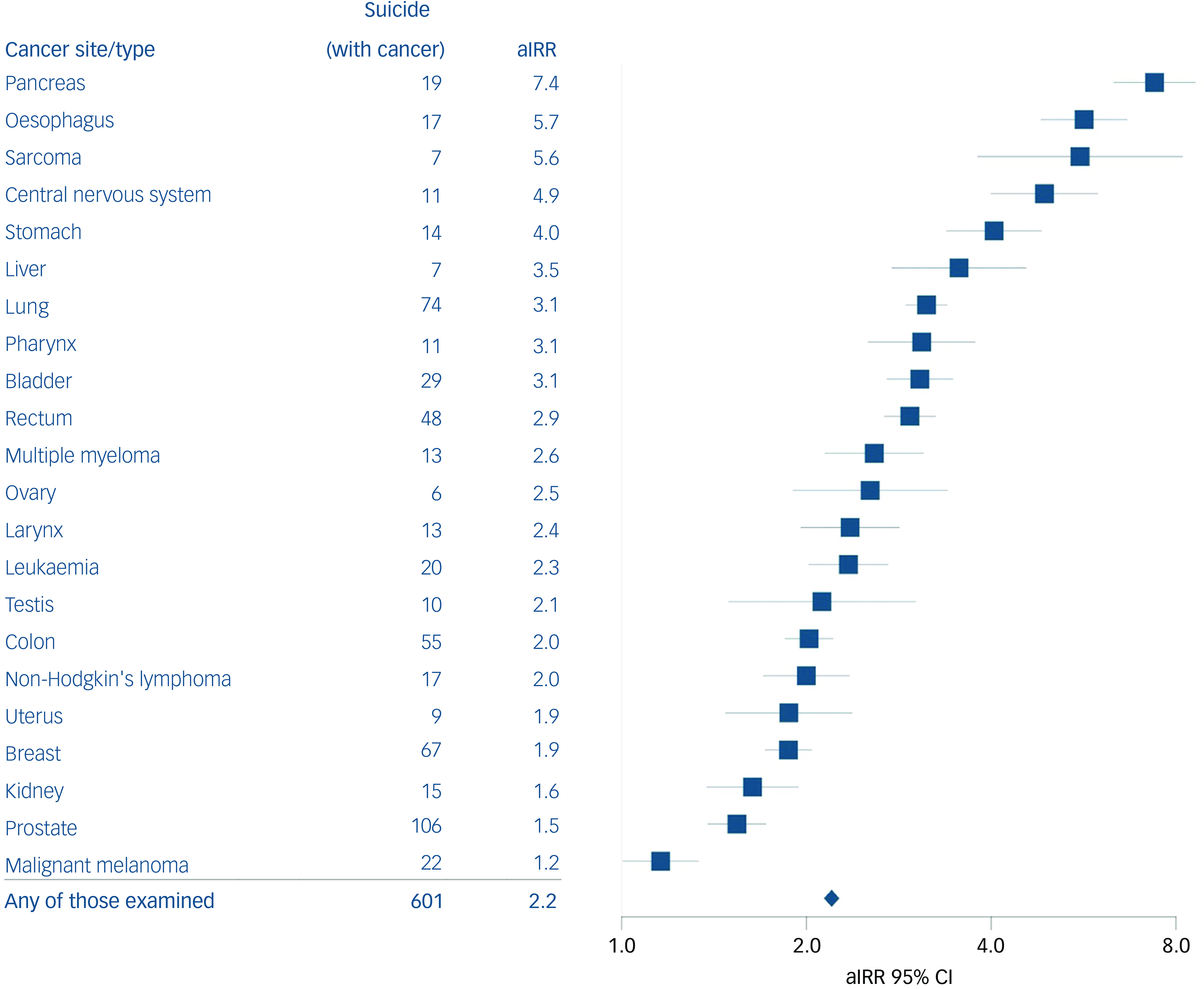
Fig. 1 Adjusted incidence rate ratio (aIRR) associated with each type of cancer analysed. The fully adjusted model was adjusted for calendar period, gender, age group, civil status, educational level, household income level, Charlson comorbidity index, psychiatric disorder prior to any cancer diagnosis and suicide attempt prior to any cancer diagnosis. All models were calculated in comparison with the same reference group, consisting of individuals not diagnosed with any cancer during the past 5 years. Results for basic adjusted analyses can be found in Supplementary Table 2.
For all sites combined, when compared with suicide rates of cancer-free individuals, the aIRR within the first 6 months of diagnosis was 3.9 (95% CI: 3.6–4.2) (Fig. 2). Individuals diagnosed within 6–11 months also had elevated rates (aIRR 2.4, 95% CI: 2.1–2.7). Compared with individuals diagnosed with a stage 1 tumour, individuals with a stage 4 tumour had significantly higher rates of suicide (aIRR 3.1, 95% CI: 2.8–3.4), whereas individuals with stage 2 and 3 tumours had aIRRs of 1.0 (95% CI: 0.9–1.2) and 1.7 (95% CI: 1.5–1.9), respectively (Table 2).
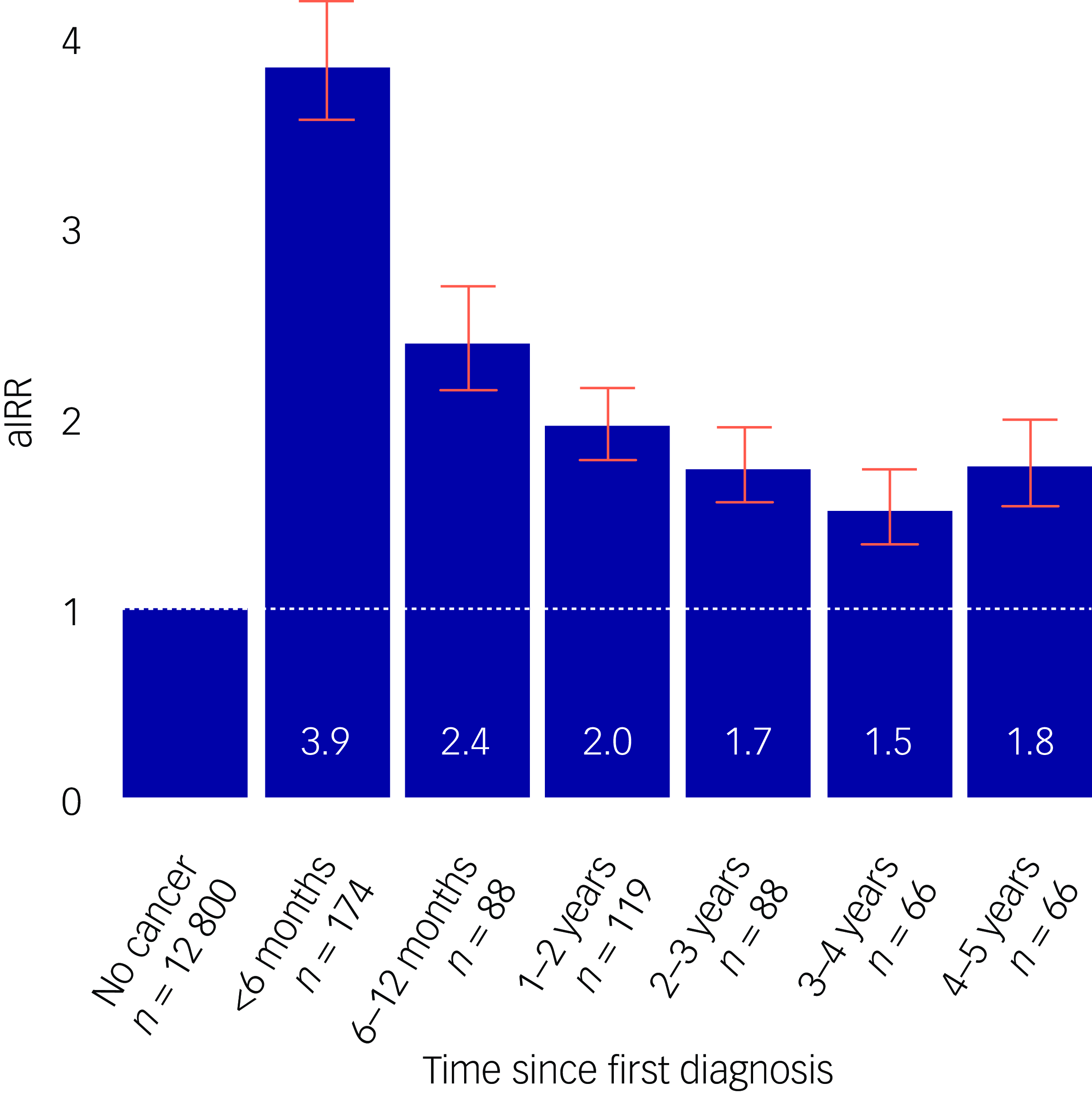
Fig. 2 Adjusted incidence rate ratio (aIRR) for any cancer according to time since diagnosis. The fully adjusted model was adjusted for calendar period, gender, age group, civil status, educational level, household income level, Charlson comorbidity index, psychiatric disorder prior to any cancer diagnosis and suicide attempt prior to any cancer diagnosis.
Table 2 Adjusted IRR a for associations of any cancer and suicide with respect to stage of tumour and calendar period
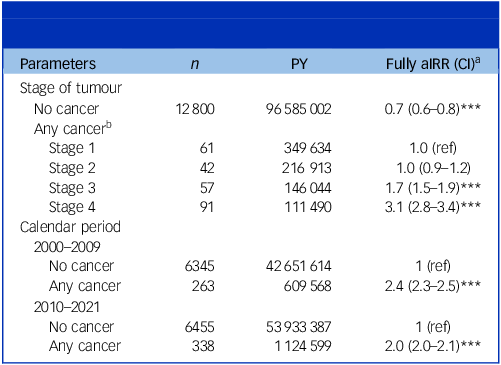
IRR, incidence rate ratio; PY, person-years; aIRR, adjusted incidence rate ratio.
a The fully adjusted model was adjusted for calendar period, gender, age group, civil status, educational level, household income level, Charlson comorbidity index, psychiatric disorder prior to any cancer diagnosis and suicide attempt prior to any cancer diagnosis.
b Information on stage of cancer was missing for 265 individuals who died by suicide. Of these, 24% had been diagnosed with prostate cancer, 8% with breast cancer, 7% with bladder cancer, 7% with colon cancer, 6% each from lung cancer and leukaemia, 5% were diagnosed in 2021 (for which data on stage were not available) and 43% were due to other types of cancer.
***P < 0.001.
Most of the cancer suicide incidents occurred among individuals with cancer of the prostate (n = 106), lung (n = 74) and breast (n = 67). Compared with cancer-free individuals, the highest rates were found for individuals with cancer of the pancreas (aIRR 7.4, 95% CI: 6.3–8.6) and oesophagus (aIRR 5.7, 95% CI: 4.8–6.7), which are cancers carrying a poor prognosis. The lowest, but still statistically significant, rates were found for prostate cancer (aIRR 1.3, 95% CI: 1.1–1.6). No statistical difference was observed for individuals with cancer of the oral cavity (aIRR 1.0, 95% CI: 0.3–3.1), thyroid (aIRR 1.3, 95% CI: 0.7–2.5) and cervix (aIRR 0.5, 95% CI: 0.2–1.4), and melanoma (aIRR 0.9, 95% CI: 0.7–1.2).
Using the cancer-free population as reference, excess rates of suicide were found to be higher during the period 2000–2009 (aIRR 2.4, 95% CI: 2.3–2.5) versus 2010–2021 (2.1, 95% CI: 2.0–2.1).
When restricting exposure to the first year following diagnosis, aIRRs remained elevated (Supplementary Table 3). Individuals diagnosed with cancer had higher cumulative rates of suicide during the first years following diagnosis in comparison with matched peers with no diagnosis (Supplementary Fig. 1).
Discussion
Having complete, nationwide and validated data on all cancers diagnosed in Denmark, individuals with a recent first primary cancer were found to have a higher rate of suicide when compared with those without cancer. During the first 6 months following diagnosis the rate was highest, thus suggesting a temporal association. Also, a dose–response association was indicated because the highest suicide rates were found for individuals with high-stage tumours. Similarly, high rates were found for cancers carrying a poor prognosis, such as pancreatic and oesophageal. A relatively lower rate was found for 2010–2021 versus 2000–2009, suggestive of a period effect.
Our findings of increased suicide rates for persons diagnosed with cancer augment those previously documented Reference Yousaf, Christensen, Engholm and Storm8,Reference Henson, Brock, Charnock, Wickramasinghe, Will and Pitman10 by restricting exposures to the first 5 years following diagnosis, implying an increased likelihood of the suicide being related to the cancer diagnosis. This was further accentuated by restricting the sample to the first year following diagnosis, to reduce the option of competing risks due to other-cause mortality related to cancer diagnosis or non-cancer comorbidity; nevertheless, rates remained significant. By accounting for socioeconomic factors, pre-existing psychiatric disorders and suicide attempts, the excess rate estimates obtained here are more precse than previous ones. Reference Guo, Zheng, Zhu, Yu, Ding and Wu24–Reference Chen, Lin, Xu, Liu, Cai and Yang26 A recent umbrella review has emphasised the need for high-quality studies. Reference Calati, Filipponi, Mansi, Casu, Peviani and Gentile9 The reliability of the current findings is supported by the overall excess rate being close to the 1.5- to 1.7-fold higher rates, which was reported previously. Reference Choi and Park6,Reference Yousaf, Christensen, Engholm and Storm8,Reference Calati, Filipponi, Mansi, Casu, Peviani and Gentile9
A temporal effect was found, with the highest rates seen in the first 6 months following diagnosis and then gradually decreasing rates with longer duration from diagnosis. The higher suicide rate associated with stage 4 tumours versus stage 1, supporting previous findings, is indicative of a dose–response relation. Reference Heynemann, Thompson, Moncur, Silva, Jayawardana and Lewin27,Reference Heinrich, Hofmann, Baurecht, Kreuzer, Knüttel and Leitzmann28 Being generally incurable, for stage 4 tumours and their associated severe symptoms, palliative treatment is typically the only treatment option and this prospect is likely to affect the patient’s quality of life. The link between cancer and suicide is, thus, supported by the observed temporal and dose–response associations. Finally, a lower suicide rate was found for persons with cancer during the period 2010–2021 than 2000–2009, which could suggest that earlier diagnostics, better treatment options, focus on subsequent depression and provision of palliative care may have improved the health-related quality of life in recent years. Reference Han, Hu, Zhao, Ma, Jemal and Yabroff29
Although our study design precludes statements regarding a causal link, the highest rates were found among individuals with mesothelioma and cancer of the pancreas and oesophagus, which often coincide with high comorbidity and low survival rates. 30 These tend to be aggressive cancers associated with short life expectancy and rapidly progressing morbidity, and which may have a severe impact on health-related quality of life. Interestingly, cancers that are more common and included in screening programmes, such as colonic and breast cancer, were associated with less elevated rates, potentially due to a better overall prognosis.
Depression has been linked to an elevated risk of suicide Reference Fazel and Runeson11 and we, therefore, adjusted for pre-existing depression. Cancer has been linked to higher rates of clinical depression, Reference Pitman, Suleman, Hyde and Hodgkiss31–Reference Klaassen, Wallis, Chandrasekar, Goldberg, Sayyid and Williams33 and an onset of depression following a cancer diagnosis could potentially increase the risk of suicide. In such a scenario, however, depression would be considered as a mediator rather than a confounder. For this reason, we opted not to adjust for post-cancer depression. Should our findings be explained through post-cancer depression, this might indicate the relevance of screening for depression at the time of cancer diagnosis and during the subsequent treatment course.
Strengths and limitations
The strengths of this study include data derived from almost complete, longitudinal and high-quality registers, which included prospectively obtained information on diagnoses. The Cancer Registry includes 90% histologically verified cancers, dates back to 1943 and undergoes continuous validation through quality control routines. Reference Gjerstorff15 Danish register data provided a large and national fully representative sample with no loss to follow-up. Also, the registration of suicide deaths in Denmark has been evaluated as reliable. Reference Tollefsen, Helweg-Larsen, Thiblin, Hem, Kastrup and Nyberg34
Certain limitations should be acknowledged. First, because detailed information regarding the motives behind suicide deaths was not available, we cannot exclude that other life events might have led to the suicide. Second, restricting the exposure to the first 5 years following diagnosis may have led to a conservative estimate of the total number of suicides related to cancers, because potential relapse or progression may occur later in the trajectory. This also precluded assessment of any long-term effects as a response to psychological distress. Reference Fazel and Runeson11 Also, restricting the sample to persons with only one cancer resulted in conservative estimates. Third, information on cancer diagnoses for 2021 was derived from the National Patient Register, which has been validated for other diseases, Reference Christensen, Vestergaard, Olsen and Sidenius35 albeit neither validated by TNM classification nor cross-referenced to pathology records. Fourth, the stage analysis included fewer cases because the register is not complete according to staging; those stages found to be lacking might not be evenly distributed among the different cancer sites; the distribution of missing cases is listed in Table 2. Fifth, for some cancers, risk estimates have a relatively wide margin of uncertainty due to few observations. Sixth, socioeconomic status has been inversely associated with all-cause mortality following a cancer diagnosis, which might be explained through lifestyle, health literacy and access to healthcare. Reference Jang and Chang36,Reference Byers, Wolf, Bauer, Bolick-Aldrich, Chen and Finch37 We therefore opted to adjust for educational and household income levels. Seventh, it would have been preferable to account for individual patient factors, such as available social support and psychological distress arising from the cancer or its treatment. Finally, treatment for depression was not explored and, further, we cannot exclude treatment-related mood disturbances such as corticosteroid use and hormones. Reference Ismail, Lavelle and Cassidy38,Reference Donovan, Walker, Wassersug, Thompson and Robinson39 These aspects might be addressed in future analyses.
Clinical implications
Healthcare providers should be attentive towards levels of psychological distress in patients with cancer, especially at the time of first diagnosis and in cases with severe cancer stages and prognosis. Although high-quality evidence regarding effective suicide preventive interventions is scarce, Reference Bulotiene and Pociute40,Reference Kawashima, Yonemoto, Inagaki, Inoue, Kawanishi and Yamada41 detection and treatment of depression would be a first priority. Reference Li, Kennedy, Byrne, Gérin-Lajoie, Katz and Keshavarz42 Collaborative care, where psychological and pharmacological interventions might be combined and further enhanced through integrated delivery, have shown promising findings. Reference Bulotiene and Pociute40 Proactive and timely introduction of palliative care in the case of advanced disease may help reduce levels of anxiety and improve relief of symptoms, which is supported by the observed reduction in suicide levels among persons with cancer in the USA, in parallel with an intensified focus on palliative and psychosocial care. Reference Han, Hu, Zhao, Ma, Jemal and Yabroff29 Removal of access to means of suicide is indicated for individuals at risk. Reference Fazel and Runeson11 Patients with cancer may have access to specific methods, i.e. poisoning, and future studies may examine this.
In summary, individuals recently diagnosed with cancer have higher rates of suicide, when accounting for important confounders, and compared with individuals with no such diagnosis. The first months following diagnosis were found to be the most vulnerable period. The diagnosis of aggressive cancers such as pancreatic or oesophageal and, in general, advanced stage of disease, were associated with elevated rates.
Supplementary material
The supplementary material is available online at https://doi.org/10.1192/bjp.2025.10363
Data availability
The data that support the findings of this study are available from the corresponding author, C.F., on reasonable request.
Acknowledgements
The study was supported by a research grant from the Danish Health Foundation (no. 21-B-0197), the P Carl Petersen Foundation and The Danish Medical Association.
Author contributions
Conceptualisation: A.E., C.W.S., S.O.D., L.S.M., H.F. Funding acquisition: C.F., A.E. Data handling and analyses: C.F., A.E. Drafting of manuscript: C.F. Critical review: A.E., C.W.S., S.O.D., L.S.M., H.F., M.N.
Declaration of interest
None.







eLetters
No eLetters have been published for this article.