Tick taxonomy
Ticks are divided into three extant families, Ixodidae or hard ticks 731-742 species (Dantas-Torres Reference Dantas-Torres2018; Guglielmone et al., Reference Guglielmone, Petney and Robbins2020), Argasidae or soft ticks 216 species (Dantas-Torres Reference Dantas-Torres2018; Mans et al., Reference Mans, Featherston, Kvas, Pillay, de Klerk, Pienaar, de Castro, Schwan, Lopez, Teel, Pérez de León, Sonenshine, Egekwu, Bakkes, Heyne, Kanduma, Nyangiwe, Bouattour and Latif2019), and Nuttalliellidae (single species Nuttalliella namaqua limited to the Afrotropic region, Latif et al., Reference Latif, Putterill, de Klerk, Pienaar and Mans2012). The latter family has aspects of both soft and hard ticks and has been described as the evolutionary link between these two large families. Thus, phylogenetic and taxonomic studies continue to better understand how the current extant tick species evolved. In this Special Issue, Chitimia-Dobler et al. (Reference Chitimia-Dobler, Handschuh, Dunlop, Pienaar and Mans2024b) examine extinct tick species found in amber to suggest that the distribution of Nuttalliella likely stretched from Africa over Antarctica and much of Australia before the rift with Burma at ~150 mya (https://doi.org/10.1017/S0031182024000477). From eight fossils in Burmese amber the Nuttalliellidae were found to be comprised of three genera: Deinocroton, Legionaris nov. gen. and Nuttalliella, and the following new species: Deinocroton bicornis sp. nov.; Deinocroton lacrimus sp. nov.; Nuttalliella gratae sp. nov.; Nuttalliella tuberculata sp. nov. Nuttalliella placaventrala sp. no.; Nuttalliella odyssea sp. nov.; Nuttalliella tropicasylvae sp. nov.; and Legionaris robustus sp. nov. The authors suspect that the Australian continent may have extant Nuttalliellidae yet to be discovered. A separate study in this Special Issue by Mans et al. (Reference Mans, Chitimia-Dobler, Pienaar, de Castro, Khan, Almutairi, Alouffi and Ali2024) using mitochondrial genome and nuclear ribosomal RNA sequencing, demonstrated that Alveonasus genus is paraphyletic and that Alveonasus lahorensis is better placed within the soft tick sub-family of Argasinae rather than Ornithodorinae (https://doi.org/10.1017/S0031182024000441). In addition, after sampling animal shelters in Khyber Pakhtunkhwa in Pakistan, Ali et al. (Reference Ali, Khan, Numan, Alouffi, Almutairi, Pienaar, de Castro, Chitimia-Dobler, Muñoz-Leal and Mans2024) identified a new tick species using mitogenome sequencing and morphological comparisons: Ornithodoros pakistanensis sp. nov. in the Pavlovskyella subgenus (https://doi.org/10.1017/S0031182024000982). Applying similar mitogenomic sequencing methods and morphological identifications, Chitimia-Dobler et al. (Reference Chitimia-Dobler, Barboutis, Bounas, Kassara, Mans and Saratsis2024a) discovered a new hard tick species from Eleonora's falcons on Antikythira Island in Greece (https://doi.org/10.1017/S0031182024000866): Ornithophysalis subgenus Haemaphysalis doenitzi. The significance of this finding is that this falcon species is a long-distance migrant of the Afro-Palearctic flyway breeding during summer in the Mediterranean and winter in North-West Africa, and this is the first identification of this tick genus in the Western Palearctic region.
Tick and TBD vector control
Lyme disease (borreliosis) is the most prevalent vector-borne disease in both Europe and the United States, presenting a significant public health concern. The main causative agent in the United States is Borrelia burgdorferi transmitted by Ixodes scapularis ticks, while the predominant species in Europe are Borrelia afzelii, Borrelia garinii, and B. burgdorferi transmitted by Ixodes ricinus (Marques et al., Reference Marques, Strle and Wormser2021). Ostfeld et al. Reference Ostfeld, Adish, Mowry, Bremer, Duerr, Evans, Fischhoff, Keating, Pendleton, Pfister, Teator and Keesing2024 (https://doi.org/10.1017/S0031182024000349) examined the effects of acaricide treatments in 24 residential neighbourhoods of Dutchess County (New York, USA) on the subsequent pathogen coinfection in I. scapularis ticks known to carry multiple medically important pathogens such as Anaplasma phagocytophilum, Babesia microti and B. burgdorferi. The use of fungus based biopesticides showed coinfections of B. microti and B. burgdorferi to be more common than single infections. However, when using tick control system bait boxes, the bias towards coinfections was eliminated. The authors concluded that control methods directed at ticks attached to small mammals may influence human exposure to coinfected ticks and the probability of exposure to multiple tick-borne infections. Chemical acaricides have proven effective in reducing tick infestation loads on livestock and pets primarily targeting the tick central nervous system (Obaid et al., Reference Obaid, Islam, Alouffi, Khan, da Silva Vaz, Tanaka and Ali2022). In previous studies, passive topical application of fipronil significantly reduced the burden of nymphs and larvae of I. scapularis on small reservoir hosts and decreased the abundance of nymphs in treated areas. In addition, infection rates of B. burgdorferi and A. phagocytophilum in reservoir animals were significantly reduced after treatment (Dolan et al., Reference Dolan, Maupin, Schneider, Denatale, Hamon, Cole, Zeidner and Stafford2004, Reference Dolan, Schulze, Jordan, Schulze, Ullmann, Hojgaard, Williams and Piesman2016). Šíma et al. (Reference Šíma, Palusová, Hatalová, Robbertse, Berková, Moos, Kopáček, Urbanová and Perner2024) used a mouse model to demonstrate the nanomolar efficiency of Fipronil (phenylpyrazole chemical class) against I. ricinus ticks and its rapid speed-or-kill aimed at blocking the transmission of B. afzelii pathogens (https://doi.org/10.1017/S0031182024001136).
Table 1. Summary of the 17 articles included in this Special Issue ‘Ticks & Tick-Borne Parasites and Diseases’ and the section title associated with this Editorial

Rhipicephalus simus, classified within the genus Rhipicephalus and the family Ixodidae is a highly capable vector of pathogens of critical importance in both medical and veterinary fields (Shekede et al., Reference Shekede, Chikerema, Spargo, Gwitira, Kusangaya, Mazhindu and Ndhlovu2021; Phiri et al., Reference Phiri, Kattner, Chitimia-Dobler, Woelfel, Albanus, Dobler and Küpper2023). This hard tick species not only thrives in diverse habitats but also exhibits a remarkable ability to infest and feed on humans, thus potentially facilitating the transmission of a wide range of infectious agents (Horak et al., Reference Horak, Fourie, Heyne, Walker and Needham2002). Phleboviruses belonging to the genus Phlebovirus and family Phenuiviridae are frequently identified in ticks of the genus Rhipicephalus worldwide (Li et al., Reference Li, Bao, Hu, Liu, Wang, Zhang, Ji, Feng, Li, Shen, Liu, Zhao, Tan, Zhou, Qi, Zhu, Tang, Cardona and Xing2016; Pereira et al., Reference Pereira, Figueira, Nunes, Esteves, Cotão, Vieira, Maia, Campino and Parreira2017; López et al., Reference López, Miranda, Mattar, Gonzalez and Rovnak2020) but have not been reported in R. simus. Tick-borne phleboviruses (TBPVs) were largely neglected until recently when severe fever with thrombocytopenia syndrome virus (SFTSV) and Heartland virus (HRTV) were confirmed as causative agents of severe disease in humans (McMullan et al., Reference McMullan, Folk, Kelly, MacNeil, Goldsmith, Metcalfe, Batten, Albariño, Zaki, Rollin, Nicholson and Nichol2012; Li et al., Reference Li, Bao, Hu, Liu, Wang, Zhang, Ji, Feng, Li, Shen, Liu, Zhao, Tan, Zhou, Qi, Zhu, Tang, Cardona and Xing2016). Munjita et al. Reference Munjita, Mubemba, Tembo, Bates and Munsaka2024 (https://doi.org/10.1017/S0031182024001033) used metagenomic next-generation sequencing to determine the viral diversity in tick populations from a dormant commercial farm in the riverine area in Lusaka, Zambia. This is the first report of a phlebovirus found in R. simus ticks.
The knowledge, attitudes and control practices of farmers in the Dhar district of Madhya Pradesh (India) was assessed by Jamra et al. (Reference Jamra, Shakya, Jayraw, Agrawal, Singh, Sharma, Bhangale, Jatav and Jamra2024) to mitigate acaricide resistance and tick-borne diseases covering 200 livestock owners using a questionnaire (https://doi.org/10.1017/S0031182024001331). Jamra et al. (Reference Jamra, Shakya, Jayraw, Agrawal, Singh, Sharma, Bhangale, Jatav and Jamra2024) concluded that 75% of respondents were not aware of TBDs and that 36.5% showed favourable attitudes towards adopting tick control practises. In addition, grazing animals were six times more susceptible to ticks compared to livestock held in mixed feeding or manger systems. Rhipicephalus microplus and Hyalomma anatolicum ticks most commonly affecting livestock in India (Ghosh et al., Reference Ghosh, Azhahianambi and de la Fuente2006) were assessed and found to be resistant to deltamethrin in all five different sub-divisions due to the easy availability of this acaricide. The study recommended the development of targeted educational programs to enhance farmers' knowledge of sustainable tick control practices to explore alternatives to chemical acaricides to minimise acaricide resistance and TBDs in livestock.

Figure 1. Word cloud generated using the article titles in this Special Issue – Ticks & Tick-borne Parasites and Diseases.
Using the BME26 tick embryonic cell line (Esteves et al., Reference Esteves, Lara, Lorenzini, Costa, Fukuzawa, Pressinotti, Silva, Ferro, Kurtti, Munderloh and Daffre2008), Moraes et al. Reference Moraes, Gomes, Saramago, Braz, Parizi, Braz, da Silva Vaz, Logullo and Moraes2024 (https://doi.org/10.1017/S003118202400101X) targeted R. microplus aurora kinases (AURK) using a pan AURK inhibitor (CCT137690). AURK play a central role in controlling the cell cycle in a range of organisms and belong to the family of serine-threonine kinase proteins. Their roles in the cell cycle include entry into mitosis, maturation of the centrosome and formation of the mitotic spindle. The authors identified two AURK coding sequences in the transcriptome of R. microplus (Rm-AURKA and Rm-AURKB) and cell viability decline was demonstrated in BME26 cells using the pan AURK inhibitor. The authors suggest that AURK inhibitors could be exploited to develop species specific tick control strategies.
Epidemiology and ecology
Enzyme-linked-immunosorbent-assays (ELISAs) to determine the seroprevalence of bovine tick fever pathogens have been use for almost 30 years in various regions of the world. Countries with high live cattle export industries have routinely vaccinated using the milder Anaplasma centrale for bovine anaplasmosis and attenuated strains of Babesia bigemina and Babesia bovis (reviewed by Salinas-Estrella et al., Reference Salinas-Estrella, Amaro-Estrada, Cobaxin-Cárdenas, Preciado de la Torre and Rodríguez2022). South Africa, Australia, Argentina, Brazil, Uruguay, and Israel have used A. centrale to control A. marginale infections. Zim et al. Reference Zim, Ahmed, Ahmed, Miah, Sajib, Rabbi, Rahman, Roy and Talukder2024 (https://doi.org/10.1017/S0031182024001495) have demonstrated the emergence of bovine anaplasmosis in commercial livestock and dairy farms in Bangladesh and may consider vaccination as a future control measure.
Several studies in this Issue have investigated or predicted the ecological spread of four different tick species in four different geographical regions respectively. The recent U.S. invasion of Haemaphysalis longicornis (longhorn tick) has led to studies of human and livestock tick-borne disease transmission and its relationship with wildlife tick species on affected cattle farms. Butler et al. Reference Butler, Muller, Grove and Fryxell2024 (https://doi.org/10.1017/S0031182024001380) concluded that farmer controlled integrated pest management strategies, and the reduction of tick populations led to better tick management. In Europe, the spread of the castor bean tick I. ricinus (significant vector of various diseases including Lyme borreliosis to humans) was determined using microclimatic and macroclimatic models (https://doi.org/10.1017/S003118202400132X). Through the application of this mixed modelling, Kuyucu and Hekimoglu (Reference Kuyucu and Hekimoglu2024) suggest significant expansion of I. ricinus into northern and eastern Europe, with declines in southern Europe. In Saudi Arabia and United Arab Emirates, Hyalomma dromedarii is the most abundant tick species affecting primarily camels and other livestock to a lesser extent. Maximum Entropy Species Distribution Modelling (MaxEnt.) used species presence, land use/landcover, elevation, slope and 19 bioclimatic variables to model current and future distribution of H. dromedarii ticks (https://doi.org/10.1017/S0031182024001161). Willingham et al. (Reference Willingham, Perveen, Muzaffar, Jaradat and Sparagano2024) highlighted those areas in the north, east and south-western parts that were highly suitable for this tick species. Finally, Godinho et al. Reference Godinho, van Lieshout, Griffiths and Kwak2024 (https://doi.org/10.1017/S0031182024000817) studied the ecology of one of the 12 native soft tick species (Argasidae) in Australia, Argas dewae. This tick parasitises several insectivorous bat species and has also been recorded on humans. A. dewae populations were monitored on two bat hosts (Chalinolobus gouldii; Austronomus australis) at three sites in the southern state of Victoria for 28 months showed that tick load increased throughout winter and peaked in the first month of spring before remaining low during late spring and summer. This paper also reports the first records of A. dewae from six bat species in three bat families (Miniopteridae; Molossidae; Vespertilionidae) and a second record of A. dewae from a human. Godinho et al., also document the first distribution records for A. dewae in an additional three Australian states. This data will contribute to improvements in wildlife health management and public health preparedness.

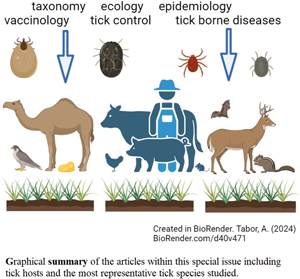
Figure 2. Graphical representation of articles collected for the ‘Ticks & Tick-borne Parasites and Diseases’ Special Issue demonstrating the tick species (Hard tick species Ixodidae: Ixodes spp., Rhipicephalus spp., Haemaphysalis spp., Soft tick species: Argasidae), the hosts and the main topics covered by the article collection.
Vaccinology
Three articles in this Special Issue reviewed tick vaccinology (de la Fuente and Ghosh, Reference de la Fuente and Ghosh2024) or described potential vaccine antigens. Rhipicephalus microplus is the most significant tick species impacting livestock industries worldwide estimated at USD22-30b annually (Lew-Tabor and Rodriguez-Valle, Reference Lew-Tabor and Rodriguez-Valle2016). Overreliance on chemical treatments for tick control has led to the emergence of acaricide-resistant ticks and environmental contamination while vaccine strategies offer an alternative approach for tick control. Perez-Soria et al. Reference Perez-Soria, López-Díaz, Jiménez-Ocampo, Aguilar-Tipacamú, Ueti and Mosqueda2024 (https://doi.org/10.1017/S0031182024000143) predicted four R. microplus B-cell epitopes based on the enzyme chitinase. Chitinases degrade older chitin at the time of tick moulting. Immunization experiments demonstrated that Chitinase peptide 3 reduced weight and oviposition of engorged ticks and reduced larval viability at a 71% overall vaccine efficacy.
Ferreira et al. Reference Ferreira, de Souza, Valentina, Leal and Oliveira2024 (https://doi.org/10.1017/S0031182024001410) reviewed tick glycine-rich proteins (GRPs). The authors described the functions of tick GRPs historically associated with salivary gland secretion to form the tick cement cone enabling host attachment and highlighted other GRP roles. GRPs have been identified in a diverse array of organisms and shown to possess several distinctive biological characteristics, including nucleic acid binding, adhesive glue-like properties, antimicrobial activity, involvement in the stress response and in the formation of cuticle components. The authors highlight that GRPs are present in all tick developmental stages, and that expression is modulated by physiological processes and immune challenges such as feeding and pathogen infection. The authors further discuss possible roles of tick GRPs and highlight the vaccine potential of these proteins by summarizing published vaccination experiments in rabbits, mice, cattle and guinea pigs against H. longicornis, Rhipicephalus haemaphysaloides, R. microplus or Rhipicephalus appendiculatus ticks.
Finally, de la Fuente and Ghosh (https://doi.org/10.1017/S003118202400043X) describe the challenges of tick vaccines including: (1) Ticks are difficult to control, (2) Vaccines control tick infestations by reducing ectoparasite fitness and reproduction, (3) Vaccine efficacy against multiple tick species, (4) Impact of tick strain genetic diversity on vaccine efficacy, (5) Antigen combination to improve vaccine efficacy, (6) Vaccine formulations and delivery platforms and (7) Combination of vaccines with transgenesis and para-transgenesis. Their review suggests that advances in tick organ antigen recombinant proteins and chimeras designed using vaccinomics and quantum vaccinomics will be combined with technologies such as multi-omics, AI and Big Data, mRNA vaccines, microbiota-driven probiotics and vaccines. In addition, the authors predict that tick vaccines could be combined with other interventions associated with regional ticks' infestations and tick-borne diseases for a personalized medicine approach.
Data availability statement
All data used in the study is disclosed in the paper and corresponding references.
Acknowledgements
I thank the Parasitology journal for the opportunity to coordinate this Special Issue on ticks & tick-borne parasites and diseases.
Author contributions
AT wrote the article.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Competing interests
None declared.
Ethical standards
Not applicable.