Statement of Research Significance
Research Question(s) or Topic(s): This study examined whether neurocognitive function in neuro-oncology patients is associated with caregiver burden in a sample of distressed caregivers. While caregivers provide essential functional support, leading to distress in other neurologic conditions, few studies have explored this relationship in neuro-oncology. Main Findings: In a sample of 78 patient-caregiver dyads, findings demonstrate that poorer cognitive performance – particularly delayed memory – is associated with greater interference in caregivers’ daily schedules, underscoring the potential for interdependence between patient functioning and caregiver well-being. Study Contributions: By identifying cognitive mechanisms linked to caregiver burden, this work supports the integration of cognitive rehabilitation strategies into neuro-oncology care to improve outcomes for both patients and caregivers.
Introduction
A primary malignant brain tumor diagnosis has significant implications for neurocognitive functioning, which can limit a patient’s quality of life and daily independence. Mild-to-moderate neurocognitive impairment, including slowed processing speed, executive dysfunction, aphasia, and memory loss, is estimated to impact more than 80% of patients with primary malignant brain tumors (Boone et al., Reference Boone, Roussel, Chauffert, Le Gars and Godefroy2016). These impairments result from the tumor itself (e.g., brain invasion, cerebral edema) and/or the aggressive standard-of-care treatments that follow (e.g., surgical resection, cranial irradiation, chemotherapy) (Ng et al., Reference Ng, See, Ang, Tan, Ang and King2019), and often lead to decreased functioning (Oh et al., Reference Oh, Song, Kwon, Lee, Choi and Jeon2022), unemployment (Silvaggi et al., Reference Silvaggi, Leonardi, Raggi, Eigenmann, Mariniello, Silvani, Lamperti and Schiavolin2020), driving restrictions (Thomas et al., Reference Thomas, Mehta, Kuo, Ian Robins and Khuntia2011), and poorly maintained social roles (Loughan et al., Reference Loughan, Reid, Willis, Davies, Boutté, Barrett and Lo2022). Cognitive and functional declines may also result in sudden and significant role shifts, requiring informal and uncompensated caregiver involvement (e.g., friends, family) with substantial physical, emotional, social, and financial demands – responsibilities for which caregivers feel unprepared to manage (Schubart et al., Reference Schubart, Kinzie and Farace2008).
Neuro-oncology caregivers are burdened by compounding sources of stress, juggling the demands and disruptions to daily life that arise from supporting their loved one through cancer treatment and navigating the unique challenges of cognitive and functional decline common throughout the trajectory of a primary malignant brain tumor. In general, caregiving often entails unexpected additional responsibilities and changing relationships, contributing to caregiver burden – a construct defined as the objective and subjective impact of providing care on one’s [caregiver’s] schedule, self-esteem, lack of family support, physical health, and financial health (Given et al., Reference Given, Given, Stommel, Collins, King and Franklin1992). Patients with progressive cognitive and functional deficits require additional support, further amplifying the stressors contributing to caregiver burden. Thus, the stress resulting from disease-related cognitive and functional decline and the stress of the broader demands of caregiving are additive, both contributing to the severity of burden for neuro-oncology caregivers.
Understandably, caregivers of those with primary malignant brain tumors report the need for increased supportive care services to ensure their ability to provide high-quality patient care, which requires self-management of their own mental and physical health (Boele et al., Reference Boele, Rooney, Bulbeck and Sherwood2019; Choi et al., Reference Choi, Stone, Kim, Ren, Schulz, Given, Given and Sherwood2012). Findings regarding the psychological impact of neuro-oncology caregiving are variable. While some caregivers report personal growth or deriving new meaning and hope from their experiences (Applebaum et al., Reference Applebaum, Kryza-Lacombe, Buthorn, DeRosa, Corner and Diamond2016; Schubart et al., Reference Schubart, Kinzie and Farace2008), many others underscore the prevalence of emotional distress (e.g., depression, anxiety) in addition to burnout (Braun et al., Reference Braun, Aslanzadeh, Thacker and Loughan2021; Choi et al., Reference Choi, Stone, Kim, Ren, Schulz, Given, Given and Sherwood2012; Willis et al., Reference Willis, Ravyts, Lanoye, Reid, Aslanzadeh, Braun, Svikis, Rodin and Loughan2022). While not all caregivers at large experience increased levels of distress, it is well-documented in the field of neuro-oncology with a systematic review reporting levels of depression ranged from 10 – 50% in caregivers and increased symptoms of anxiety in 40 – 75% of neuro-oncology caregivers (Sherwood et al., Reference Sherwood, Cwiklik and Donovan2016). For caregivers of individuals with primary malignant brain tumors, the incidence of anxiety was found to be present in 79% of the individuals (Sherwood et al., Reference Sherwood, Cwiklik and Donovan2016). Further, previous biobehavioral research found that neuro-oncology caregivers with high baseline depressive symptoms are at sustained risk throughout the patient’s disease trajectory, with 95% following a high-risk trajectory for depressive symptoms, 86% for anxiety, and 78% for caregiver burden (Bayen et al., Reference Bayen, Laigle-Donadey, Prouté, Hoang-Xuan, Joël and Delattre2017; Choi et al., Reference Choi, Stone, Kim, Ren, Schulz, Given, Given and Sherwood2012). Caregivers’ experience of chronic stress has been found to produce a cascade of events that increases their vulnerability to mental and physical illness; this, in turn, has been shown to threaten the quality of patient care and associated outcomes (Goldberg et al., Reference Goldberg, Sherwood, Sereika, Donovan, Weimer, Drappatz, Boele, Shi and Loughan2023; Sherwood et al., Reference Sherwood, Given, Doorenbos and Given2004, Reference Sherwood, Given, Given, Schiffman, Murman, Lovely, von Eye, Rogers and Remer2006, Reference Sherwood, Given, Given, Schiffman, Murman, Von Eye, Lovely, Rogers and Remer2007; Sherwood et al., Reference Sherwood, Price, Weimer, Ren, Donovan, Given, Given, Schulz, Prince, Bender, Boele and Marsland2016; Zeleníková et al., Reference Zeleníková, Ren, Schulz, Given and Sherwood2016).
Negative associations between caregiver burden and neurocognitive functioning have been documented across neurological populations including neurodegenerative diseases (Black et al., Reference Black, Ritchie, Khandker, Wood, Jones, Hu, Ambegaonkar and Ginsberg2018; Chang et al., Reference Chang, Huang, Chang, Hsu, Lee, Huang, Wang and Chang2023; Dauphinot et al., Reference Dauphinot, Ravier, Novais, Delphin-Combe, Moutet, Xie, Mouchoux and Krolak-Salmon2016; Hergert & Cimino, Reference Hergert and Cimino2021; O’Caoimh et al., Reference O’Caoimh, Calnan, Dhar and Molloy2021), traumatic brain injury (Ilaghi et al., Reference Ilaghi, Gharib, Pirani, Vahabie, Grafman, Shariat, Shariati, Jahanbakhshi and Mirfazeli2024), and stroke (Stolwyk et al., Reference Stolwyk, Mihaljcic, Wong, Hernandez, Wolff and Rogers2024). However, scientific limitations exist such as the reliance on global cognitive screening tools (e.g., Mini Mental Status Examination [MMSE], Montreal Cognitive Assessment [MoCA]) which have demonstrated limited sensitivity in detecting dysfunction in brain tumor patients (Aslanzadeh et al., Reference Aslanzadeh, Braun, Brechbiel, Willis, Parker, Lanoye and Loughan2022), reliance on subjective reports of patient’s neurocognitive functioning despite evidence that subjective and objective cognition represent two distinct constructs (Pranckeviciene et al., Reference Pranckeviciene, Deltuva, Tamasauskas and Bunevicius2017), and inconsistencies in selection of objective neurocognitive tests and testing procedures. While patient neurocognitive functioning has been examined in relation to a variety of neuro-oncology caregiver characteristics (e.g., quality of life, emotional health) (Boele et al., Reference Boele, Weimer, Marsland, Armstrong, Given, Drappatz, Donovan and Sherwood2022), the few studies that have specifically assessed caregiver burden rely mostly upon the experience of those with low-grade brain tumors and even fewer include the experience of those with malignant tumors (Yang et al., Reference Yang, Rushton, Park, Son, Woodward, Mcconnell and Hendrix2020; Zamanipoor et al., (Reference Zamanipoor Najafabadi, van der Meer, Boele, Taphoorn, Klein, Peerdeman, van Furth and Dirven2021) – a group more likely to experience cognitive impairment (van Kessel et al., Reference van Kessel, Baumfalk, van Zandvoort, Robe and Snijders2017). The existing limited literature suggests that there is a varying relationship between a patient’s cognitive status and caregiver burden, with some studies reporting a negative association – where greater cognitive impairment is linked to higher caregiver burden – while others highlight factors that may moderate this relationship (Yang et al., Reference Yang, Rushton, Park, Son, Woodward, Mcconnell and Hendrix2020).
Therefore, the purpose of the present study was to explore the relationship between patients’ domain-specific neurocognitive functioning and dimensions of caregiver burden (i.e., negative effects on caregivers’ daily schedule, self-esteem, and perceived family support) within a sample of primary malignant brain tumor dyads. It is hypothesized that greater patient neurocognitive impairment (e.g., executive function, memory deficits) will be associated with higher levels of caregiver burden across multiple dimensions. Given the limited work in this area, this study aims to explore specific cognitive domains including verbal fluency, memory recall, inhibition, divided attention, and fine motor functioning as potential predictors of caregiver burden, with the goal of understanding which areas of cognitive impairment may contribute to increased caregiver challenges. As this is an exploratory aim, no a priori hypotheses were made regarding domain-specific effects. Considering these patients’ high risk for cognitive impairment and the considerable demands of caregiving, findings from the present study may inform treatment targets for the development of dyadic supportive care interventions.
Materials and methods
Standard protocol approvals, registrations, and patient consents
This cross-sectional secondary data analysis was conducted using patient neurocognitive testing and their caregivers’ self-reported burden from the baseline assessment of participants enrolled in an intervention targeting caregiver distress (NR013170) (SmartCare; Boele et al., Reference Boele, Weimer, Marsland, Armstrong, Given, Drappatz, Donovan and Sherwood2022). This study was reviewed and approved by the Virginia Commonwealth University and University of Pittsburgh Institutional Review Boards. The research was completed in accordance with the Helsinki Declaration.
Participants
Original data collection occurred between March 2014 and July 2016. The parent study (Boele et al., Reference Boele, Weimer, Marsland, Armstrong, Given, Drappatz, Donovan and Sherwood2022) was a three-arm parallel-group randomized-controlled trial to address the effectiveness of SmartCare – a nurse-led online need-based intervention for distressed caregivers compared to enhanced care as usual. Recruitment occurred at two NCI-designated cancer centers. Caregivers of adult ( > 21) patients who were diagnosed with a primary malignant brain tumor were screened and invited to participate in the SmartCare trial if they: 1) were over 21 years old; 2) were a non-professional, non-paid person who provided a majority of emotional, financial, and/or physical support; 3) were not currently the caregiver for anyone else over the age of 21; 4) reported elevated depressive symptoms ( ≥ 6) on the shortened Center for Epidemiological Studies-Depression Scale (CES-D); 5) be proficient in English, and 6) have telephone access. Patients were invited to participate in neurocognitive testing, though this was not required for caregivers’ eligibility in the original protocol. Both the patient and caregiver had to consent to be eligible for the study.
Measures
Caregiver burden
The 24-item Caregiver Reaction Assessment (CRA; Given et al., Reference Given, Given, Stommel, Collins, King and Franklin1992) was used to measure several dimensions of caregiver burden. Caregivers responded to self-report items on a 5-point Likert-type scale to assess five different domains of their caregiving experience: (1) impact on daily schedule, (2) caregiver’s esteem, (3) lack of family support, (4) impact on health, and (5) impact on finances. Each subscale score was calculated as an average of corresponding item responses. A higher score represents a stronger impact on the domain being assessed (e.g., a higher score on schedule impact means the caregiver experiences more scheduling burden). However, a higher score on the self-esteem subscale is indicative of higher reported self-esteem – suggesting of lower burden in this domain. As part of the original data collection in the parent RCT (SmartCare: Boele et al., Reference Boele, Weimer, Marsland, Armstrong, Given, Drappatz, Donovan and Sherwood2022), caregivers completed 3 subscales: impact on daily schedule, self-esteem, and lack of family support. The CRA has demonstrated good reliability and validity in previous studies of cancer survivors (Cronbach’s α = 0.61 – 0.83) (Nijboer et al., Reference Nijboer, Triemstra, Tempelaar, Sanderman and van den Bos1999).
Neurocognitive function
Neuropsychological test batteries completed by patients comprised several standardized tests commonly used in neuro-oncology populations including the Controlled Oral Word Association Test (COWA) (Ruff et al., Reference Ruff, Light, Parker and Levin1996; Strauss et al., Reference Strauss, Sherman and Spreen2006), Trail Making Test – Part B (TMT B) (Tombaugh, Reference Tombaugh2004), Stroop Color-Word Interference – Inhibition/Switching Trial (CWI) (Golden et al., Reference Golden, Freshwater and Golden2002), Auditory Verbal Learning Test – Delayed Recall (AVLT-DR) (Schmidt, Reference Schmidt1996; Strauss et al., Reference Strauss, Sherman and Spreen2006), and Grooved Pegboard – Dominant hand (GPB) (Heaton et al., Reference Heaton, Grant and Matthews1986). The tests selected were used to assess verbal fluency, divided attention, inhibition, delayed verbal recall, and fine motor dexterity, respectively. Standardized administration and scoring procedures were used. Neurocognitive tests were administered by trained study personnel.
Analysis
All analyses were performed using SPSS version 29 (IBM Corp, Reference Corp2021). Prior to analysis, data were assessed for assumptions of normality. Bivariate correlations assessed the relationship between caregivers’ educational attainment and their level of burden, as previous literature suggests there is a negative relationship between socioeconomic status (SES) and caregiver burden within oncology (Badger et al., Reference Badger, Segrin, Crane, Morrill and Sikorskii2024; Nikbakht Nasrabadi et al., Reference Nikbakht Nasrabadi, Pahlevan Sharif, Allen, Naghavi, Sharif Nia, Salisu and Yaghoobzadeh2022; Vahidi et al., Reference Vahidi, Mahdavi, Asghari, Ebrahimi, Eivazi Ziaei, Hosseinzadeh, Namdar Areshtanab and Kermani2016). Level of education was used as a proxy for SES, as has been done in other studies (Badger et al., Reference Badger, Segrin, Crane, Morrill and Sikorskii2024). Given our primary focus on the relationship between patient cognitive status on their caregiver’s level of burden, analyses were limited to dyads with complete data. Demographic data for both patients and caregivers were calculated including age, gender, level of education, and employment status. Additional patient-specific variables include medical characteristics: type of tumor, tumor grade, hemispheric location of tumor, lateralization of tumor, and months since initial diagnosis. Little’s MCAR was performed to determine randomness of missing patient neurocognitive data.
First, independent samples t-tests were used to evaluate the differences in caregiver burden between patients with and without intact neurocognitive functioning. Patients were categorized as being cognitively intact (z-score > −1.50) or having a cognitive deficit (z-score ≤ −1.50) based on published normative samples for each neurocognitive test (Golden et al., Reference Golden, Freshwater and Golden2002; Heaton et al., Reference Heaton, Grant and Matthews1986; Ruff et al., Reference Ruff, Light, Parker and Levin1996; Schmidt, Reference Schmidt1996; Strauss et al., Reference Strauss, Sherman and Spreen2006; Tombaugh, Reference Tombaugh2004); practices often utilized in this population (Wefel et al., Reference Wefel, Vardy, Ahles and Schagen2011). Then, hierarchical regressions evaluated the relationship between patients’ neurocognitive performance on individual tests and caregiver burden for only those variables that were significant in independent t-tests (i.e., there was a significant difference in caregiver burden between patients with and without intact cognition). Scores for each caregiver burden subscale were entered into the model as the dependent variable. To control for clinical variables that may impact the relationship between patient cognition and caregiver burden, the first block included tumor lateralization (left- or right-sided) and tumor grade (low- or high-grade). Given the potential effect of motor dexterity on TMT B (a task of divided attention), post-hoc analyses controlled for patients’ fine motor capabilities (GPB) on this test only. Standardized neurocognitive test scores (e.g., z-scores) went into each model at subsequent steps using the enter method. To account for multiple comparisons, Bonferroni corrections were applied separately within each set of analyses (i.e., across 5 t-tests and across 2 regression models). Adjusted significance thresholds were maintained at p < .05.
Because this study used secondary data, an a-priori power analysis was not feasible; therefore, we emphasize effect sizes and confidence intervals to convey the precision of our estimates (Goodman & Berlin, Reference Goodman and Berlin1994). Further, a post-hoc power analysis was not conducted, as such analyses are not informative once results are known (Althouse, Reference Althouse2021; Heckman et al., Reference Heckman, Davis and Crowson2022; Heinsberg & Weeks, Reference Heinsberg and Weeks2022; Hernán, Reference Hernán2022; Wallace & Melia, Reference Wallace and Melia2008).
Results
Participants
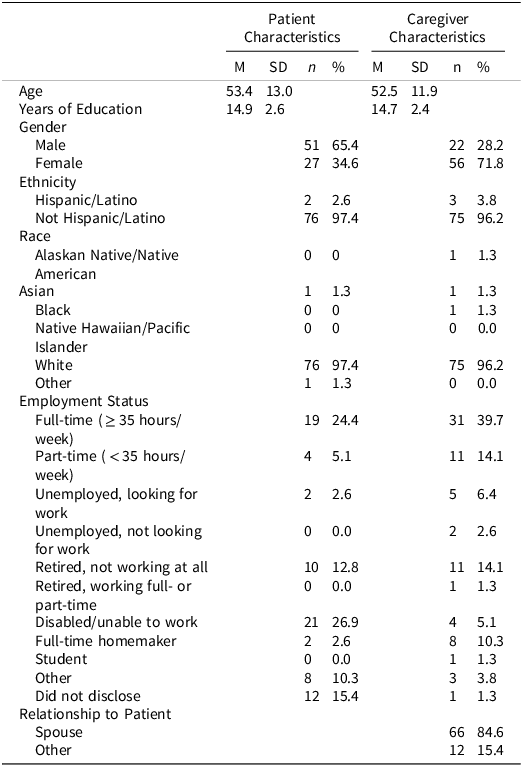
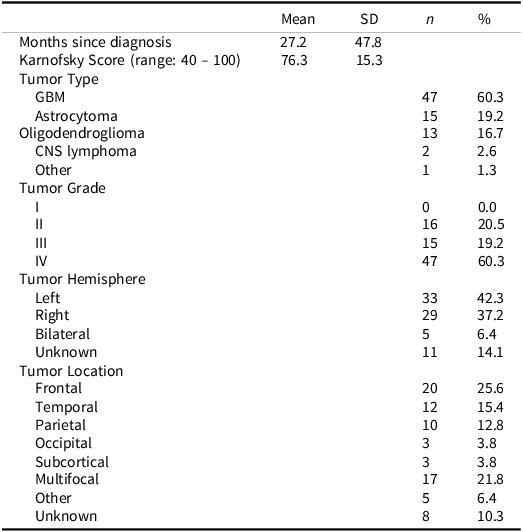
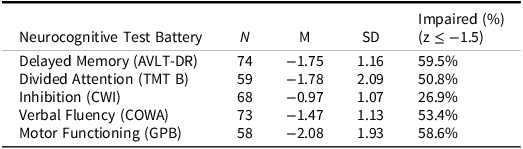
In the parent RCT (SmartCare; Boele et al. (Reference Boele, Weimer, Marsland, Armstrong, Given, Drappatz, Donovan and Sherwood2022), 120 caregivers completed baseline evaluation and were randomized to treatment. Reasons for non-participation in eligible dyads (n = 94) included lack of interest (41.5%), feeling overwhelmed (24.5%), not wanting support (19.2%), or being uncomfortable with technology and digital platforms (14.8%) (Boele et al., Reference Boele, Weimer, Marsland, Armstrong, Given, Drappatz, Donovan and Sherwood2022). Patients who enrolled in the study were not required to complete neurocognitive testing. Those who agreed to testing may not have completed all neurocognitive measures due to fatigue and disease burden at the time of the visit, contributing to the missingness of certain data points. Little’s MCAR determined that patient neurocognitive data was missing completely at random (ps > .05). Ultimately, seventy-eight dyads were included in this study. Demographic information is available in Tables 1 and 2 for patients and caregivers. Information regarding patient performance on neurocognitive measures is in Table 3. As expected per eligibility for the parent study, caregivers reported elevated levels of depression at baseline (CES-D: M = 10.32, SD = 5.53), which is comparable to levels reported in previous research (M = 11.82, SD = 11.53) (Badger et al., Reference Badger, Segrin, Crane, Morrill and Sikorskii2024). Levels of caregiver burden in this sample (Schedule Burden: M = 3.43, SD = 1.17; Self-Esteem: M = 4.20, SD = 0.50; Lack of Family Support: M = 1.88, SD = 1.00) were comparable to caregiver burden in other oncology caregivers demonstrated in a psychometric validation study (Nijboer et al., Reference Nijboer, Triemstra, Tempelaar, Sanderman and van den Bos1999).
Table 1. Patient and caregiver demographics (N=78)

Note: Demographic information was self-reported.
Table 2. Patient tumor characteristics
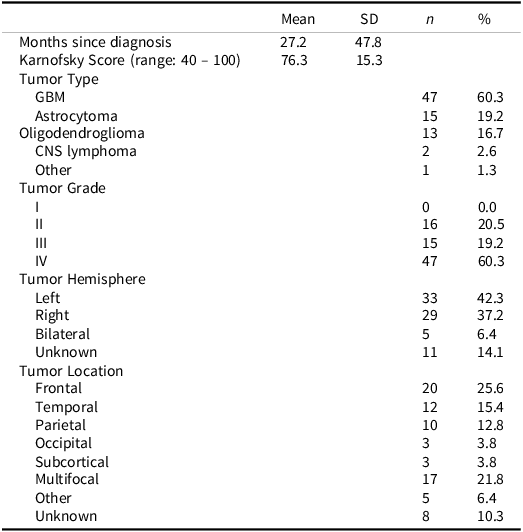
CNS = central nervous system, GBM = glioblastoma.
Table 3. Patient neurocognitive performance

AVLT-DRc = Auditory Verbal Learning Test-Delayed Recall, COWA = Controlled Oral Word Association, CWI = Stroop Color-Word Interference – Inhibition/Switching Trial, GPB = Grooved Pegboard, TMT B = Trail Making Test – Part B.
Caregiver educational attainment was not associated with their level of burden in this sample (all subscales ps > .05).
Differences in caregiver burden between patients with intact vs. below expectation neurocognitive function
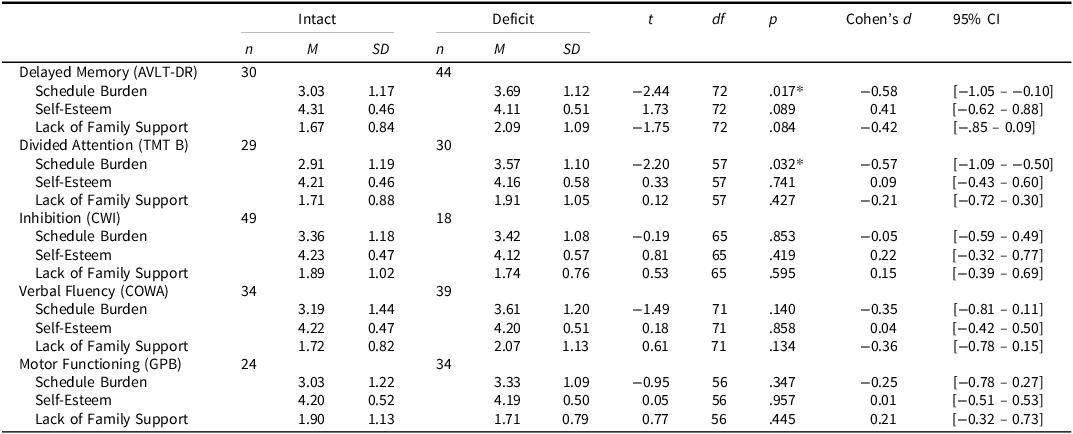
There was a difference in caregiver schedule burden for patients with intact delayed memory (M= 3.03, SD = 1.17) versus those with a deficit (M = 3.69, SD = 1.12), t(72) = −2.44, p = 0.017, Cohen’s d= −0.58, and for patients with intact divided attention (TMT B) (M= 2.91, SD = 1.19) compared to those with a deficit (M = 3.57, SD = 1.10), t(57) = −2.20, p= 0.032, Cohen’s d= −0.57; however, neither effect remained significant after Bonferroni correction, though delayed memory remained trending towards significance. There were no differences in caregiver self-esteem or lack of family support regardless of the patient’s cognitive status (all p’s > .05). T-test results are summarized in Table 4.
Table 4. Differences in caregiver burden based on patient cognitive status

*p < .05.
Intact: z > −1.5 SD, Deficit: z ≤ −1.5 SD; AVLT-DR = Auditory Verbal Learning Test-Delayed Recall, COWA = Controlled Oral Word Association, CWI = Stroop Color-Word Interference– Inhibition/Switching Trial, GPB = Grooved Pegboard, TMT B = Trail Making Test – Part B.
Relationship between patient neurocognitive function and caregiver burden
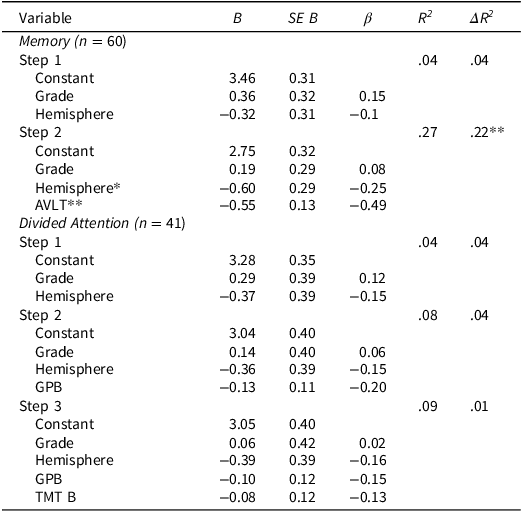
Informed by the t-test results, individual hierarchical regressions were used to evaluate the relationship between caregiver schedule burden and patients’ delayed verbal memory and divided attention. Time since diagnosis was not associated with caregiver burden or patients’ neurocognitive performance, thus it was not included in subsequent models (ps > .05).
Delayed verbal memory
The first overall regression model including tumor hemisphere and grade did not predict caregiver burden (p > .05). However, adding delayed memory performance in the second model significantly improved the model, ΔR 2 = .23, p < .001. Better delayed memory performance (β = −.49, p < .001) and left-sided tumor lateralization (β = −.26, p = .034) were both associated with reduced caregiver schedule burden. After applying Bonferroni correction for multiple comparisons, delayed memory remained a significant predictor, whereas the effect of left-lateralization did not.
Divided attention
The initial model, which included tumor hemisphere and grade, was not significantly associated with caregiver schedule burden (p > .05). Adding performance on a motor task did not improve the model (p > .05), nor did the addition of a divided attention task (p > .05). Regression results are summarized in Table 5.
Table 5. Association between patient neurocognitive performance and caregiver schedule burden

* p < .05; ** p < .001
AVLT-DR = Auditory Verbal Learning Test-Delayed Recall, GPB = Grooved Pegboard, TMT B = Trail Making Test – Part B.
Discussion
Neuro-oncology caregiver burden is significant and related to a host of negative health sequelae for both the patient and the caregiver. Specifically, patients with primary malignant brain tumors experience cognitive effects from both the tumor and its treatment. Together, these insults increase the burden and responsibility of informal caregivers. Therefore, the present study sought to explore the relationship between objective neurocognitive functions in patients with primary malignant brain tumors and their caregiver’s subjective burden. Although lower SES has been linked to greater caregiver burden in oncology populations (Badger et al., Reference Badger, Segrin, Crane, Morrill and Sikorskii2024; Nikbakht Nasrabadi et al., Reference Nikbakht Nasrabadi, Pahlevan Sharif, Allen, Naghavi, Sharif Nia, Salisu and Yaghoobzadeh2022), this was not observed in our sample. This may be due to the use of education as a proxy for SES rather than direct measure of income; however, income data could not be reliably analyzed, as 50% of caregivers declined to report their income.
Although differences in caregiver schedule burden – or the strain on a caregiver’s time and daily functioning – by delayed memory and divided attention performance did not remain significant after Bonferroni correction, both associations demonstrated medium effect sizes, suggesting potentially meaningful trends. Caregivers of patients with cognitive deficits in these areas reported greater interference with daily routines. These preliminary findings warrant further investigation in larger samples to determine whether specific cognitive domains contribute uniquely to caregiver schedule burden. Executive functions including inhibition, divided attention, and verbal fluency were not related to any domain of caregiver burden.
Interestingly, time since diagnosis was not associated with caregiver burden or patients’ neurocognitive performance. This suggests that both caregiver burden and patient cognitive difficulties may be relatively stable or robust across the disease course in this sample, rather than increasing or decreasing systematically with time. These findings highlight the importance of early identification and support as burden and cognitive impairment may persist regardless of how long it has been since diagnosis. After controlling for tumor hemisphere and grade and dominant-sided fine motor dexterity, delayed verbal memory, but not divided attention, remained significantly related to caregiver schedule burden. Specifically, caregiver schedule burden increased with worse patient delayed verbal memory performance. Poor delayed verbal memory, or difficulty with free recall of unstructured verbal information over time, may contribute to daily forgetfulness and reduced independence of instrumental activities of daily living, such as missed appointments or difficulty managing one’s schedule.
Taken together, difficulty with retrieval was the most salient predictor of strain on neuro-oncology caregivers, particularly their daily schedule and time burden (e.g., “Stop work to care”; “Activities centered on care”), whereas caregiver’s daily burden appeared to be less sensitive to changes in patient’s higher order, executive functions. Similar findings regarding the impact of memory on caregiver burden have been observed in both objective (Bajaj & Sinha, Reference Bajaj and Sinha2009) and subjective memory assessments (Shim et al., Reference Shim, Kang, Kim and Kim2015) of individuals with mild cognitive impairment. Deficits in retrieval of verbal information may be an indicator of an amnestic profile or temporal lobe dysfunction or may represent a frontal network disruption in which cues, context, or mnemonics are required for retrieval, with storage relatively spared. Although the association between left-sided tumor lateralization and caregiver burden did not survive correction for multiple comparisons, the unadjusted result suggests a potential trend worth exploring in future studies to clarify whether tumor laterality contributes uniquely to caregiver burden strain beyond cognitive performance itself. While left temporal lesions can often be associated with language dysfunction, this region also plays a broader role in memory, social cognition, and emotion regulation (Olson et al., Reference Olson, McCoy, Klobusicky and Ross2013). This means that a lesion classified as “left temporal” may not produce a strictly amnestic profile but could be a broader disruption of neural networks and may impact the brain’s functional connectivity and efficiency (Parsons & Sabsevitz, Reference Parsons and Sabsevitz2023). Another important consideration is that patients with left temporal tumors may have more advanced disease (Fyllingen et al., Reference Fyllingen, Bø, Reinertsen, Jakola, Sagberg, Berntsen, Salvesen and Solheim2021), meaning that both the patient and caregiver have had more time to adapt. Caregivers may have developed coping strategies or accessed additional support, which could contribute to lower reported burden. Further, if patients present with significant cognitive decline, caregivers may have already adjusted their expectations, resulting in perceived lower burden.
Lastly, research suggests that gliomas can prompt structural and functional reorganization in the contralateral hemisphere, particularly within the cognitive control network (Liu et al., Reference Liu, Hu, Yu, Jiang, Yang, Hu, Li, Liu, Zou, Liu and Chen2020). This compensatory mechanism may help mitigate cognitive deficits, which could partly explain why left temporal tumors were associated with lower caregiver burden. If similar network-level compensation occurs in temporal regions, it may preserve certain cognitive functions despite tumor presence, reducing the observable impact on daily functioning and, in turn, caregiver strain.
The strong association between delayed verbal memory and caregiver schedule burden is likely due to the increase in patient dependence on their informal caregivers for schedule and time management. Cognitive rehabilitation interventions, tailored to patients’ specific cognitive deficits are likely to also impart benefit to both the patient and the caregiver. Cognitive strategies – such as external memory aids (e.g., calendars, alarms, structured planners, and a memory notebook), errorless learning techniques, and spaced retrieval practice – can help compensate for verbal memory deficits and improve daily functioning. Additionally, strategy-based interventions, such as teaching caregivers to provide effective cueing or establishing structured routines may further alleviate the functional demands that are placed on caregivers. Implementing these approaches in patients with primary malignant brain tumors is likely to not only enhance patient autonomy but also significantly reduce caregivers’ functional burden (e.g., daily scheduling and time-related burden).
Neither caregiver self-esteem nor family support differed between caregivers of patients with and without intact neurocognitive functioning, across any domain of neurocognitive performance. It is possible that, regardless of the patient’s level of cognitive impairment, caregivers are still able to experience reward and fulfillment. Existing literature does support the notion that neuro-oncology caregivers report personal growth (Choi et al., Reference Choi, Stone, Kim, Ren, Schulz, Given, Given and Sherwood2012; Schubart et al., Reference Schubart, Kinzie and Farace2008), so this may explain why caregiver self-esteem was unaffected by patient cognitive status. Further, caregivers in this sample reported high levels of self-esteem. Instead, caregiver self-esteem may be linked to their perspective of the patient’s illness. Caregivers may have the appropriate insight into the probability of eventual cognitive decline as a natural part of the disease process. This perception may offer caregivers the ability to separate the patient’s condition from their own self-worth and caregiving abilities. Furthermore, issues of family support are more likely to be influenced by external factors outside of a patient’s cognitive status, such as family dynamics, physical distance, and SES.
Several limitations should be noted within the context of this study. First, the study is limited by its cross-sectional design and small sample size. This renders all conclusions correlational and warrants future longitudinal analysis of caregiver burden and patient neurocognitive functioning to adequately identify the ideal timing for dyadic cognitive rehabilitation intervention. The present sample was predominantly White, highly educated, and had the time and means to participate in an online, behavioral intervention. Additionally, the caregiver sample in this study exhibited treatment-seeking behaviors to alleviate their increased levels of distress and depressive symptoms. Further, there was limited information in the caregiver sample regarding socioeconomic position. Research supports that socioeconomic position impacts the level of caregiver burden, such that those with lower position incur higher objective burden (Tough et al., Reference Tough, Brinkhof, Siegrist and Fekete2019). As a result, the generalizability of the findings is limited. While the sample of patients all had neuro-oncology diagnoses, there was variability in disease characteristics. Although variability in time since diagnosis, tumor type, grade, and hemisphere, as well as treatment history, decreases internal validity, it increases generalizability. Larger samples with adequate power to investigate differences based on tumor molecular profile and grade would be helpful to investigate differences in pursuit of intervention development and implementation as these disease characteristics are related to disease prognosis and functional status (Li et al., Reference Li, Qin, Zhang and Cao2022). Finally, a measure of premorbid functioning (e.g., Test of Premorbid Functioning) and a measure of delayed recognition would make for a better-rounded battery of patients’ objective cognitive functioning. Specifically, without data on delayed recognition of verbal information, it is unclear whether the relationship between delayed memory and caregiver schedule burden is due to a temporally mediated amnestic dysfunction or a frontal-mediated retrieval deficit. These data would be important to guide intervention efforts.
Conclusion
Patients with primary malignant brain tumors experience a myriad of cognitive sequelae from the immediate effects of the tumor and its prolonged treatments – often leaving them with deficits across multiple cognitive domains. These insults increase the burden and responsibility of informal caregivers who are tasked with navigating cognitive decline alongside the cancer treatment of their loved ones. In this sample, difficulty with memory retrieval was the most salient predictor of strain on neuro-oncology caregiver’s daily schedule and time burden. Our findings speak to the interdependence of patient outcomes and caregiver quality of life. There is an immediate need for dyadic interventions that support patient cognition and caregiver well-being as they both adjust to these functional changes. Cognitive rehabilitation interventions, tailored to the specific deficits of patients with malignant brain tumors and the caregiver’s experience of the deficits, are likely to impart significant benefit on the caregiver’s functional quality of life. Supporting both patients and caregivers as they navigate the complexities of a neuro-oncology diagnosis is essential to ensuring high-quality care.
Funding Statement
This project was funded by the National Institute for Nursing Research R01 NR013170.
Competing interests
There are no conflicts of interest disclosed by any author.