Introduction
Ebstein’s anomaly is rare, with an incidence of 1.2 to 5 in 100 000 live births. It accounts for 0.3–0.5% of CHDs and represents 40% of congenital tricuspid valve abnormalities. No gender difference in incidence has been reported. It was first described by Dr Wilhelm Ebstein, in 1866, in a post-mortem examination of a young adult with cyanosis, dyspnoea, and palpitations. Reference Ebstein1
Ebstein’s anomaly is caused by failed delamination (separation of the valve tissue from the myocardium) of the tricuspid valve leaflets with variable degrees of fibrous and muscular attachments to the right ventricular myocardium. The posterior (inferior) and septal tricuspid valve leaflets are generally most severely affected. Downward displacement of septal and posterior leaflets into the right ventricular cavity causes atrialization of a portion of right ventricle and consequently functional right ventricular hypoplasia. The tricuspid valve typically shows variable degrees of regurgitation; tissue defects within the leaflets (“fenestrations”) may contribute to the regurgitation and tricuspid valve stenosis is rare. Reference Lamers, Virágh, Wessels, Moorman and Anderson2,Reference Cetta, Dearani, O’Leary, Shaddy, Penny, Feltes, Cetta and Mital3
Presentation can vary from the most extreme form in fetal life to asymptomatic diagnosis late in adult life. Clinical symptoms are usually age-dependent: neonates may present with cyanosis, congestive heart failure, and significant cardiomegaly; children may show signs of progressive right ventricular failure; and adolescents and adults frequently present with palpitations due to arrhythmias. Reference Cetta, Dearani, O’Leary, Shaddy, Penny, Feltes, Cetta and Mital3–Reference Karagöz, Ertuğrul and Aypar14
Studies in literature about clinical features, management, and outcome of paediatric patients with Ebstein’s anomaly are limited. Reference Celermajer, Cullen, Sullivan, Spiegelhalter, Wyse and Deanfield4,Reference Geerdink, Delhaas and Helbing8–Reference Holst, Dearani and Said17 In this study, we evaluated a large series of children and young adults with Ebstein’s anomaly. Our aim was to evaluate clinical characteristics, treatment modalities (medical, ablation, and surgery), and follow-up results of the patients. We also aimed to determine factors affecting arrhythmia presence and mortality.
Materials and methods
Demographic, clinical data, echocardiography, catheterisation, ablation, surgery, and outcome results of patients diagnosed and followed as Ebstein’s anomaly in our centre, between 2000 and 2017, were retrospectively evaluated from the hospital files. The study protocol was approved by the Hacettepe University, School of Medicine Ethics Committee (Ethical consent number: GO 17/762-08).
Inclusion criteria included diagnosis of Ebstein’s anomaly by transthoracic echocardiography. The Mayo Clinic criteria were used for diagnosis and included apical displacement of the septal tricuspid valve leaflet from the insertion of the anterior leaflet of the mitral valve by 0.8 cm/m2 body surface area in systole. Reference O’Leary, Eidem, O’Leary and Cetta18 Exclusion criteria included Ebstein’s anomaly patients with missing data and incomplete hospital files.
Valve insufficiencies were graded as mild, moderate, or severe by Doppler and colour imaging according to the recommended guidelines. Reference Lancellotti, Tribouilloy and Hagendorff19
All patients had electrocardiogram and 24-hour electrocardiogram recordings. Transesophageal and/or invasive electrophysiological studies were performed in all patients with symptomatic tachycardia or documented arrhythmia (Wolff-Parkinson-White pattern or sustained tachycardia) in electrocardiograms or in 24-hour electrocardiogram recordings. Ablation or cryoablation procedures were performed in all patients more than 15 kgs with documented supraventricular tachycardia by electrophysiological studies. Three-dimensional imaging techniques (Ensite NavX navigation display technology) and Coil-Assisted Retrograde Transvenous Obliteration were used in some of the patients during the ablation procedures. Patients less than 15 kgs and patients without successful ablation were treated with anti-arrhythmic drugs.
Cardiac magnetic resonance imaging (MRI) were used in some of the patients as a complementary imaging with echocardiography.
Indications for surgery included severe or progressive cyanosis, congestive heart failure due to severe valvular regurgitation despite medical treatment, decreasing or severe limitation of activity, severe ventricular outflow tract obstruction, and recurrent or intractable life-threatening arrhythmia despite medical treatment.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics for Windows v.22.0 (IBM Corp., Armonk, NY, USA). Descriptive statistics were presented as median (minimum-maximum), frequency distribution, and percentage. Pearson’s Chi-Square Test or Fisher’s exact test was used for between-group comparisons. The Mann–Whitney U Test was used as a statistical method for statistical significance between two independent groups for variables that were found to be inconsistent with normal distribution. The effects of diagnosis age, gender, presence of genetic diseases and/or congenital anomalies, tricuspid regurgitation, arrhythmias, and surgery history on survival were evaluated using log-rank test. Cox regression analysis was used to investigate the independent effect of variables on survival. The level of statistical significance was set at p < 0.05 in all statistical tests.
Results
A total of 79 patients were included in the study. The median diagnosis age was 1.5 years (1 day–24 years); 61 patients (77.2%) were 18 years, 51.9% were male, and 7.6% of the patients were diagnosed prenatally. The median age of the surviving patients was 12.8 years (10 months–34 years).
Common presenting symptoms were cyanosis (29.1%) and palpitations (13.9%). Others included respiratory distress (3.8%), syncope (3.8%), fatigue (3.8%), and chest pain (1.3%). The rest of the patients were asymptomatic (44.3%) and referred to our department with diagnosis of murmur (34.2%), arrhythmia (6.3%) diagnosed incidentally in another centre, or abnormal fetal ultrasound (3.8%). Associated genetic diseases were present in 5% (4/79 patients) and included 1p36.33 deletion (one patient), Carpenter syndrome (one patient), cystic fibrosis (one patient), and autoinflammatory disease (one patient). Congenital anomalies were present in 3.8% (3/79 patients) and included cleft lip and/or palate (two patients) and congenital scoliosis (one patient).
Most common associated anomalies were atrial septal defect/patent foramen ovale (56.9%), mild-moderate degree mitral regurgitation (21.5%), and ventricular septal defect (16.5%). Other common associated anomalies included pulmonary stenosis (10.1%), mitral valve prolapse (8.9%), transposition of the great arteries (8.9%), pulmonary atresia (7.6%), patent ductus arteriosus (6.3%), cardiomyopathy (5.0%), coarctation of the aorta (3.8%), severe mitral regurgitation (3.8%), and bicuspid aortic valve (2.5%). Tricuspid regurgitation was present in 75.9%; mild degree in 11.7%, moderate in 38.3%, and severe in 50.0%.
Cardiac MRI was used as a complementary imaging method in 17.7% of the patients and 50% of them had surgery.
Arrhythmias were detected in 30.4% (24/79 patients) of the patients. Of these, 20/24 patients (83.3%) had tachycardia. Supraventricular tachycardia was present in 19/20 patients and one patient had ventricular tachycardia diagnosed during cardiac catheterization. In patients with supraventricular tachycardia, 16/19 had accessory pathway mediated re-entrant tachycardia and 3/19 had non-accessory pathway mediated re-entrant tachycardia (intra-atrial re-entrant tachycardia). In 16 patients with accessory pathway mediated re-entrant tachycardia, 15/16 patients had atrioventricular re-entrant tachycardia and one patient had atrioventricular nodal re-entrant tachycardia. In 15 patients with atrioventricular re-entrant tachycardia, 10 had Wolff-Parkinson-White syndrome, four patients had non-Wolff-Parkinson-White related tachycardia, and one had Mahaim tachycardia. Nine patients with Wolff-Parkinson-White syndrome had ablation: radiofrequency ablation was performed in eight patients and cryoablation in one patient. One patient, in whom the ablation procedure was not performed, was followed up with anti-arrhythmic drug therapy.
Bradycardia was observed in 4/24 patients (16.6%) due to congenital (two patients) and post-operative (two patients) complete atrioventricular block. In patients with congenital complete atrioventricular block, one patient had epicardial pacemaker implantation at one month and the other had permanent transvenous pacemaker implantation at 11 years. Anti-arrhythmic drug therapy was given to 18 patients and included oral metoprolol (37.5%), propafenone (20%), sotalol (20%), propranolol (16.7%), and amiodarone (12.5%).
Cardiac catheterization and interventional procedures were performed in 75% (59/79 patients) of the patients; the median age was 7.6 years (0–23). Procedures included diagnostic angiography (31/59 patients, 52.5%), electrophysiologic study and ablation (21/59 patients, 35.6%), other interventional procedures (7/59 patients, 11.9%) which included transvenous permanent pacemaker implantation (three patients), pulmonary valvuloplasty (two patients), stent implantation for aortic coarctation (one patient), and transcatheter atrial septal defect closure (one patient).
A total of 21 ablation procedures were performed in 16 patients with supraventricular tachycardia. Median age was 13.3 years (4.9–17). Radiofrequency ablation (85.7%) or cryoablation (14.3%) were performed and were successful in 14/16 patients (87.5%). Median procedure duration was 120 min (65–300), and floroscopy duration was 67 min (3–136). Three-dimensional imaging techniques (Ensite NavX navigation display technology; five procedures) and Coil-Assisted Retrograde Transvenous Obliteration; two procedures were used during 33.3% (7/21) of ablation procedures. Recurrence rate was 25%. Four patients required second ablation procedure (median age was 14.1 years [7.3–17.0]) and one patient (13.4 years) required third ablation procedure. An implantable cardiac defibrillator was implanted in one patient with ventricular tachycardia.
Cardiac surgery
Cardiac surgery was performed in 31.6% of the patients (25/79); the median age at first surgery was 6.5 years (4 days–29 years). Second surgery was performed in 28% (7/25) of the patients and median age at second surgery was 6.6 years (6 months–11 years). Common associated anomalies in operated patients were tricuspid regurgitation (88%), atrial septal defect/patent foramen ovale (72%), mild-moderate mitral regurgitation (28%), ventricular septal defect (20%), patent ductus arteriosus (16%), transposition of the great arteries (16%), pulmonary atresia (16%), and pulmonary stenosis (12%). Preoperatively, 59% of the patients had severe, 27.2% had moderate, and 13.6% had mild degree tricuspid regurgitation. During the neonatal period, 7.6% of the patients were operated on, and during the first year of life, 12.6% were operated on.
Types of surgical procedures included biventricular repair (11/25 patients, 44%), one-and-a-half ventricular repair (one patient, 4%), single ventricular repair (one patient, 4%), and other palliative surgical procedures (12/25 patients, 48%). Out of 11 patients with biventricular repair, five patients had Danielson repair, three had tricuspid valve replacement, two had tricuspid valvuloplasty, and one had mitral valve replacement and atrial septal defect closure and epicardial pacemaker insertion. Other surgical procedures included atrial septal defect closure (four patients), ventricular septal defect closure (one patient), pulmonary valvulotomy and patent ductus arteriosus closure (one patient), Brock procedure and atrial septal defect closure (one patient), pulmonary valvulotomy and left Blalock–Taussig shunt (one patient), atrial plication (one patient), epicardial pacemaker implantation (one patient), intrapericardial Blalock–Taussig shunt (one patient), and pulmonary banding (one patient).
Second surgical procedures included tricuspid valve replacement and epicardial pacemaker implantation (one patient), epicardial pacemaker implantation (two patients), second tricuspid valve replacement due to trombus-related valve dysfunction (one patient), mitral valve replacement and atrial septal defect closure (one patient), residual pulmonary stenosis removal with patch (one patient), and atrial septal defect closure (one patient).
Follow-up and mortality
The median follow-up period was 5.3 years (1 day–32 years). During the study period, eight patients (10.1%) were deceased. In total, 62.5% were female. The median mortality age was 25 days (1 day–17 years). Causes were heart failure (five patients), sudden cardiac death (one patient), necroting enterocolitis (one patient), and renal failure (one patient). Three patients died during the perioperative period, and two of them were in neonatal period. Perioperative mortality rate was 12%. The causes were heart failure (two patients) and renal failure (one patient). Surgical procedures performed in these patients were single ventricular repair (one patient), biventricular repair (mitral valve replacement and atrial septal defect closure and epicardial pacemaker insertion) (one patient), and intrapericardial Blalock–Taussig shunt (one patient).
Factors affecting arrhythmia presence and mortality
Older diagnosis age (p = 0.005) and presence of mild-moderate mitral regurgitation (p = 0.036) were significantly associated with presence of arrhythmias. Gender, presence, or degree of tricuspid regurgitation and other associated cardiac anomalies did not significantly affect presence of arrhythmias (p > 0.05) (Table 1).
Table 1. Comparison of the patients according to presence of arrhythmias

n = Number of patients; % = Column percentage; min-max = minimum-maximum; *Chi-Square Test; **Mann–Whitney U Test; # Fisher’s Exact Test; +Others include patients(1) with persistent left superior vena cava (one patient) and tetralogy of Fallot (one patient) and patients(2) with interventricular septal malignment (one patient), mild degree aortic insufficiency (one patient), patent ductus arteriosus (one patient).
Younger age at diagnosis (p = 0.012), younger age at first surgery (p = 0.004), and presence of pulmonary atresia (p = 0.000014) had a significant effect on mortality. In addition, surgery before age three years was also calculated as a risk factor for mortality (p = 0.037). Gender, diagnosis period (prenatal or postnatal), associated genetic diseases or congenital anomalies, associated cardiac anomalies other than pulmonary atresia, presence and degree of tricuspid regurgitation, surgery history, surgery type, and second surgical intervention did not significantly affect mortality (p > 0.05) (Table 2).
Table 2. Comparison of alive and demised patients
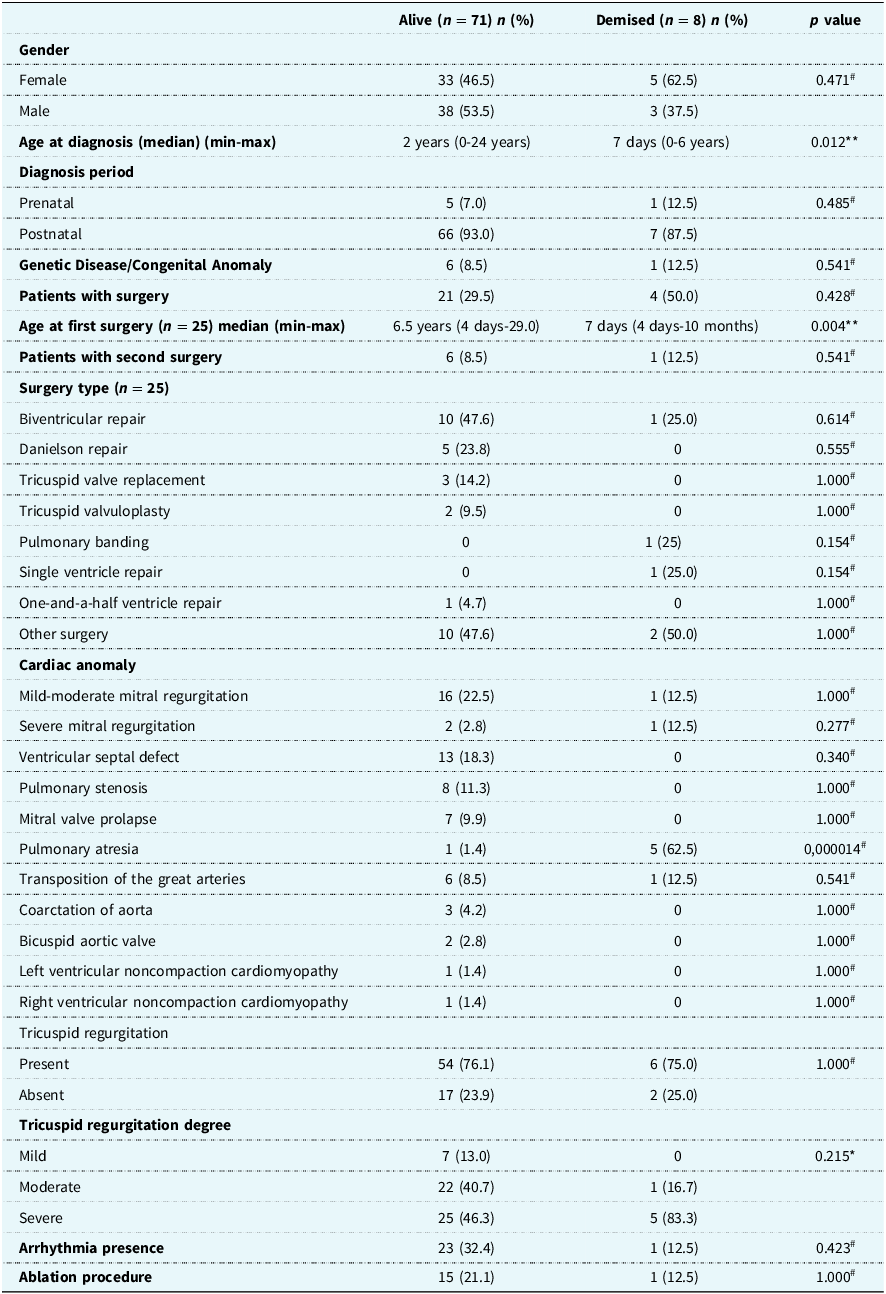
n = Number of patients; % = percentage; *Pearson Chi-square Test; **Mann–Whitney U Test; # Fisher’s Exact Test.
Presence of arrhythmias and arrhythmia types and ablation procedures did not significantly affect mortality (p > 0.05) (Table 2).
Survival analysis was made using log rank distribution curves. No statistically significant difference was found between the survival rates of the patients with respect to gender (p = 0.188), presence of arrhythmias (p = 0.149), and surgery history (p = 0.499). Presence of moderate and severe tricuspid regurgitation compared with mild and no tricuspid regurgitation did not have a significant effect on survival (p = 0.988).
According to univariate and multivariate analysis, gender, diagnosis age, presence of congenital anomalies and genetic disorders, tricuspid regurgitation, arrhythmias, and surgery history did not have an independent effect on survival (p > 0.05) (Table 3).
Table 3. Independent effects of some variables on survival
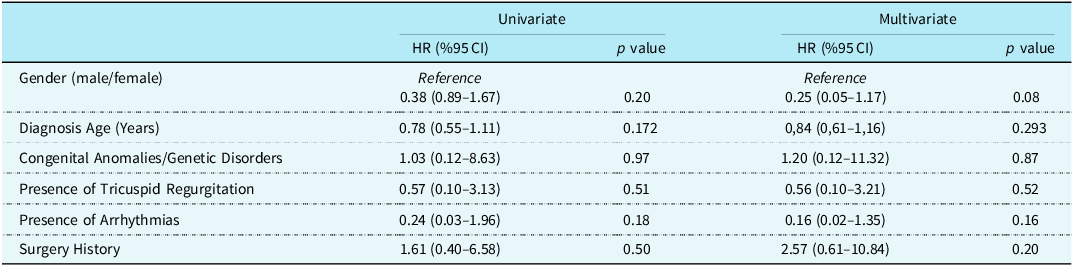
HR = Hazard ratio; CI = Confidence interval.
Discussion
Studies in literature about management and outcome of paediatric patients with Ebstein’s anomaly are limited. Reference Celermajer, Cullen, Sullivan, Spiegelhalter, Wyse and Deanfield4,Reference Geerdink, Delhaas and Helbing8–Reference Holst, Dearani and Said17 In this study, we evaluated a large cohort of children and young adults with Ebstein’s anomaly and analysed factors affecting arrhythmia presence and mortality.
In a retrospective multicentre study by Kapusta et al., which evaluated 93 paediatric patients diagnosed and followed as Ebstein’s anomaly, median diagnosis age was less than 30 days (1 day–162 months). Reference Kapusta, Eveleigh and Poulino9 Median follow-up period was 86 months (maximum, 216 months) and 81% of the patients survived. Younger age (12 months) at presentation, mechanical ventilation, diuretic and prostaglandin requirement at presentation, presence of hepatomegaly, moderate/severe pulmonary stenosis, pulmonary atresia, patent ductus arteriosus, and ventricular septal defect were significantly associated with death. Gender, gestational age, birth weight, degree of tricuspid regurgitation, need for operation, cyanosis, and arrhythmias did not significantly affect mortality. Boston et al. analysed risk factors for mortality in 52 children younger than 12 years who underwent tricuspid valve repair and annuloplasty for Ebstein’s anomaly. Reference Boston, Dearani, O'Leary, Driscoll and Danielson15 Mean age at surgery was 7.1 ± 3.9 years (5 months to 12 years) and follow-up period was 12.2 ± 7.4 years (10 months–24.3 years). Early mortality rate was 5.8%. Risk factors for mortality were found as age younger than 2.5 years and weight less than 10.7 kg. In accordance with these studies, we also observed that younger age at diagnosis (p = 0.012), surgery before age three years (p = 0.037), and pulmonary atresia (p = 0.000014) were significantly associated with mortality. This may be explained by the fact that patients who have mortality have more severe form of disease, become symptomatic earlier, and therefore diagnosed at a younger age. Our median mortality age was 25 days. Higher mortality rates with fetal and neonatal presentations were also reported in other studies. Reference Celermajer, Cullen, Sullivan, Spiegelhalter, Wyse and Deanfield4,Reference Geerdink, Delhaas and Helbing8,Reference Shinkawa, Polimenakos and Gomez-Fifer10–Reference Oxenius, Attenhofer Jost and Prêtre11 . Gender, presence and degree of tricuspid regurgitation, and arrhythmias also did not affect mortality in our study.
Attie et al. analysed risk factors for mortality in 174 Ebstein’s anomaly patients (148 operated and 26 non-operated) with a follow-up from 6 months to 25.3 years. Reference Attie, Casanova and Zabal20 Functional class III or IV and cardiothoracic ratio ≥65% with either cyanosis or arrhythmias were found to be associated with mortality. However, the mortality in patients who had only one of these variables was lower. We also did not observe any significant difference between alive and demised patients in terms of arrhythmia presence and arrhythmia types. We did not analyse functional class, cardiothoracic ratio, cyanosis, and combination of variables as risk factors for mortality in our study.
Geerdink et al. analysed factors associated with death during childhood in a cohort of 176 patients diagnosed with Ebstein’s anomaly. Reference Geerdink, Delhaas and Helbing8 Thirty-one patients (18%) died before the age of 18 years. The authors also observed that the median age at death was less than one month (range, 1 day–49 months). Presentation with Modified Ross Heart Failure Class IV was the most important univariate factor associated with death and others included neonatal diagnosis, severe tricuspid valve regurgitation, severe right ventricular outflow tract obstruction, and patent ductus arteriosus at presentation. Multivariable analysis showed that presentation with Modified Ross Heart Failure Class IV and a ventricular septal defect as additional comorbidity were the strongest predictors of death in childhood and adolescence. The authors included patients with pulmonary atresia in the severe right ventricular outflow tract obstruction group. We also observed that pulmonary atresia was strongly associated with death in our cohort. We did not analyse Modified Ross Heart Failure class and also did not find ventricular septal defect as an additional risk factor. These differences may be because our cohort consisted of a mixed population of children and young adults. Patients diagnosed in young adulthood might have different outcomes from those patients diagnosed during the neonatal period or later in childhood.
Most commonly associated cardiac anomalies reported in Ebstein’s anomaly are atrial septal defect or patent foramen ovale (80–94%). Reference Cetta, Dearani, O’Leary, Shaddy, Penny, Feltes, Cetta and Mital3,Reference Ramcharan, Goff, Greenleaf, Shebani, Salazar and Corno6,Reference Oxenius, Attenhofer Jost and Prêtre11 In Oxenius et al.’s study, atrial septal defect/patent foramen ovale (79%), pulmonary stenosis/atresia (17%), left ventricular noncompaction (12%), and ventricular septal defect (7%) were the most common associated anomalies. Reference Oxenius, Attenhofer Jost and Prêtre11 In our study, atrial septal defect/patent foramen ovale (57%), mitral regurgitation (25.3%), pulmonary stenosis/atresia (17.7%), and ventricular septal defect (16.5%) were also the most common anomalies. Attenhofer et al. stated that Ebstein anomaly should not be regarded as a disease confined to the right side of the heart. Reference Attenhofer Jost, Connolly, O'Leary, Warnes, Tajik and Seward21 They observed left heart abnormalities involving the myocardium or valves in 39% of their patients; 17.9% had left ventricular myocardial changes resembling noncompaction, left ventricular dysfunction was present in 43%, ventricular septal defect in 8%, bicuspid aortic valve in 8%, mitral valve prolapsus in 15%, and mitral valve dysplasia in 4%. We also observed left heart lesions in 58% of our patients, including ventricular septal defect (16.5%), mitral regurgitation (25.3%), mitral valve prolapsus (8.9%), aortic coarctation (3.8%), bicuspid aortic valve (2.5%), and left ventricular noncompaction in one patient.
In Ebstein’s anomaly, chromosomal or Mendelian disorders are detectable in about 20% of the patients, and in 80%, it is an isolated non-syndromic malformation. Reference Digilio, Silvestri, Dallapiccola, Marino, Giamberti and Chessa22 Deletion 1p36, deletion 8p23.1, and terminal deletion 18q are the most frequently reported chromosomal disorders associated with Ebstein’s anomaly. In our series, chromosomal and Mendelian disorders were present in three patients. We also had one patient with deletion 1p36. Apert, Noonan, CHARGE, Holt-Oram, Cornelia de Lange, Kabuki syndromes, and VACTERL association have also been reported monogenic syndromes. Reference Digilio, Silvestri, Dallapiccola, Marino, Giamberti and Chessa22 Congenital anomalies such as low-set ears, micrognathia, cleft lip and/or palate, renal agenesis, megacolon, undescended testis, and bilateral inguinal hernia have also been reported. We observed cleft lip and/or palate in two patients and congenital scoliosis in one patient. In our observation, presence of associated genetic diseases or congenital anomalies did not significantly affect mortality.
Attenhofer et al. compared the use of echocardiography and cardiac MRI in Ebstein’s anomaly patients and concluded that both methods provided complementary data and should be performed together before surgery. Reference Attenhofer Jost, Edmister and Julsrud23 We also used cardiac MRI as a complementary imaging method before surgery.
Accessory pathways are common and present in approximately one-third of the Ebstein’s anomaly patients and may be even multiple. Reference Cetta, Dearani, O’Leary, Shaddy, Penny, Feltes, Cetta and Mital3,Reference Ramcharan, Goff, Greenleaf, Shebani, Salazar and Corno6,Reference Geerdink and Kapusta7,Reference Reich, Alud and Hulse12–Reference Karagöz, Ertuğrul and Aypar14 The prevalence of arrhythmias in children and adolescents with Ebstein’s anomaly was reported lower than in adults. Reference Cetta, Dearani, O’Leary, Shaddy, Penny, Feltes, Cetta and Mital3,Reference Ramcharan, Goff, Greenleaf, Shebani, Salazar and Corno6,Reference Delhaas, du Sarvaas and Rijlaarsdam13 In younger age, atrioventricular re-entrant tachycardia related to accessory pathways or atrioventricular nodal re-entrant tachycardia is common. Later in life, atrial tachycardia, atrial flutter, intra-atrial re-entrant tachycardia, and atrial fibrillation are more common, due to a dilated right atrium and postoperative atrial incisions. Reference Cetta, Dearani, O’Leary, Shaddy, Penny, Feltes, Cetta and Mital3 Studies about arrhythmias and ablation in paediatric Ebstein’s anomaly patients are limited. Reference Reich, Alud and Hulse12–Reference Karagöz, Ertuğrul and Aypar14 Delhaas et al. reported prevalence of arrhythmias as 17% in 93 children with Ebstein’s anomaly, and most were supraventricular tachycardia with a preexcitation prevalence of 15%. Reference Delhaas, du Sarvaas and Rijlaarsdam13 In another study from our centre, in which 89 paediatric Ebstein’s anomaly patients were evaluated, arrhythmias were present in 30.3, 31% required ablation, and most were due to accessory pathways (82%). Reference Karagöz, Ertuğrul and Aypar14 We observed a high incidence of arrhythmias (30.4%) in our study. This finding may be because some of our patients had been referred for ablation, as our centre is a tertiary referral centre. Another reason may be the use of electrophysiological studies in all of our patients with symptomatic tachycardia or documented arrhythmia (Wolff–Parkinson–White pattern or sustained tachycardia) in electrocardiograms or in 24-hour electrocardiogram recordings. Ventricular tachycardia has been reported to be rare and can more commonly be induced during electrophysiological studies. Reference Delhaas, du Sarvaas and Rijlaarsdam13,Reference Karagöz, Ertuğrul and Aypar14 However, following tricuspid valve surgery, there is a higher incidence of ventricular arrhythmias, related to ventriculotomy incisions. Reference Cetta, Dearani, O’Leary, Shaddy, Penny, Feltes, Cetta and Mital3 We had only one patient with ventricular tachycardia diagnosed during catheterisation in the pre-operative period.
It has been reported that although acute success rates for catheter ablation of accessory pathways in Ebstein’s anomaly are high, recurrence rates are also high. Reference El-Assaad, DeWitt and Mah24 In a multicentre study, catheter ablation was successful in 8/9 patients. Reference Delhaas, du Sarvaas and Rijlaarsdam13 In another study, in which 65 paediatric Ebstein’s anomaly patients with 82 accessory pathways (median age: 9.8 ± 5.4 years [4 months–20 years]) were evaluated, acute success rates and recurrence rates for right free wall, right septal, and other mechanisms were 79/32%, 89/29%, and 75/27%, respectively. Reference Reich, Alud and Hulse12 Our acute success rate was higher and recurrence rate was lower compared to this study, the differences may be due to use of three-dimensional imaging techniques during some of our ablation procedures and added procedural experience at our centre. Successful results with surgical ablation in accessory pathway-related tachycardias without recurrence have also been reported. Reference Stulak, Sharma, Cannon, Ammash, Schaff and Dearani25
Surgical results of Ebstein’s anomaly patients have been reported both in children and adults. Reference Boston, Dearani, O'Leary, Driscoll and Danielson15–Reference Holst, Dearani and Said17,Reference Stulak, Sharma, Cannon, Ammash, Schaff and Dearani25–Reference Davies, Pasquali and Jacobs27 In a multicentre study, in which 63 children with Ebstein’s anomaly with 109 operations were evaluated, biventricular repair was performed in 59%, one-and-half ventricular repair in 5.8%, and univentricular repair in 33%. The median age at first operation was 26 months (range: 0–203 months) and median follow-up period was 121 months. Second surgery was required in 42.8% of the patients. Reference Geerdink, du Sarvaas and Kuipers16 The most common post-operative complication was arrhythmia (21%). Perioperative mortality rate was 14%. In our series, we mostly performed biventricular repair (44%) and palliative surgery (48%). These differences in surgery types may be due to fact that surgery type is primarily determined by the tricuspid anatomy in Ebstein’s anomaly and also by the surgeon and the institution experience. Our lower rate of second surgical interventions compared to this study may be because we only performed 32 operations in 25 patients with a higher median age at first surgery (6 years) and our median follow-up period was shorter. Our post-operative arrhythmia (19.2%) and perioperative mortality (12.1%) rates were similar.
In a study in which 498 Ebstein’s anomaly patients with a total of 595 operations from different centres were analysed: 116 were neonates (19%), 122 were infants (21%), 264 were children (44%), and 93 were adults (16%). Reference Davies, Pasquali and Jacobs27 Neonates mostly had palliative procedures: systemic-to-pulmonary artery shunts, with or without tricuspid valve closure (37.1%), tricuspid valve closure (8.6%), and Ebstein’s repair/tricuspid valvuloplasty (32%). Infants mostly had superior cavopulmonary connections (50.8%) and systemic-to-pulmonary artery shunts (8.2%). In older patients, tricuspid valve surgery (children, 54.5%; adults, 68.8%), arrhythmia procedures (children, 8.7%; adults, 17.3%), and Fontan surgery (children, 14.8%) were applied. Overall mortality rate was 5.9%. Highest in-hospital mortality was in neonatal period (23.4%). We also performed mostly palliative procedures in neonates and infants, and biventricular repair in older children and young adults. We also observed highest in-hospital mortality in the neonatal period.
Limitations of the study
Our study is a retrospective study of all 79 patients diagnosed with Ebstein’s anomaly and followed in a single tertiary care university hospital over a 17-year period. All limitations of retrospective studies apply.
Although we presented a large cohort with a rare disease, as out of 79 patients, only eight patients were deceased, the number is statistically still too small for analysing risk factors for mortality and performing an extended multivariable analysis.
Conclusions
In children and young adults presenting with Ebstein’s anomaly, younger age at presentation and at surgery, surgery before age three years, and presence of pulmonary atresia were associated with death. Ablation procedures can be successfully performed but recurrence rate is still high.
Acknowledgements
None.
Financial support
None.
Competing interests
None.





