Children and adolescents are increasingly prescribed antipsychotic medications. In 2014, the prevalence of antipsychotic use among persons aged 0–19 years ranged from 0.5 to 30.8 per 1000 individuals (0.05–0.38%) globally. Reference Hálfdánarson, Zoëga, Aagaard, Bernardo, Brandt and Fusté1 In England, the prevalence of antipsychotic prescriptions for children and adolescents doubled, from 0.057% in 2000 to 0.105% in 2019. Reference Radojčić, Pierce, Hope, Senior, Taxiarchi and Trefan2 Increases of 137 and 110% were reported in the Netherlands and Denmark, respectively, from 2000 to 2010. Reference Kaguelidou, Holstiege, Schink, Bezemer, Poluzzi and Mazzaglia3 In Canada, the prevalence of antipsychotic use in children and adolescents increased about fourfold over a similar period. Reference Alessi-Severini, Biscontri, Collins, Sareen and Enns4 A comprehensive study of data obtained from 16 countries, from 2005 to 2014, reported increased antipsychotic use in children and adolescents in 14 of the 16 countries examined. Reference Hálfdánarson, Zoëga, Aagaard, Bernardo, Brandt and Fusté1 It is likely that this global increase is related to a surge in off-label prescribing practices. Reference Klau, Gonzalez-Chica, Raven and Jureidini5 This includes the prescription of antipsychotic medications to children and adolescents for anxiety or mood disorders, neurodevelopmental conditions and conduct and behavioural problems. Reference Carton, Cottencin, Lapeyre-Mestre, Geoffroy, Favre and Simon6–Reference Dörks, Bachmann, Below, Hoffmann, Paschke and Scholle11
Antipsychotic medications carry a significant risk of adverse physical health effects. Reference Loy, Merry, Hetrick and Stasiak7,Reference Iasevoli, Barone, Buonaguro, Vellucci and de Bartolomeis12,Reference Cheng-Shannon, McGough, Pataki and McCracken13 Adverse cardiometabolic effects, such as weight gain and increased risk of type 2 diabetes, are of particular concern in children and adolescents due to the potential for continued complications into adulthood and associated long-term morbidity. Reference Bobo, Cooper, Stein, Olfson, Graham and Daugherty14–Reference Pozzi, Ferrentino, Scrinzi, Scavone, Capuano and Radice16 Younger age for the use of antipsychotics promotes vulnerability to the adverse cardiometabolic effects of antipsychotics, Reference Iasevoli, Barone, Buonaguro, Vellucci and de Bartolomeis12,Reference De Hert, Dobbelaere, Sheridan, Cohen and Correll17–Reference Ratzoni, Gothelf, Brand-Gothelf, Reidman, Kikinzon and Gal21 with children and adolescents gaining disproportionately more weight than adults. Reference Ratzoni, Gothelf, Brand-Gothelf, Reidman, Kikinzon and Gal21,Reference Kaguelidou, Valtuille, Durrieu, Delorme, Peyre and Treluyer22
Additionally, those with neurodevelopmental conditions who are often prescribed antipsychotics Reference Park, Cervesi, Galling, Molteni, Walyzada and Ameis10,Reference Dörks, Bachmann, Below, Hoffmann, Paschke and Scholle11 are at risk of specific lifestyle-related challenges including heightened sedentary behaviour, Reference McCoy, Jakicic and Gibbs23 poor diet and nutrition, Reference Howard, Robinson, Smith, Ambrosini, Piek and Oddy24,Reference Barnhill, Gutierrez, Ghossainy, Marediya, Devlin and Sachdev25 disrupted sleep patterns Reference Devnani and Hegde26 and frequent tobacco use, Reference Tiikkaja and Tindberg27,Reference Casseus, Cooney and Wackowski28 all of which could compound the adverse cardiometabolic effects. Indeed, reports suggest that autistic youth are the most susceptible to antipsychotic-induced weight gain, Reference De Hert, Dobbelaere, Sheridan, Cohen and Correll17 with as many as one in six potentially receiving treatment with antipsychotic medications. Reference Park, Cervesi, Galling, Molteni, Walyzada and Ameis10 Weight gain is also likely to impact self-esteem and reduce the willingness of children and adolescents to continue taking antipsychotic medications. Reference Noguera, Ballesta, Baeza, Arango, de la Serna and González-Pinto29–Reference Eapen and John32 Thus, interventions to prevent adverse cardiometabolic effects during childhood have the potential to sustain the use of antipsychotic pharmacotherapy, in addition to preventing continued complications in adulthood.
‘Lifestyle’ interventions are non-pharmacological approaches aimed at modifying behaviour. They are typically multifaceted, including educational, psychotherapeutic or social intervention to modify lifestyle behaviour such as physical activity and dietary habits. Lifestyle interventions are effective at preventing and treating antipsychotic-induced weight gain in adults with serious mental illness. Reference Naslund, Whiteman, McHugo, Aschbrenner, Marsch and Bartels33–Reference Mohanty, Gandhi, Krishna Prasad, John, Bhaskarapillai and Malo37 They are also the mainstay for preventing and managing childhood obesity. Reference Pandita, Sharma, Pandita, Pawar, Tariq and Kaul38,Reference Salam, Padhani, Das, Shaikh, Hoodbhoy and Jeelani39 Given the unique context and characteristics of children and adolescents with neurodevelopmental and other mental health conditions who are regularly prescribed antipsychotics, Reference Park, Cervesi, Galling, Molteni, Walyzada and Ameis10,Reference Dörks, Bachmann, Below, Hoffmann, Paschke and Scholle11,Reference McCoy, Jakicic and Gibbs23–Reference Casseus, Cooney and Wackowski28,Reference Bowling, Blaine, Kaur and Davison40 effective strategies in general paediatrics probably require substantial adaptation. There is limited research on the effectiveness of such strategies, and no systematic analysis of the literature is available. Therefore, this study aims to systematically review and evaluate the effectiveness of lifestyle interventions for preventing or managing cardiometabolic risk and/or other adverse physical health outcomes in children and adolescents taking antipsychotic medications.
Specific research questions were:
-
(a) For children and adolescents taking antipsychotic medications, do lifestyle interventions reduce the risk of compromised physical health (any physical health outcome) compared with treatment as usual (TAU)?
-
(b) Which individual or combined components of a lifestyle intervention are most effective in reducing the risk of physical health decline?
Method
This systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Reference Matthew, David, Patrick, Isabelle, Tammy and Cynthia41 The PRISMA checklists are available in Supplementary Material 1.1–1.2 available at https://doi.org/10.1192/bjo.2025.10919. The study protocol was prospectively registered with PROSPERO (no. CRD42019136568) and has been published elsewhere. Reference Hawker, Bellamy, McHugh, Wong, Williams and Wood42
Eligibility criteria
Randomised controlled trials comparing lifestyle interventions with a TAU comparator group were eligible for inclusion if they met the following criteria: the study population included children and adolescents aged 6–17 years (mean age <20 years); ≥55% of the participants were taking an antipsychotic medication (adjusted from the initial criterion of ≥70% in the registered protocol, prior to screening); a physical health outcome (aside from motor development) was measured; and the study was published in English. Lifestyle interventions were defined as any educational, psychotherapeutic, social and behavioural intervention that aims to increase exercise or physical activity, optimise dietary intake, aid nicotine cessation or improve sleep quality and duration. Reference Hawker, Bellamy, McHugh, Wong, Williams and Wood42 For comparator groups, TAU was operationalised according to recommended care standards for children and adolescents prescribed antipsychotic medications (i.e. the implementation of metabolic monitoring and/or psychoeducation about weight and lifestyle Reference Walkup43,Reference Dinnissen, Dietrich, van der Molen, Verhallen, Buiteveld and Jongejan44 ). For a detailed description of the study eligibility criteria, developed using the Population, Intervention, Comparison, Outcomes and Study design (PICOS) framework, Reference Amir-Behghadami and Janati45 see Supplementary Material 2.
Information sources and search strategy
Four online bibliographic databases (PubMed, PsycINFO, Embase and Cochrane CENTRAL) were initially searched in March 2023, with searches re-run up to 22 April 2025. The search strategy was developed in PubMed and adapted for the other databases. Search terms were developed using the PICOS framework as a guideline. The search strategy was piloted by identifying a test set of three target papers known to meet the eligibility criteria. Using PubMed unique identifiers, the search strategy was iteratively refined to maximise the sensitivity and specificity for identification of the target articles, until reaching 100% sensitivity. The search strategy conducted in PubMed is available in Supplementary Material 3. The reference lists of relevant studies were searched to identify additional articles.
Following duplicate removal, at least two reviewers (J.B., P.J.H. and/or T.Y.W.). independently conducted title and abstract screening and full-text screening using Covidence systematic review software, version 2014 for Windows (Veritas Health Innovation, Melbourne, Australia; https://www.covidence.org/), with the PICOS framework as an eligibility criterion. Eligibility concerns were resolved by discussion. Interrater reliability for title/abstract screening and full-text screening was moderate (Cohen’s κ = 0.41) and excellent (Cohen’s κ = 0.87), respectively. Disagreements were primarily related to the study population, due to difficulties in elucidating participants’ antipsychotic medication status during title and abstract screening. Reviewers addressed this by marking records as ‘maybe’ during title and abstract screening when antipsychotic use was suspected (based on participant diagnoses). Medication status was then confirmed during full-text screening.
Outcomes
While all relevant physical health outcomes were considered, the primary outcome measure was participants’ cardiometabolic health as reflected by the difference in mean body mass index (BMI) change (kg/m2) between intervention and TAU groups at the post-intervention time point. BMI was selected as the primary outcome because it is the most robust indicator for identification of individuals whose excess adiposity places them at increased cardiometabolic risk. Reference Abbasi, Blasey and Reaven46 It is a validated and replicable proxy measure of adiposity in children and adolescents. Reference Simmonds, Llewellyn, Owen and Woolacott15,Reference Javed, Jumean, Murad, Okorodudu, Kumar and Somers47,Reference Freedman and Sherry48 Additional physical health outcomes included participants’ anthropometric data; blood pressure; any relevant hepatic, metabolic, endocrine and/or haematological data; presence of cardiovascular or respiratory disease; indicators of physical fitness; and physical health-related behaviours.
Data collection
Data extraction began on 4 January 2024. P.J.H. and J.B. independently extracted study data using a custom template created in Covidence. Physical health outcomes were extracted at all available time points. Study characteristics, including funding information, methods, population characteristics and intervention details, were extracted.
Data analysis and synthesis
A random-effects meta-analysis model was used to synthesise any physical health outcome reported at a post-intervention time point by three or more studies. The DerSimonian and Laird random-effects model was implemented using the metafor package in R version 4.4.0 for Windows (R Foundation, Vienna, Austria; https://www.r-project.org/). Reference Viechtbauer49 Standard mean difference (SMD) was calculated from the means and standard deviations comparing intervention and comparator group outcomes at the post-intervention time point, using aggregate data as reported in each study. Authors were contacted on two separate occasions (several weeks apart) when these data were not available. If no response was received, standard deviations were calculated assuming a z-score of 1.96 for a 95% confidence interval. The magnitude of effect size was interpreted according to Cohen’s guidelines. Reference Cohen50 Heterogeneity was assessed using Higgin’s I 2, with higher values indicating greater heterogeneity. Reference Higgins, Thompson, Deeks and Altman51 Substantial heterogeneity was defined as an I 2 value greater than 75%. Reference Deeks, Higgins, Altman, McKenzie and Veroniki52 Subgroup and sensitivity analyses were planned a priori to explore variations according to intervention characteristics, study risk of bias and participant characteristics. A narrative synthesis was used to summarise evidence of effect where results were not reported in a manner conducive to meta-analysis (insufficient data and/or presented in a format incompatible with other studies).
Assessments of bias and confidence in evidence
Publication bias was evaluated by visual inspection of funnel plots and Egger and colleagues’ test of asymmetry. Reference Egger, Davey Smith, Schneider and Minder53 Risk of bias was independently assessed by two reviewers (P.J.H. and J.B.) via the Cochrane risk-of-bias tool for randomised trials version 2 (RoB 2). Reference Sterne, Savović, Page, Elbers, Blencowe and Boutron54 Each outcome reported by a minimum of three studies was evaluated. Judgements of ‘low’ or ‘high’ risk of bias, or expression of ‘some concerns’, across domains were generated by the Cochrane algorithm following reviewers’ answers to signalling questions, with discrepancies resolved by discussion. The Grading of Recommendations, Assessment, Development and Evaluations (GRADE) Reference Guyatt, Oxman, Vist, Kunz, Falck-Ytter and Alonso-Coello55 was used to assess the cumulative body of evidence for the primary outcome measure.
Results
Searches
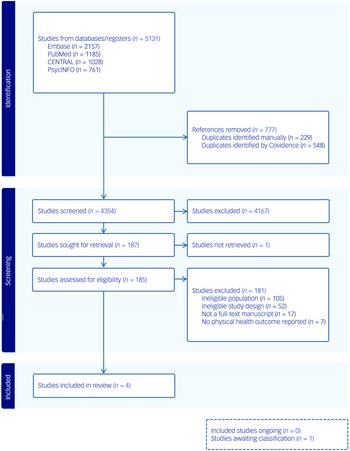
Searches revealed 5131 records. Following duplicate removal and title and abstract screening, 185 full texts were assessed for eligibility. Of these, four studies Reference Detke, DelBello, Landry, Hoffmann, Heinloth and Dittmann56–Reference Toscano, Carvalho and Ferreira59 met the inclusion criteria by reporting a physical health outcome measure in populations that included children and adolescents engaged in a lifestyle intervention during antipsychotic pharmacotherapy (see Fig. 1). One study Reference Correll, Sikich, Reeves, Johnson, Keeton and Spanos60 initially appeared to meet the inclusion criteria but was excluded because it did not contain an eligible comparator group. Another study Reference Moiniafshari, Gholami, Dalvand, Effatpanah and Rezaei61 was unable to be classified due to the full text being published in a non-English language.
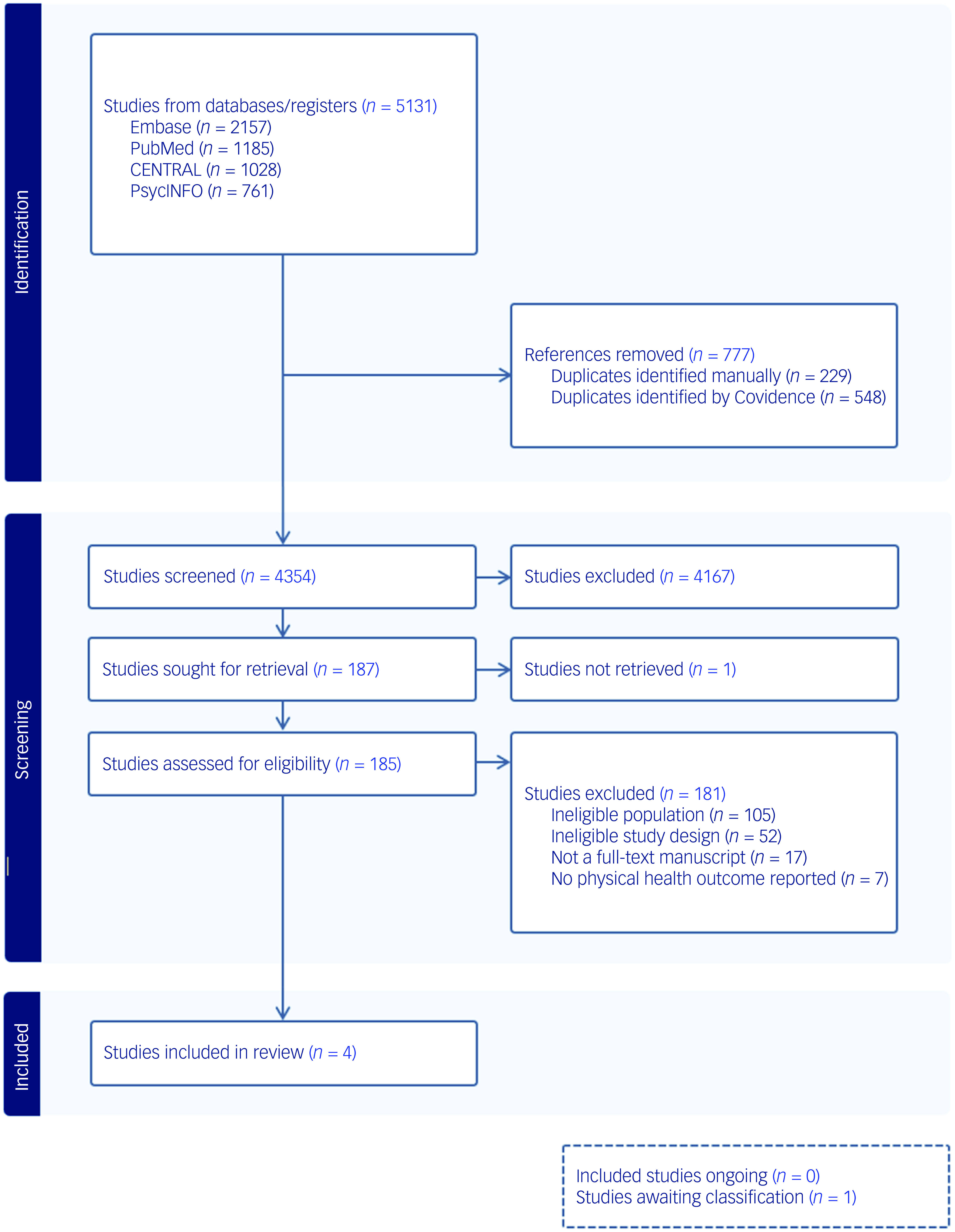
Fig. 1 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart.
Participant characteristics
Across all studies, 370 participants were included, with sample sizes ranging from 26 to 203 participants. Of these participants, 204 (55%) received a lifestyle intervention and 166 (45%) received TAU; 221 (60%) participants were male and 149 (40%) were female. The mean age of participants was 14.3 years (range 6–24). Ethnicity data were reported by only one study, Reference Detke, DelBello, Landry, Hoffmann, Heinloth and Dittmann56 in which 24 participants (12% of their sample) were described as Hispanic or Latino.
In the pooled data from all studies, 340 (91%) participants were taking antipsychotic medications. Data on the type of antipsychotic medication taken were available for 297 (87%) participants. Three studies Reference Detke, DelBello, Landry, Hoffmann, Heinloth and Dittmann56–Reference O’Donoghue, Mifsud, Castagnini, Langstone, Thompson and Killackey58 reported the type of antipsychotic medication taken by participants, with second-generation antipsychotics being prescribed in all cases. Olanzapine, aripiprazole and risperidone were the most frequently reported, representing 209 (70%), 41 (14%) and 21 (7%) instances, respectively. The remaining 26 (9%) antipsychotic prescriptions included paliperidone, lurasidone, quetiapine, amisulpride and ziprasidone.
Across all studies, two Reference Nicol, Kolko, Lenze, Yingling, Miller and Ricchio57,Reference O’Donoghue, Mifsud, Castagnini, Langstone, Thompson and Killackey58 reported a proportion of their sample as diagnosed with a psychotic disorder (the proportion of participants with a psychotic disorder across all studies was 41%). Other diagnoses included mood disorders (31%) Reference Detke, DelBello, Landry, Hoffmann, Heinloth and Dittmann56,Reference Nicol, Kolko, Lenze, Yingling, Miller and Ricchio57 and neurodevelopmental conditions (i.e. attention-deficit hyperactivity disorder, autism, learning disabilities and intellectual disabilities; 26%). Reference Nicol, Kolko, Lenze, Yingling, Miller and Ricchio57,Reference Toscano, Carvalho and Ferreira59 The remaining data included participants with anxiety disorders and other psychiatric diagnoses (2%). Reference Nicol, Kolko, Lenze, Yingling, Miller and Ricchio57
Study characteristics
One study was a multi-site trial conducted within the USA, Russia, Poland and Germany; Reference Detke, DelBello, Landry, Hoffmann, Heinloth and Dittmann56 the remaining studies were conducted in the USA, Reference Nicol, Kolko, Lenze, Yingling, Miller and Ricchio57 Australia Reference O’Donoghue, Mifsud, Castagnini, Langstone, Thompson and Killackey58 and Brazil. Reference Toscano, Carvalho and Ferreira59 The median duration from baseline to post-intervention was 32 weeks (range 12–52 weeks). One study Reference O’Donoghue, Mifsud, Castagnini, Langstone, Thompson and Killackey58 included a 6-month follow-up post-intervention. Two studies Reference Detke, DelBello, Landry, Hoffmann, Heinloth and Dittmann56,Reference O’Donoghue, Mifsud, Castagnini, Langstone, Thompson and Killackey58 reported lifestyle interventions to prevent antipsychotic-induced weight gain and/or metabolic complications, while the remaining two studies Reference Nicol, Kolko, Lenze, Yingling, Miller and Ricchio57,Reference Toscano, Carvalho and Ferreira59 employed a ‘treatment’ approach to ascertain the physical health benefits of lifestyle interventions delivered to children and adolescents taking medications.
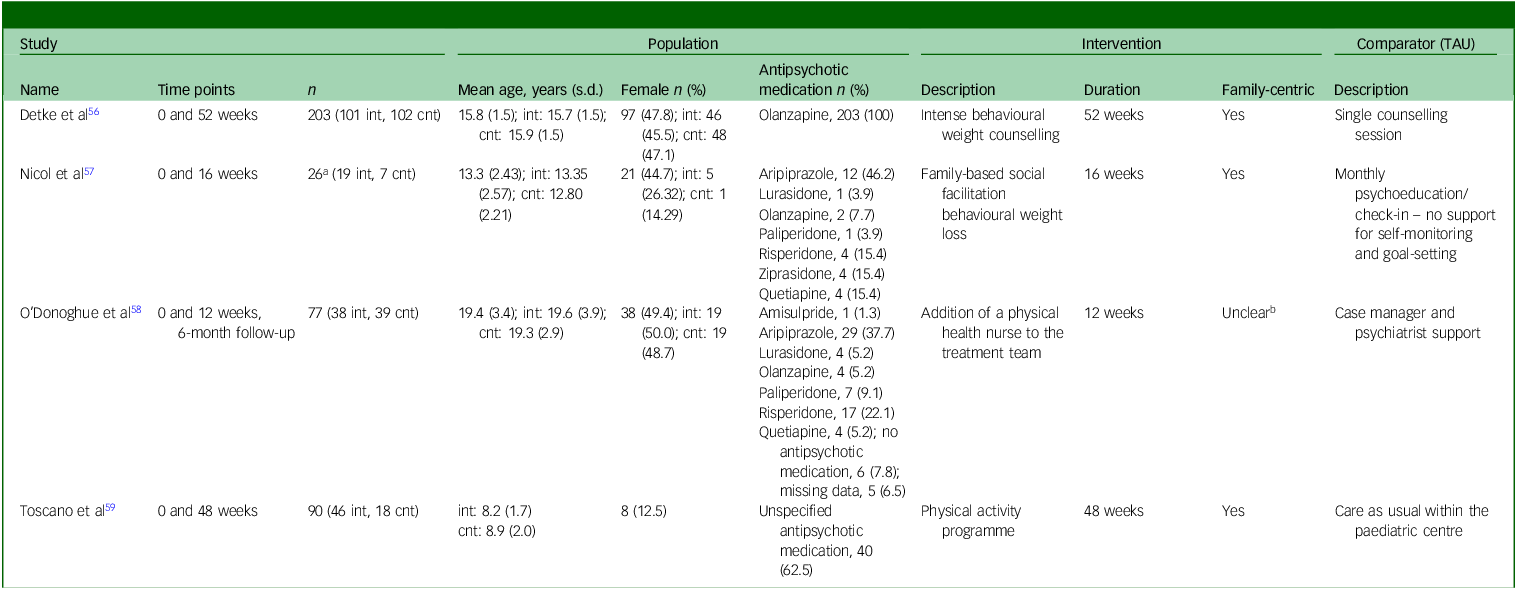
Three studies Reference Detke, DelBello, Landry, Hoffmann, Heinloth and Dittmann56,Reference Nicol, Kolko, Lenze, Yingling, Miller and Ricchio57,Reference Toscano, Carvalho and Ferreira59 reported the primary outcome measure of participants’ BMI at a post-intervention time point. Laboratory analytes, including hepatic, metabolic, endocrine and haematological data, were reported by all studies. Participants’ waist circumference was reported by three studies. Reference Detke, DelBello, Landry, Hoffmann, Heinloth and Dittmann56,Reference Nicol, Kolko, Lenze, Yingling, Miller and Ricchio57,Reference Toscano, Carvalho and Ferreira59 Clinically significant weight gain, defined as an increase of ≥7% from baseline weight, was provided by 2 studies. Reference Detke, DelBello, Landry, Hoffmann, Heinloth and Dittmann56,Reference O’Donoghue, Mifsud, Castagnini, Langstone, Thompson and Killackey58 Health behaviours were assessed through standardised questionnaires in two studies. Reference Nicol, Kolko, Lenze, Yingling, Miller and Ricchio57,Reference O’Donoghue, Mifsud, Castagnini, Langstone, Thompson and Killackey58 One study Reference Detke, DelBello, Landry, Hoffmann, Heinloth and Dittmann56 provided data on blood pressure, electrocardiograms and the side-effects of antipsychotic medications. One study Reference Toscano, Carvalho and Ferreira59 reported on participants’ health-related quality of life as rated by primary caregivers. Further details on study characteristics are provided in Table 1.
Table 1 Study characteristics
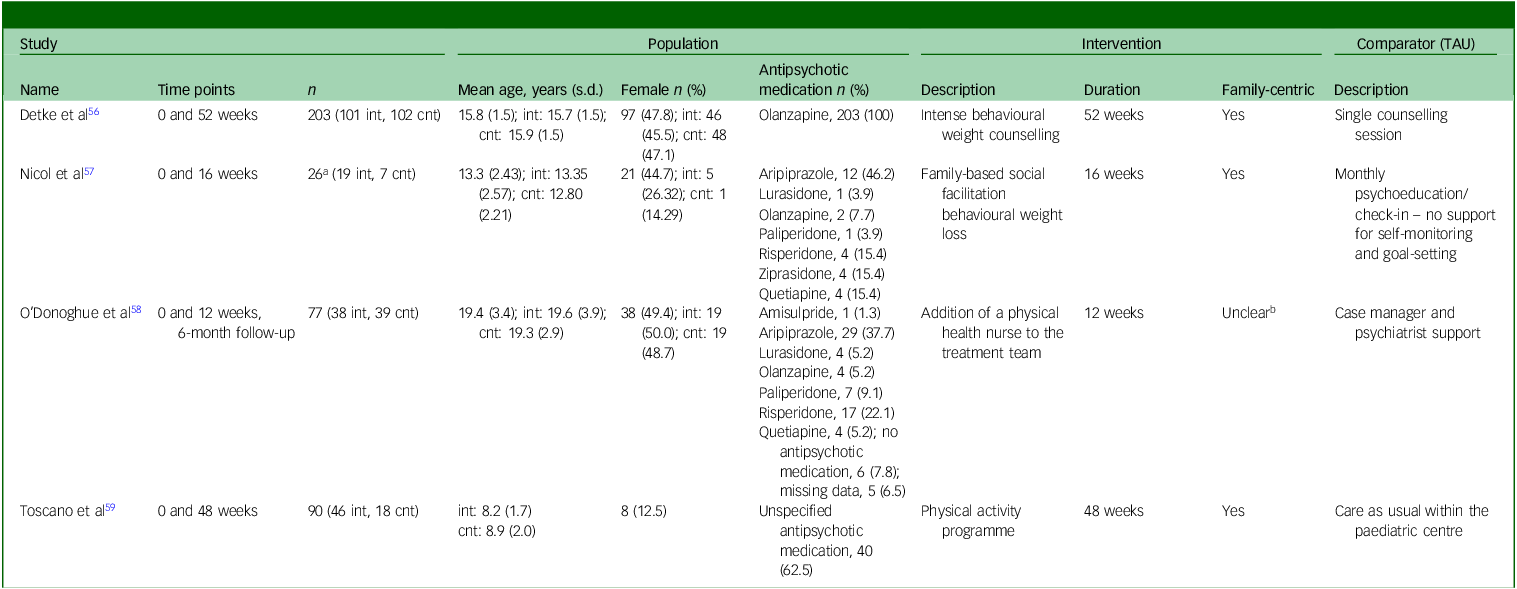
TAU, treatment as usual; int, intervention group; cnt, control group.
a. Excludes the ‘non-psychiatric’ group.
b. Parents/guardians/care-givers also consented to the study if the children were aged under 18 years.
Intervention and comparator strategies
Three studies Reference Detke, DelBello, Landry, Hoffmann, Heinloth and Dittmann56–Reference O’Donoghue, Mifsud, Castagnini, Langstone, Thompson and Killackey58 employed counselling as the core intervention strategy, and one Reference Toscano, Carvalho and Ferreira59 used a structured exercise programme. Of the three studies that utilised counselling, all included behavioural modification strategies comprising self-monitoring of physical activity and diet, goal-setting around health behaviours and problem-solving. Three included lifestyle education covering diet/nutrition, physical activity, smoking cessation and the metabolic impacts of antipsychotics. Three Reference Detke, DelBello, Landry, Hoffmann, Heinloth and Dittmann56–Reference O’Donoghue, Mifsud, Castagnini, Langstone, Thompson and Killackey58 included routine physical health monitoring at study visits, and one Reference O’Donoghue, Mifsud, Castagnini, Langstone, Thompson and Killackey58 included referral to allied health providers (dietitians and physiotherapists) and supported participants’ attendance at smoking cessation and sexual health interventions, when appropriate. The median frequency of participant engagement in lifestyle interventions was once per week (range, weekly to monthly) over a 32-week period (range, 12–52 weeks). Two studies Reference Detke, DelBello, Landry, Hoffmann, Heinloth and Dittmann56,Reference Toscano, Carvalho and Ferreira59 reported the length of intervention sessions with participants, with a median time of 27.5 min per engagement (range, 15–40 min).
Comparator (TAU) groups typically included brief education on weight gain and lifestyle adjustments and/or the implementation of monitoring practices. Further details on intervention and comparator strategies are available in Supplementary Material 4.
Random-effects meta-analysis
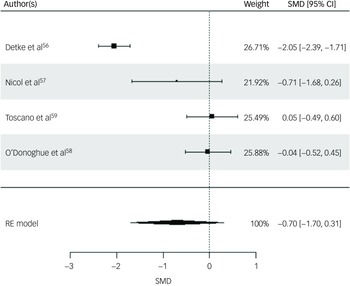
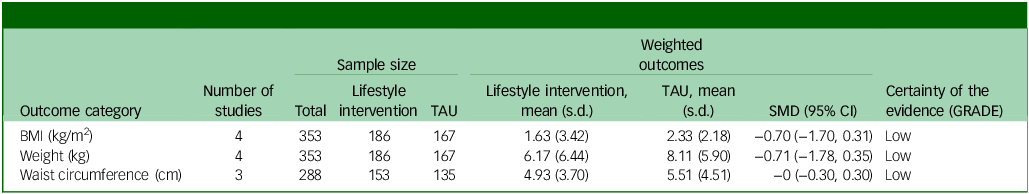
Three physical health variables, including participants’ BMI, weight and waist circumference, were included in the random-effects model. All four studies were included for participants’ BMI post-intervention. The random-effects model indicated a moderate but statistically non-significant intervention effect on BMI (SMD = −0.70, s.e. = 0.51, z = −1.36, P = 0.17, 95% CI: −1.70 to 0.31; Fig. 2), with substantial heterogeneity reported (I 2 = 93%, t 2 = 0.95, P < 0.001). The risk of bias for BMI as rated via RoB 2 was high across all studies. Reference Detke, DelBello, Landry, Hoffmann, Heinloth and Dittmann56,Reference Nicol, Kolko, Lenze, Yingling, Miller and Ricchio57,Reference Toscano, Carvalho and Ferreira59 Egger’s test indicated no evidence of publication bias (t = 1.0852, P = 0.39). While interpretation is limited due to the small sample, visual inspection of the funnel plot suggested relative symmetry around the pooled effect size, with study precision inversely related to the magnitude of effect size (Supplementary Material 5.1).
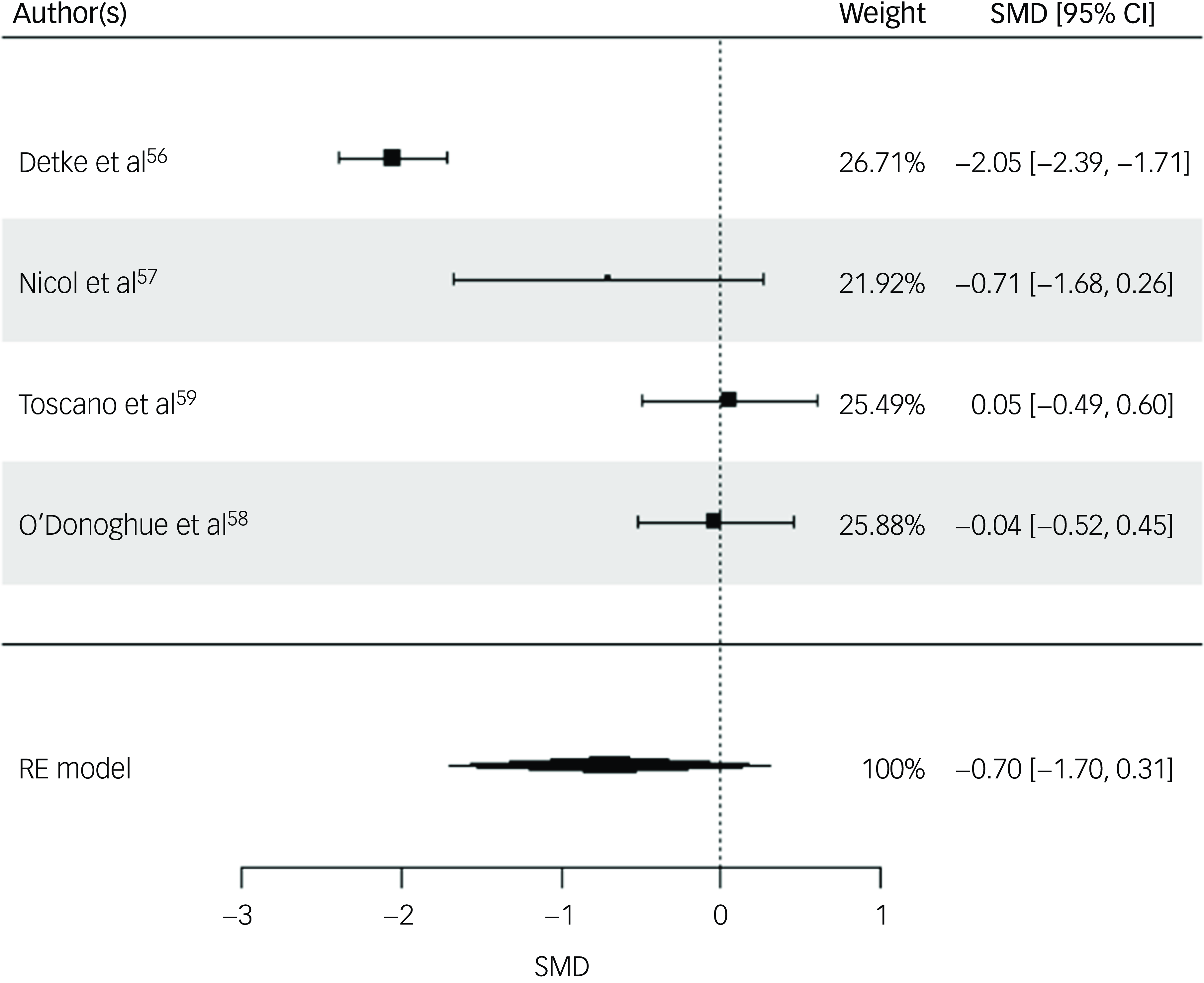
Fig. 2 Forest plot for BMI post-intervention. BMI, body mass index; RE, random effects; SMD, standard mean difference.
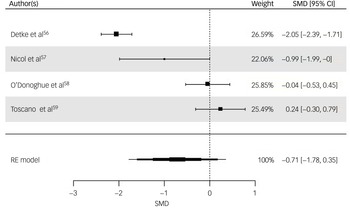
All four studies were included for weight (kg) post-intervention. The random-effects model indicated a moderate but statistically non-significant intervention effect (SMD = −0.71, z = −1.32, s.e. = 0.54, P = 0.19, 95% CI: −1.78 to 0.35; Fig. 3), with substantial heterogeneity reported (I 2 = 94%, t 2 = 1.07, P < 0.001). The risk of bias for weight as rated via RoB 2 was ‘high’ for three studies, Reference Detke, DelBello, Landry, Hoffmann, Heinloth and Dittmann56,Reference Nicol, Kolko, Lenze, Yingling, Miller and Ricchio57,Reference Toscano, Carvalho and Ferreira59 with ‘some concerns’ for O’Donoghue et al. Reference O’Donoghue, Mifsud, Castagnini, Langstone, Thompson and Killackey58 Egger’s test indicated no evidence of publication bias (t = 0.871, P = 0.476). While interpretation is limited due to the small sample, visual inspection of the funnel plot of weight suggested a slight pattern of asymmetry, with the most precise study reporting a small effect size and less precise studies indicating a wider variation in effect size (Supplementary Material 5.2).
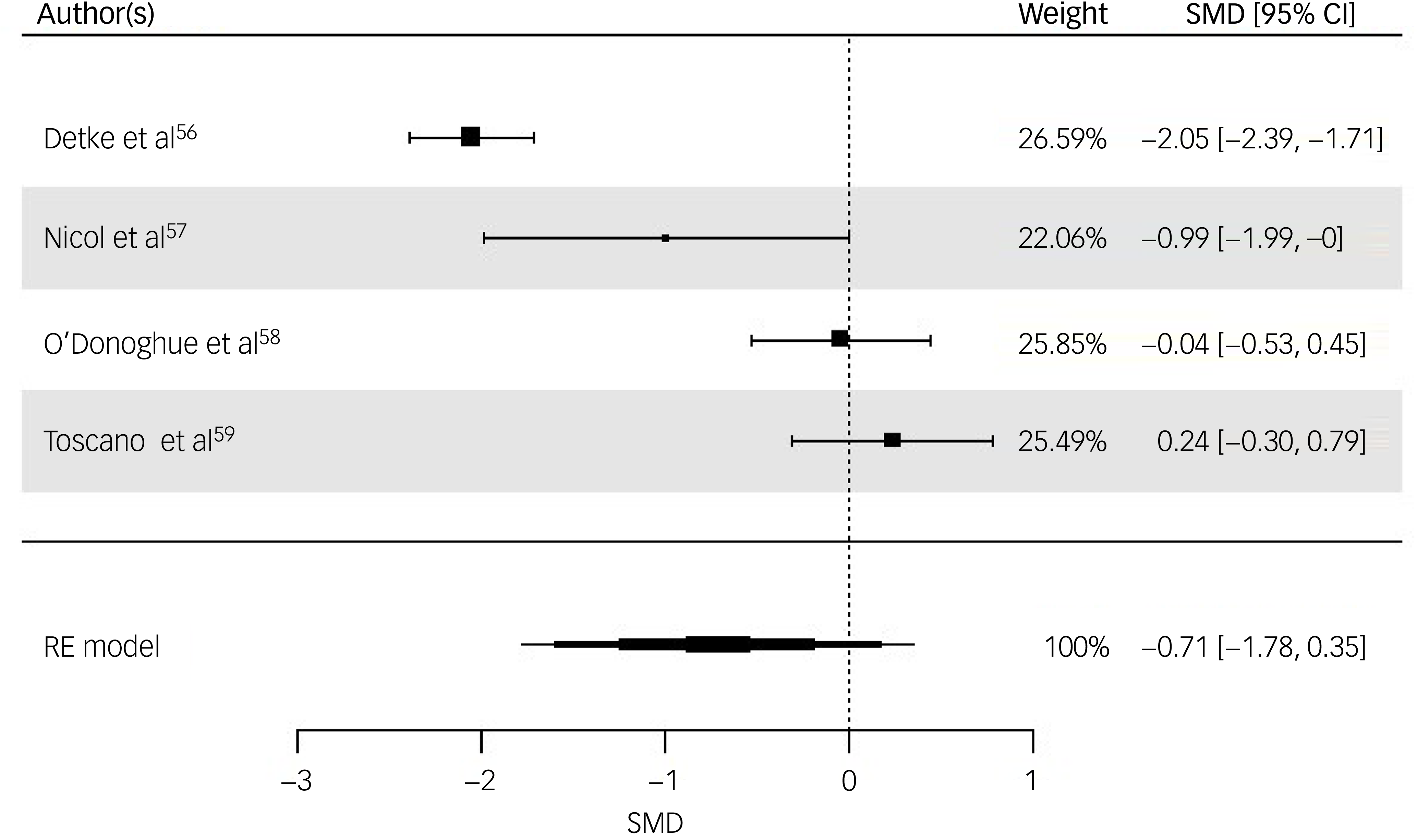
Fig. 3 Forest plot for weight post-intervention. RE, random effects; SMD, standard mean difference.
Three studies were included for waist circumference (centimetres) post-intervention. The random-effects model indicated no effect (SMD = −0, s.e. = 0.1520, P = 0.99, 95% CI: −0.30 to 0.30). The risk of bias for waist circumference as rated via RoB 2 was high across all studies. Reference Detke, DelBello, Landry, Hoffmann, Heinloth and Dittmann56,Reference Nicol, Kolko, Lenze, Yingling, Miller and Ricchio57,Reference Toscano, Carvalho and Ferreira59
The small number of studies limits the ability to perform subgroup analyses that could elucidate factors as sources of heterogeneity for weight and BMI outcomes. The summary statistics for the meta-analysis are presented in Table 2.
Table 2 Summary of findings
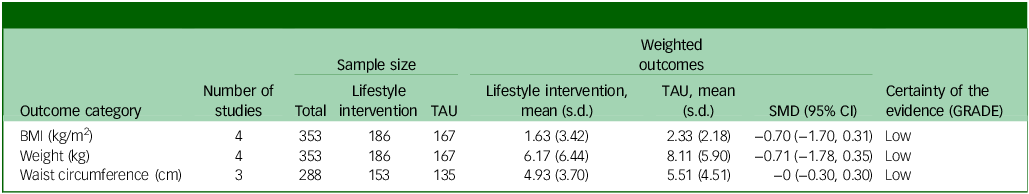
TAU, treatment as usual; SMD, standard mean difference; GRADE, Grading of Recommendations, Assessment, Development and Evaluations; BMI, body mass index.
Narrative synthesis
Clinically significant weight gain
Two studies Reference Detke, DelBello, Landry, Hoffmann, Heinloth and Dittmann56,Reference O’Donoghue, Mifsud, Castagnini, Langstone, Thompson and Killackey58 reported the proportion of participants who had experienced clinically significant weight gain (≥7% of baseline weight). Both studies reported no significant differences for clinically significant weight gain between intervention and TAU groups.
Presence of metabolic syndrome
One study Reference O’Donoghue, Mifsud, Castagnini, Langstone, Thompson and Killackey58 included data on the presence of metabolic syndrome, describing no differences in its prevalence between groups either following the 12-week intervention period or at 6-month follow-up.
Anthropometric and cardiovascular data
Anthropometric data were collected in three studies, Reference Detke, DelBello, Landry, Hoffmann, Heinloth and Dittmann56,Reference Nicol, Kolko, Lenze, Yingling, Miller and Ricchio57,Reference Toscano, Carvalho and Ferreira59 with all reporting on participants’ waist circumference and one Reference Detke, DelBello, Landry, Hoffmann, Heinloth and Dittmann56 assessing blood pressure. No studies reported group-related differences in waist circumference. Detke et al Reference Detke, DelBello, Landry, Hoffmann, Heinloth and Dittmann56 reported a significant reduction in supine diastolic (P = 0.017) and standing systolic pressures (P = 0.031) among intervention participants compared with TAU, with no differences observed for supine systolic pressure or standing diastolic pressure.
One study Reference Detke, DelBello, Landry, Hoffmann, Heinloth and Dittmann56 provided electrocardiogram data, reporting a significant reduction in heart rate (P < 0.001) among participants in the intervention group compared with TAU, with no effect observed for QT-interval.
Laboratory analytes and imaging
Three studies Reference Detke, DelBello, Landry, Hoffmann, Heinloth and Dittmann56,Reference Nicol, Kolko, Lenze, Yingling, Miller and Ricchio57,Reference Toscano, Carvalho and Ferreira59 measured an array of hepatic, metabolic, endocrine and/or haematologic analytes. Detke et al Reference Detke, DelBello, Landry, Hoffmann, Heinloth and Dittmann56 reported no changes for any markers across groups. While Nicol et al Reference Nicol, Kolko, Lenze, Yingling, Miller and Ricchio57 reported a reduction in both hepatic triglyceride content and dual-energy X-ray absorptiometry-measured fat in intervention participants, the effectiveness of the intervention was overestimated by the inclusion of a ‘non-psychiatric’ participant group who engaged in the intervention but were not taking antipsychotic medications. Accounting for this group, calculated differences between intervention and TAU participants were non-significant (P > 0.05) for all hepatic, metabolic, endocrine and/or haematologic indicators (Supplementary Material 6). Toscano et al Reference Toscano, Carvalho and Ferreira59 reported significant reductions in glucose levels and low-density lipoprotein (LDL) cholesterol, as well as an increase in high-density lipoprotein (HDL) cholesterol, among intervention participants compared with TAU (P < 0.05; Supplementary Material 6).
Adverse effects of antipsychotics
One study Reference Detke, DelBello, Landry, Hoffmann, Heinloth and Dittmann56 included data on the adverse effects of antipsychotics. No significant between-group differences were reported for any adverse event (P > 0.999).
Health-related behaviours
Two studies Reference Nicol, Kolko, Lenze, Yingling, Miller and Ricchio57,Reference O’Donoghue, Mifsud, Castagnini, Langstone, Thompson and Killackey58 reported on physical health-related behaviours via the use of standardised questionnaires. Physical activity levels were assessed through the International Physical Activity Questionnaire Reference Nicol, Kolko, Lenze, Yingling, Miller and Ricchio57 and the Simple Physical Activity Questionnaire. Reference O’Donoghue, Mifsud, Castagnini, Langstone, Thompson and Killackey58 Smoking and substance use were evaluated with the Alcohol, Smoking and Substance Involvement Screening Test. Reference O’Donoghue, Mifsud, Castagnini, Langstone, Thompson and Killackey58 No significant statistical differences were reported between intervention and TAU participants for any domain of health behaviour. No studies reported on health behaviours related to participants’ diet, nutrition or sleep practices. No studies reported on family and/or primary care-giver health behaviours, or on other obesity risk factors linked to the home environment.
Health-related quality of life
One study Reference Toscano, Carvalho and Ferreira59 reported on participants’ health-related quality of life as captured by the parent-completed Child Health Questionnaire. Reference Landgraf, Maunsell, Nixon Speechley, Bullinger, Campbell and Abetz62 All of the quality-of-life domains (psychosocial health, physical health, motor profile and autistic traits) were significantly (P < 0.05) improved in the intervention group compared with TAU (Supplementary Material 6).
Quality assessment
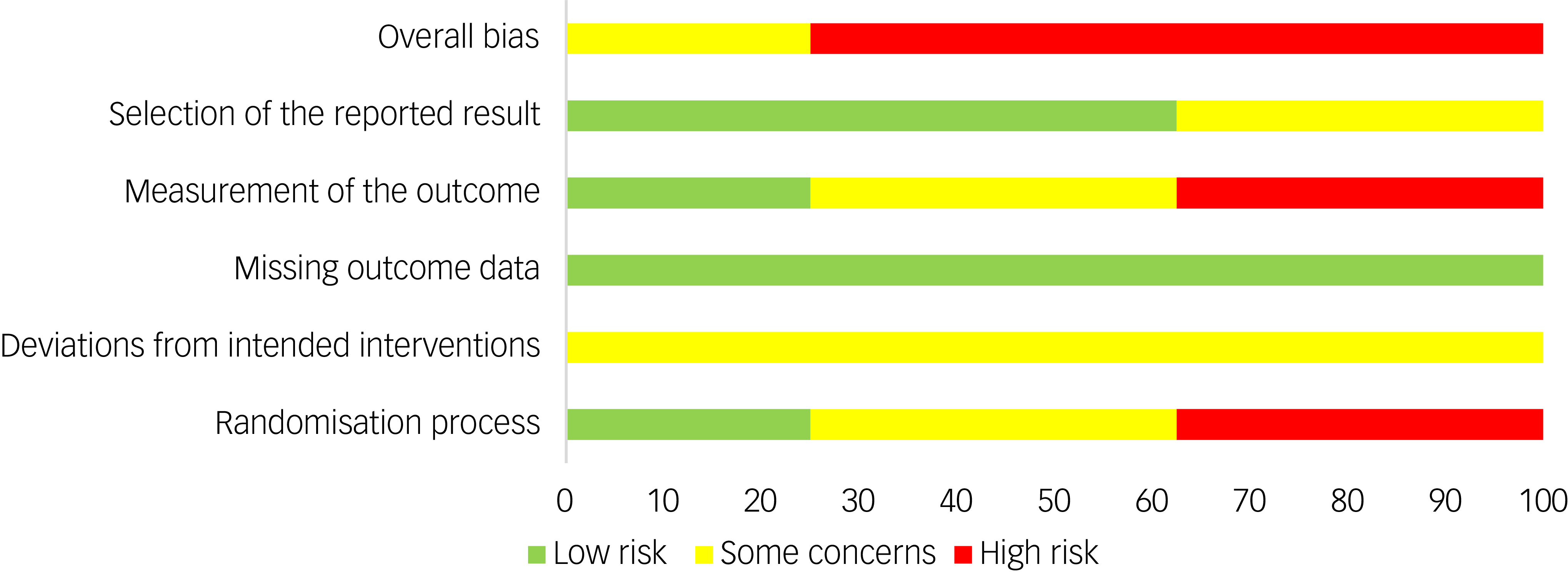
The summary risk of bias for included studies is available in Fig. 4. The quality of evidence (GRADE) of the primary outcome (BMI) was downgraded to ‘low’ due to substantial heterogeneity and high risk of bias.
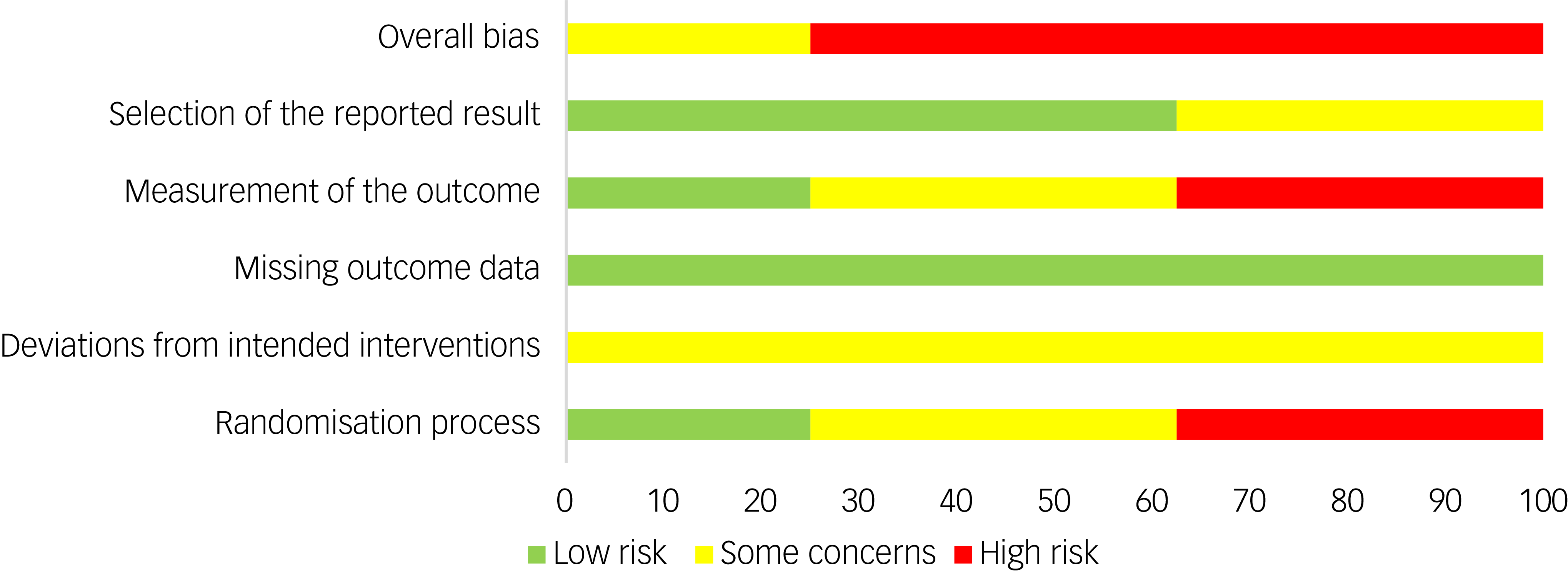
Fig. 4 Risk of bias summary.
Discussion
To our knowledge, this is the first systematic review and meta-analysis to examine the effectiveness of lifestyle intervention strategies aimed at preventing or managing cardiometabolic risk or other adverse physical health outcomes in children and adolescents taking antipsychotic medications. The random-effects models revealed a moderate but statistically non-significant intervention effect on BMI (kg/m2) and mean weight (kg), suggesting that the sample was too small and variable for the observed effect to reach statistical significance. The confidence in aggregate outcomes is limited by the small number of included trials and the presence of substantial heterogeneity. The results should be interpreted with caution due to the low quality of evidence.
Similar meta-analytic reviews in different population groups report large effect sizes of lifestyle interventions on weight that are statistically significant (P < 0.05). For example, lifestyle interventions conducted in adults taking antipsychotic medications have been shown to lead to significant reductions in weight compared with TAU (weighted mean difference −0.63 to −2.56 kg/m2; Reference Álvarez-Jiménez, Hetrick, González-Blanch, Gleeson and McGorry63–Reference Speyer, Jakobsen Ane, Westergaard, Nørgaard Hans Christian, Pisinger and Krogh65 SMD −0.2 to −0.24 Reference Naslund, Whiteman, McHugo, Aschbrenner, Marsch and Bartels33 ). Obese children without psychiatric diagnoses and participating in a lifestyle intervention may lose anything from 1.30 to 1.49 kg/m2 compared with TAU. Reference Ho, Garnett, Baur, Burrows, Stewart and Neve66,Reference Li, Gao, Bao and Li67 The current study, however, encompasses a smaller sample size with substantial heterogeneity that probably contributed to the lack of statistical significance observed.
Previous systematic reviews of lifestyle interventions suggest that geographical context, participant populations, intensity and duration of interventions and the variety of diet or exercise regimes employed are potential modulating factors of heterogeneity. Reference Naslund, Whiteman, McHugo, Aschbrenner, Marsch and Bartels33,Reference Speyer, Jakobsen, Westergaard, Nørgaard, Jørgensen and Pisinger35,Reference Ho, Garnett, Baur, Burrows, Stewart and Neve66 The present study included research predominantly conducted in Western countries. Participants were diagnostically heterogenous, and the type of antipsychotic medication taken was highly variable (if reported). The type of lifestyle interventions delivered varied in duration, content and delivery, consisting of approaches from behavioural modification and educational strategies to structured physical activity programmes. Thus, heterogeneity probably arises from factors related to participant characteristics and intervention variability.
Our study also aimed to ascertain modulators of treatment effect. The study with negligible effect on participants’ weight and BMI Reference Toscano, Carvalho and Ferreira59 employed a structured physical exercise programme with no integration of behaviour modification or educational components. That study did not, therefore, follow evidence-based treatment for weight loss including multidisciplinary and behaviour modification, Reference Salam, Padhani, Das, Shaikh, Hoodbhoy and Jeelani39,Reference Kaufman, Lynch and Wilkinson68 and may not have been intended to stimulate weight change. The study with the largest effect on weight and BMI Reference Detke, DelBello, Landry, Hoffmann, Heinloth and Dittmann56 employed a prevention approach incorporating behaviour-modification strategies, lifestyle (diet and physical activity) education and physical health monitoring over a 12-month period. Participants in that study Reference Detke, DelBello, Landry, Hoffmann, Heinloth and Dittmann56 were treated with olanzapine, which has a high propensity to cause weight gain. Reference Bak, Fransen, Janssen, van Os and Drukker69 The findings are largely consistent with reports that lifestyle interventions may be more effective when either delivered over long durations (>6–12 months), Reference Naslund, Whiteman, McHugo, Aschbrenner, Marsch and Bartels33,Reference Ho, Garnett, Baur, Burrows, Stewart and Neve66 a prevention approach is taken Reference Álvarez-Jiménez, Hetrick, González-Blanch, Gleeson and McGorry63 or patients are treated with an antipsychotic associated with significant weight gain. Reference Dayabandara, Hanwella, Ratnatunga, Seneviratne, Suraweera and de Silva70 However, the scarcity of studies in the present analysis limits the ability to perform subgroup analyses that could elucidate factors related to treatment effects or sources of heterogeneity. Thus, we are unable to address our secondary research question further.
Other measures of physical health
Waist circumference is increasingly recognised as an indicator of obesity, metabolic syndrome and cardiovascular disease risk. Reference Savva, Tornaritis, Savva, Kourides, Panagi and Silikiotou71–Reference Camhi, Kuo and Young73 Interestingly, lifestyle interventions showed no effect on participants’ waist circumference, despite the trend for a reduction in BMI. This is unexpected, because waist circumference typically correlates with BMI measured simultaneously, Reference Spolidoro, Pitrez Filho, Vargas, Santana, Pitrez and Hauschild72 as observed in lifestyle interventions delivered to overweight or obese children and adolescents. Reference Mead, Brown, Rees, Azevedo, Whittaker and Jones74 It is worth nothing that there are many limitations to relying solely on waist circumference to ascertain cardiometabolic risk in children and adolescents: for example, age- and gender-dependent cut-offs are required, or the children’s height may overestimate or underestimate the degree of central adiposity. Reference Spolidoro, Pitrez Filho, Vargas, Santana, Pitrez and Hauschild72,Reference Yoo75 Thus, typically, the waist-to-height ratio (WtHR), which avoids these limitations, should be collected to identify cardiometabolic risk in children and adolescents. Reference Yoo75–Reference Maffeis, Banzato and Talamini77 Unfortunately, no studies have reported outcomes related to WtHR.
Measurements of other hepatic, metabolic, endocrine and haematologic markers suggested unclear effects, which contrasts with reports in other populations. Reference Ho, Garnett, Baur, Burrows, Stewart and Neve66 Only one study Reference Toscano, Carvalho and Ferreira59 reported significant benefits, showing reductions in glucose levels and LDL cholesterol and an increase in HDL cholesterol among intervention participants compared with TAU. The absence of observed effects in other studies may be attributed to limited sample size, because comparisons against non-psychiatric populations reveal the positive benefits of lifestyle interventions on central adiposity markers. Reference Nicol, Kolko, Lenze, Yingling, Miller and Ricchio57 These findings highlight the need for further research to better understand the variability in responses to lifestyle interventions among different age groups and populations.
While no data were collected on participants’ dietary behaviour, nutrition or sleep practices, several studies have reported on physical activity outcomes. No significant differences between groups were reported, even though behavioural modification is a core strategy of lifestyle interventions. However, participants’ physical activity was captured via questionnaires not validated in child or adolescent populations, Reference Hagströmer, Oja and Sjöström78,Reference Rosenbaum, Morell, Abdel-Baki, Ahmadpanah, Anilkumar and Baie79 suggesting that the results should be interpreted with caution.
For participants supported by a parent or guardian during the study period, factors related to the family and home environment may have influenced their outcomes. Children with obese parents are at a greater risk of obesity compared with those having non-obese parents. Reference Lake, Power and Cole80 The risk of obesity is thought to be transmitted to the next generation through a complex interaction of genetic, metabolic, behavioural, cultural and environmental factors. Reference Albuquerque, Nóbrega, Manco and Padez81 Importantly, modifiable factors such as parental health behaviour and the home environment are predictors of children’s physical activity, sedentary behaviour and dietary intake. Reference Verloigne, Van Lippevelde, Maes, Brug and De Bourdeaudhuij82,Reference Spurrier, Magarey, Golley, Curnow and Sawyer83 In the present study, no trials captured outcomes related to parental behaviour change or the home environment. Given the influence of these factors on children’s health behaviour, future studies should aim to assess obesogenic factors related to the home environment and parental health behaviours alongside those of children and adolescents participating in lifestyle interventions.
The report from one study Reference Toscano, Carvalho and Ferreira59 suggesting that lifestyle interventions may positively impact health-related quality of life is promising, but additional data are needed to confirm any treatment effect. Some studies have shown a significant relationship between quality of life and health-related behaviours. Reference Wu, Ohinmaa, Veugelers and Maximova84,Reference Dumuid, Olds, Lewis, Martin-Fernández, Katzmarzyk and Barreira85 Therefore, routine data collection should include assessments of quality of life for both children and their families undertaking lifestyle interventions. Such data may provide a better understanding of predictors of success and potential incidental benefits.
Clinical implications
While no studies have reported a weight loss of ≥7% in participants’ weight, the potential for even modest weight reductions in the developmental period to confer significant health advantages should be appreciated. However, the trends for weight loss were not statistically significant. Previous reports suggest that lifestyle interventions alone are insufficient to prevent BMI increase in children and adolescents with serious mental illness (schizophrenia spectrum disorder, bipolar spectrum disorder or psychotic depression Reference Correll, Sikich, Reeves, Johnson, Keeton and Spanos60 ). In this cohort, adding metformin or switching to a lower-risk antipsychotic reduced the risk of overweigh/obesity compared with lifestyle intervention alone. Reference Correll, Sikich, Reeves, Johnson, Keeton and Spanos60 Thus, the clinical significance of lifestyle interventions for children and adolescents prescribed an antipsychotic is unclear and requires further investigation.
Limitations
This review is limited by several factors. The search process revealed moderate interrater reliability during title and abstract screening, with the diagnostic heterogeneity of children and adolescents taking antipsychotic medications posing barriers to making accurate judgements about participants’ medication status without a full-text report. Additionally, the small sample size and high heterogeneity of the meta-analysis limit the confidence in aggregate outcomes. With only four studies, the DerSimonian and Laird random-effects model provides imprecise estimates of between-study variance (τ 2), and measures of heterogeneity (I 2) are unstable. As a result, the pooled effect is sensitive to single-study influence and is best interpreted as a cautious summary of the observed evidence rather than a definitive estimate. The strength of the body of evidence was rated as low, suggesting that the true effect might be markedly different from that estimated. Reference Guyatt, Oxman, Vist, Kunz, Falck-Ytter and Alonso-Coello55 Finally, the planned subgroup analysis could not be undertaken due to the limited number of trials available. Future studies with more trials should aim to perform such analyses. It is possible that a less rigorous eligibility criterion (i.e. inclusion of observational studies) may have provided additional insights.
Implication for future research
The scarcity of available literature juxtaposes the rapidly increasing prescription of antipsychotic medications to children and adolescents. This gap highlights an urgent need for future research to implement and evaluate lifestyle interventions for the prevention or management of antipsychotic-induced weight gain and associated cardiometabolic risks. Effort should be made to establish programmes, in partnership with primary care or other providers in rural/remote or socioeconomically disadvantaged regions where off-label prescribing practices are prevalent and there is restricted access to non-pharmacological interventions. Reference Klau, Gonzalez-Chica, Raven and Jureidini5,Reference Crump, Sundquist, Sundquist and Winkleby86,Reference Nunn, Kritsotakis, Harpin and Parker87
Additionally, studies should ensure the use of age-appropriate, validated measurement of cardiometabolic risk, health behaviours (i.e. physical activity, dietary habits and sleep patterns) and quality of life of children and adolescents and their parents or guardians, in addition to obesogenic factors related to the home environment. The inclusion of extended follow-ups will help determine the sustainability of these interventions, recognising that adopting healthy behaviours during the developmental period can have substantial long-term benefits when maintained.
Supplementary material
The supplementary material is available online at http://doi.org/10.1192/bjo.2025.10919
Data availability
The data that support the findings of this study, and the analytic code associated with the manuscript, are available from the corresponding author, V.E., upon reasonable request. The research materials associated with the manuscript are available in the supplementary material.
Acknowledgements
We thank statistical consultants Dr Nancy Briggs and Dr David Chan (University of New South Wales) for providing assistance with the data analysis.
Author contributions
P.J.H. formulated the research questions with the assistance of J.B., P.W. and V.E. P.J.H. designed the study with input from J.B., T.Y.W., C.M., P.W., A.W., B.T., K.W., M.B., T.J.S., V.A., F.A. and V.E. P.J.H., J.B. and T.Y.W. conducted study searches and screening. P.J.H. and J.B. conducted data extraction and quality assessments. P.J.H. conducted data analysis with the assistance of C.M. P.J.H. drafted the article with input from all authors. All authors reviewed and approved the final manuscript.
Funding
This research was supported by the Medical Research Future Fund (MRF2006438 Growing Minds Australia: A National Trials Strategy to Transform Child and Youth Mental Health Services) and the Growing Minds Australia Clinical Trials Network. The funder of the study had no role in the study design, data collection, data analysis, data interpretation or writing of the report.
Declaration of interest
C.M. is a member of the BJPsych Open editorial board, but did not take part in the review of decision-making process of this paper. The remaining authors have no competing interests to declare.
Transparency declaration
The lead author and manuscript guarantor affirm that the manuscript is an honest, accurate and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the published study protocol have been explained.



eLetters
No eLetters have been published for this article.