Introduction
Candelariaceae Hakul. is a family of lichenized fungi that comprises four (4) genera based on morphological and anatomical features: Candelaria A. Massal., Candelariella Müll. Arg., Candelina Poelt, and Placomaronea Räsänen, sensu Poelt (Reference Poelt1974), Westberg et al. (Reference Westberg, Arup and Kärnefelt2007, Reference Westberg, Morse and Wedin2011) and Lücking et al. (Reference Lücking, Hodkinson and Leavitt2016, Reference Lücking, Hodkinson and Leavitt2017). Recently Kondratyuk et al. (Reference Kondratyuk, Lőkös, Jeong, Oh, Kondratyuk and Hur2020), based on an analysis of the ITS, 28S nrLSU and 12S mtSSU regions downloaded from GenBank (https://www.ncbi.nlm.nih.gov/genbank/), proposed three new genera, Candelinella S. Y. Kondr., Opeltiella S. Y. Kondr. and Protocandelariella Poelt ex D. Liu et al., to accommodate groups of species currently placed in Candelaria and Candelariella. This proposal requires further revision before it can be accepted, because the phylogenetic trees presented in both of the most recent works on the subject (Westberg et al. Reference Westberg, Arup and Kärnefelt2007; Kondratyuk et al. Reference Kondratyuk, Lőkös, Jeong, Oh, Kondratyuk and Hur2020) have low support in their basal and only moderate resolution in some of the terminal branches. The available molecular data for Candelariaceae are still insufficient, mainly focusing on North America and Europe and largely absent from regions with conspicuous diversity, such as South America and Asia. Proposing substantial changes in the taxonomy of the family at this point seems premature and could lead to nomenclatural instability, especially if generic delimitations are based only on DNA, not clearly aligned with morphological or anatomical characteristics.
Until now, the study of the taxonomic diversity in the family has focused mainly on North America (Westberg & Nash Reference Westberg, Nash, Nash, Ryan, Gries and Bungartz2002a, Reference Westberg, Nash, Nash, Ryan, Gries and Bungartzb , Reference Westberg, Nash, Nash, Gries and Bungartz2007; Westberg Reference Westberg, Nash, Ryan, Diederich, Gries and Bungartz2004, Reference Westberg2007a, Reference Westbergb , Reference Westbergc ; Westberg & Arup Reference Westberg and Arup2011; Yakovchenko et al. Reference Yakovchenko, Vondrák, Ohmura, Korchikov, Vondrákova and Davydov2017), with some additional work in Europe, Asia and Australia (Filson Reference Filson and Grgurinovic1992; Westberg & Clerc Reference Westberg and Clerc2012; Westberg & Sohrabi Reference Westberg and Sohrabi2012; Dong & Hur Reference Dong and J-S2018; Dong et al. Reference Dong, Wang, Wang and Hur2019; Liu et al. Reference Liu, Wang, Wang and Hur2019). Research in South America includes descriptions of a small number of new taxa (Räsänen Reference Räsänen1939, Reference Räsänen1941; Westberg & Frödén Reference Westberg and Frödén2007; Etayo et al. Reference Etayo, Sancho, Gómez-Bolea, Søchting, Aguirre and Rozzi2021), range extensions (Osorio Reference Osorio1974), broad geographical treatments of the family with some incidental records from South America (Hakulinen Reference Hakulinen1954; Poelt Reference Poelt1974), and a monograph of Placomaronea (Westberg et al. Reference Westberg, Frödén and Wedin2009). As mentioned by Westberg & Frödén (Reference Westberg and Frödén2007), the family is presumably characterized by a great diversity in the Andean region, but there is still much work to do.
In Peru, six species of Candelariaceae have been reported (Ramos Reference Ramos2014): Candelaria concolor (Dicks.) Arnold, C. fibrosoides M. Westb. & Frödén, Candelariella andicola (Zahlbr.) Zahlbr., Placomaronea candelarioides Räsänen, P. lambii (Hakul.) R. Sant., and P. mendozae (Räsänen) M. Westb. This number is growing, with several recent new species in preparation (D. Ramos, unpublished data), principally in Candelariella which we believe has greater diversity in the Andean region than is currently known, requiring a more extensive revision.
Prior to our revision, six species of Placomaronea were known: P. candelarioides, P. fuegiana M. Westb. & Frödén, P. kaernefeltii M. Westb. et al., P. lambii, P. mendozae and P. minima M. Westb. & Frödén. These are mainly distributed in the central and southern Andean region of South America, where they have been reported from Ecuador, Peru, Bolivia, Chile and Argentina. Placomaronea mendozae has also been reported from the United States, and P. minima from Lesotho, Africa. The genus is characterized by its fructicose, foliose, squamulose to crustose thallus, with pigments in the upper cortex and epihymenium arranged as ‘hoods’ on the tips of the outer hyphae, multi-spored asci (> 20), and hyaline, simple ascospores, commonly with two oil droplets but sometimes with one or multiple droplets (Westberg et al. Reference Westberg, Frödén and Wedin2009).
The present article describes two new species, Placomaronea fruticosa and P. placoidea, both collected in the southern Andes of Peru. Both morphologically much resemble species of Candelina, but molecular data place them into Placomaronea. Westberg et al. (Reference Westberg, Frödén and Wedin2009) emphasized that among crustose to subfoliose genera of Candelariaceae, Placomaronea may best be characterized by its distinct cortex structure, and, according to Poelt (Reference Poelt1974) secondary chemistry is deemed highly variable throughout the family. For comparison, we therefore also decided to study the cortex anatomy and secondary chemistry of Candelariella, Candelina and some of the previously described species of Placomaronea. An updated key to the genera and all species of Placomaronea currently known is provided.
Materials and Methods
The present study is primarily based on material collected by the first author during a field trip in 2018, in the southern Andes of Peru; these collections are deposited in the Herbario Sur Peruano (HSP). For comparison, particularly of the secondary chemistry and cortex anatomy, we also examined material of Candelina, Candelariella and Placomaronea from the following herbaria: ASU, COLO, LSU, MSC, NY and WIS. Detailed specimen information for all material examined is available from the Consortium of Lichen Herbaria (https://lichenportal.org/).
Morphological features were observed with a EDUblue1402-S and Wild M2Z dissecting microscope. Anatomical characteristics were studied with a Labortech-2005 and Zeiss Axio Lab A1 compound microscope. Hand-cut sections of the thallus and apothecia were mounted in distilled water. Standardized reagents of 10% aqueous solution of potassium hydroxide (K), chlorine household bleach (C), para-phenylendiamine crystals dissolved in ethanol (P) and Lugol’s solution (I) were used for spot testing, and the reaction to UV light was checked. Anatomical measurements were taken using ‘Scale Bar Tools for Microscopes’ from ImageJ v. 1.52 software, previously calibrated to the microscope. A minimum of 10 measurements for each structure and 45 measurements of ascospores were made. Microphotographs were taken with a Canon EOS 12.2 MP camera mounted on the Labortech-2005 microscope and a Nikon D7000 mounted on the Zeiss Axio Lab A1. Macrophotographs were taken in a light box using a Nikon D800E and/or D810 camera with a 60 mm AF-D Micro-Nikkor lens mounted on a Novoflex macro stand.
Secondary metabolites were examined from a selection of specimens using standardized thin-layer chromatography, routinely using solvent C (Orange et al. Reference Orange, James and White2001, Reference Orange, James and White2010). Instead of the conventional upright TLC tanks, a horizontal HPTLC developing chamber was used (Arup et al. Reference Arup, Ekman, Lindblom and Mattsson1993). A protocol first suggested by Egan (Reference Egan2001) to document and conserve TLC results was modified here as follows: TLC plates were photographed with a Nikon D300 digital camera. Photographs were taken immediately after running the solvent, in long-wave (λ365 nm) and short-wave (λ254 nm) UV light, before applying 10% H2SO4. After H2SO4 treatment and charring in a laboratory oven for c. 8 min at 110 °C, a second set of photographs in visible light and long-wave UV (λ365 nm) were taken. Standard spot tests with reagents P, K and C were routinely carried out using methods described in Bungartz (Reference Bungartz, Nash, Ryan, Gries and Bungartz2002). UV-fluorescence of thalli was studied under long-wave UV light (λ365 nm). Lugol’s iodine was used to study asci following a routine protocol outlined in Bungartz (Reference Bungartz, Nash, Ryan, Gries and Bungartz2002). Plates were subsequently analyzed in Mytabolites 1.0.0.0 (Lafferty et al. Reference Lafferty, Bungartz, Elix and Schumm2024), a software package that shares many functionalities with its popular predecessor Wintabolites, originally developed at the University of Essen (Mietzsch et al. Reference Mietzsch, Lumbsch and Elix1992, Reference Mietzsch, Lumbsch and Elix1993).
Specimens of the two new species examined are cited below (see descriptions). For comparison, we also studied thallus morphology, cortex anatomy and secondary chemistry of specimens for the following species:
Candelariella kansuensis H. Magn. USA: Arizona: C. M. Wetmore 55470 (608915, ASUL011864; MIN1408597/892474), 54366 (MIN1408598/783925), 55277 (MIN1408596/892475), 54907 (MIN1408599/783453).
Candelariella rosulans (Müll. Arg.) Zahlbr. Mexico: Baja California: T. H. Nash 4369 (579860, ASUL010372), 26322 (ASUL020311), 26332 (ASUL020306).—USA: Arizona: W. C. Davis 451 (577841, ASUL011839).
Candelariella vitellina (Hoffm.) Müll. Arg. Mexico: Baja California: T. H. Nash 38248 (514395, ASUL010373).—USA: Arizona: W. C. Davis 617 (578223, ASUL011791).
Candelina mexicana (B. de Lesd.) Poelt. Mexico: Baja California Sur: T. H. Nash 39918 (514805, ASUL034850). Michoacán: R. S. Egan 10704 (580307, ASUL034847). Sonora: J. Marsh 4776 (608870, ASUL010375).—Venezuela: Mérida: K. Kalb 24021 (WIS-L-0115751), T. H. Nash 29010 (514728, ASUL034863), 29040 (ASUL034864). Lara: K. Kalb 25843 (WIS-L-0141004), T. H. Nash 28949 (ASUL034865).—USA: Arizona: T. H. Nash 41651 (514893, ASUL010374). Texas: C. Fox T120 (580243, ASUL034888).
Candelina submexicana (B. de Lesd.) Poelt. Mexico: Chihuahua: T. H. Nash 13514 (580008, ASUL034785), 36550 (514668, ASUL034815), 36104 (ASUL034797).—Peru: Ica: W. A. Weber s. n. (L-66445, 314320, COLO-L-0063762), W. A. Weber s. n. (L66445 pkt 2, 414653, COLO).—USA: Arizona: R. Kulich 16A (ASUL003433). New Mexico: B. D. Ryan 22143-a (514803, ASUL034889), R. D. Worthington 31956 (537897, ASUL024890).
Placomaronea candelarioides Räsänen. Argentina: Tucumán: T. H. Nash 28034 (604432, ASUL010349), R. C. Harris 4254461 (NY 4254461). Catamarca: I. M. Lamb 5596 (MSC0135846).—Bolivia: La Paz: D. Ugent s. n. (MIN 1298531). Potosí: D. Ugent s. n. (MIN 1298532).—Peru: Puno: D. Ugent s. n. (MIN 1298534).
Placomaronea lambii (Hakul.) R. Sant. Argentina: Tucumán: I. M. Lamb 5413 (MSC0112896—isotype).
Placomaronea mendozae (Räsänen) M. Westb. USA: Arizona: T. H. Nash 25430 (580255, ASUL010350).
We generated ITS sequences for three specimens, including the holotype of both new species: Ramos 2899 (GenBank Accession number PQ807198) and 2908a (holotype: GenBank Accession number PQ807199) for P. placoidea, and Ramos 2946 (holotype: GenBank Accession number PQ807200) for P. fruticosa. Sequences have been submitted to GenBank (https://www.ncbi.nlm.nih.gov). DNA was extracted using the Soltis Lab CTAB DNA protocol (Doyle & Doyle Reference Doyle and Doyle1987; Cullings Reference Cullings1992). ITS1 and ITS4 primers (White et al. Reference White, Bruns, Lee, Taylor, Innis, Gelfand, Sninsky and White1990) were used to select the ITS region. Standard amplification procedures were followed (McCune & Curtis Reference McCune and Curtis2012). Forward and reverse sequences were inspected and combined by hand using Geneious Prime v. 2023.0.3 (https://www.geneious.com). All sequences were checked against the NCBI database (https://www.ncbi.nlm.nih.gov) for contamination. Additional ITS sequences were obtained from GenBank, comprising all sequences available for Placomaronea, including those used by Westberg et al. (Reference Westberg, Frödén and Wedin2009) and, where available, one or two representatives for every species in Candelariaceae. Unfortunately, it was not possible to include any ITS sequences cited in Kondratyuk et al. (Reference Kondratyuk, Lőkös, Jeong, Oh, Kondratyuk and Hur2020) since it appears that none of these have been submitted to GenBank. Reviewing the recent publication by Kondratyuk et al. (Reference Kondratyuk, Lőkös, Jeong, Oh, Kondratyuk and Hur2020), we initially planned to add mtSSU and nuLSU sequences to our analysis, but only very few sequences for these loci exist and not a single one for Placomaronea or Candelina. Any attempt to construct a multilocus phylogeny of Candelariaceae therefore seems very premature.
Following Westberg et al. (Reference Westberg, Arup and Kärnefelt2007, Reference Westberg, Frödén and Wedin2009), we chose Pleopsidium chlorophanum, P. flavum and Pycnora xanthococca as outgroups for our analysis. The sequences were initially aligned using MAFFT v. 7.490 (Katoh & Standley Reference Katoh and Standley2013), then manually trimmed and adjusted, removing regions that we considered problematic because of introns. IQ-TREE v. 2.1.3 (Minh et al. Reference Minh, Schmidt, Chernomor, Schrempf, Woodhams, von Haeseler and Lanfear2020) was then used to create maximum likelihood trees, using the default settings for an ultrafast bootstrap analysis, with 1000 bootstrap repetitions and automatic selection of the substitution model. We also ran a Bayesian analysis using the Markov chain Monte Carlo (MCMC) method (Larget & Shimon Reference Larget and Shimon1999) as implemented in MrBayes v. 3.2.7 (Ronquist et al. Reference Ronquist, Teslenko, van der Mark, Ayres, Darling, Höhna, Larget, Liu, Suchard and Huelsenbeck2012), with substitution model nset=6 rates=invgamma. Two independent runs continued until the standard deviation between split frequencies dropped below 0.01. The final tree was rendered with FigTree v. 1.4.4 (Rambaut & Drummond Reference Rambaut and Drummond2012). Alignment and log files are available on FigShare (https://doi.org/10.6084/m9.figshare.28098074).
Results
Secondary chemistry
Table 1 summarizes the results of specimens analyzed by thin-layer chromatography and their cortical spot test reactions with K. The following chemotypes can be distinguished (Figs 1 & 2):
-
• Chemotype A: pulvinic acid (major), 4-hydroxypulvinic acid (minor), pulvinic dilacetone (minor or trace), calycin (minor or trace); with a series of unidentified terpenoids.
-
• Chemotype B: same chemistry as A, but no terpenoids.
-
• Chemotype C: same chemistry as chemotype A, but no pulvinic dilacetone; with the same unidentified terpenoids as chemotype A.
-
• Chemotype D: characterized only by pulvinic acid (major) and 4-hydroxypulvinic acid (minor); no other secondary metabolites present.
Table 1. Secondary chemistry and K spot tests in selected specimens of Candelariaceae. Testing the thallus surface, K+ indicates a strong red colour reaction to 10% potassium hydroxide; K± indicates a weak reddish reaction (barely visible after several minutes); K− indicates no reaction.
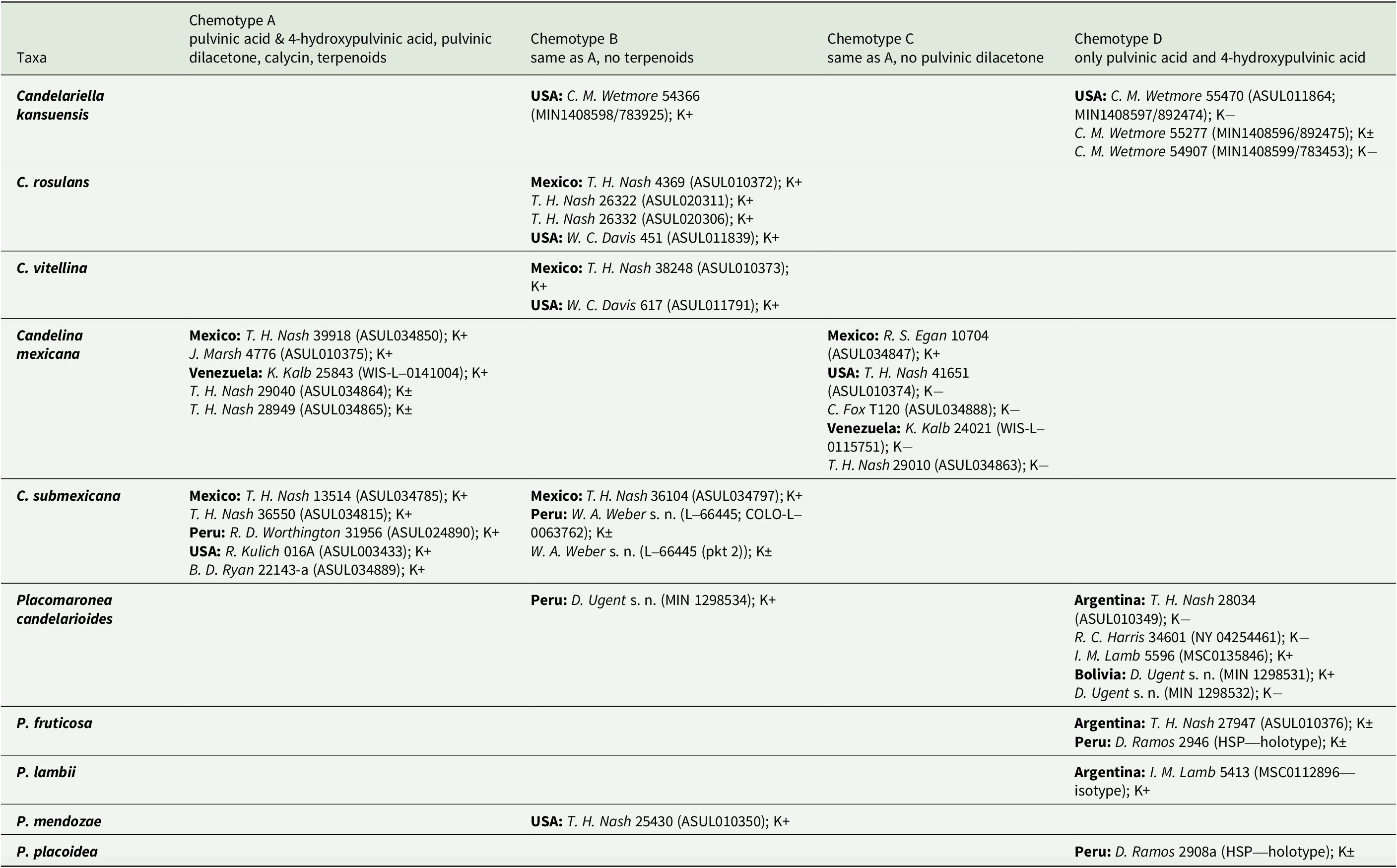
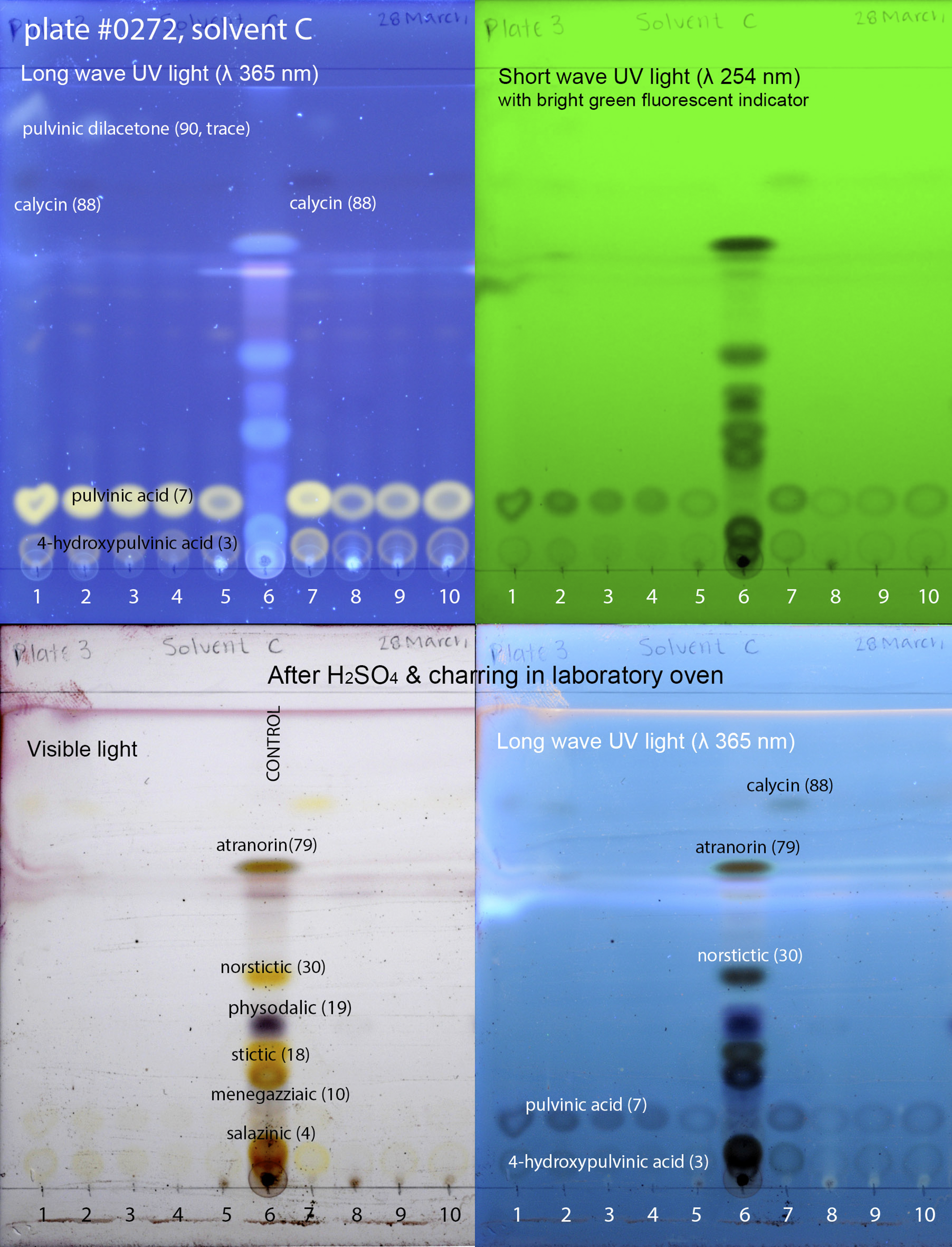
Figure 1. Thin-layer chromatography plate of selected specimens of Candelariaceae; numbers correspond to the following specimens: 1) Candelina submexicana (W. A. Weber 314320 (L-66445; COLO-L-0063762)), 2) Candelina submexicana (W. A. Weber 314320 (L-66445; pkt 2)), 3) Placomaronea placoidea (D. Ramos 2908a (HSP—holotype)), 4) Placomaronea fruticosa (D. Ramos 2946 (HSP—holotype)), 5) Placomaronea candelarioides (T. H. Nash 28034 (ASUL010349)), 6) Control (mixture of specimens with known secondary metabolites: Hypogymnia physodes (L.) Nyl., Hypotrachyna microblasta (Vain.) Hale, Parmelia sulcata Taylor, Parmotrema crinitum (Ach.) M. Choisy, Physcia adscendens H. Olivier), 7) Placomaronea mendozae (T. H. Nash 25439 (ASUL010350)), 8) Placomaronea lambii (I. M. Lamb 5413 (MSC0112896—isotype)), 9) Placomaronea candelarioides (R. C. Harris 34601 (NY 04254461)), 10) Placomaronea candelarioides (I. M. Lamb 5596 (MSC0135846)). Numbers 1, 2 & 7 = chemotype B (pulvinic dilacetone, calycin, pulvinic acid, 4-hydroxypulvinic acid, no terpenoids); 3, 4, 5, 8, 9 & 10 = chemotype D (only pulvinic acid with 4-hydroxypulvinic acid). In colour online.
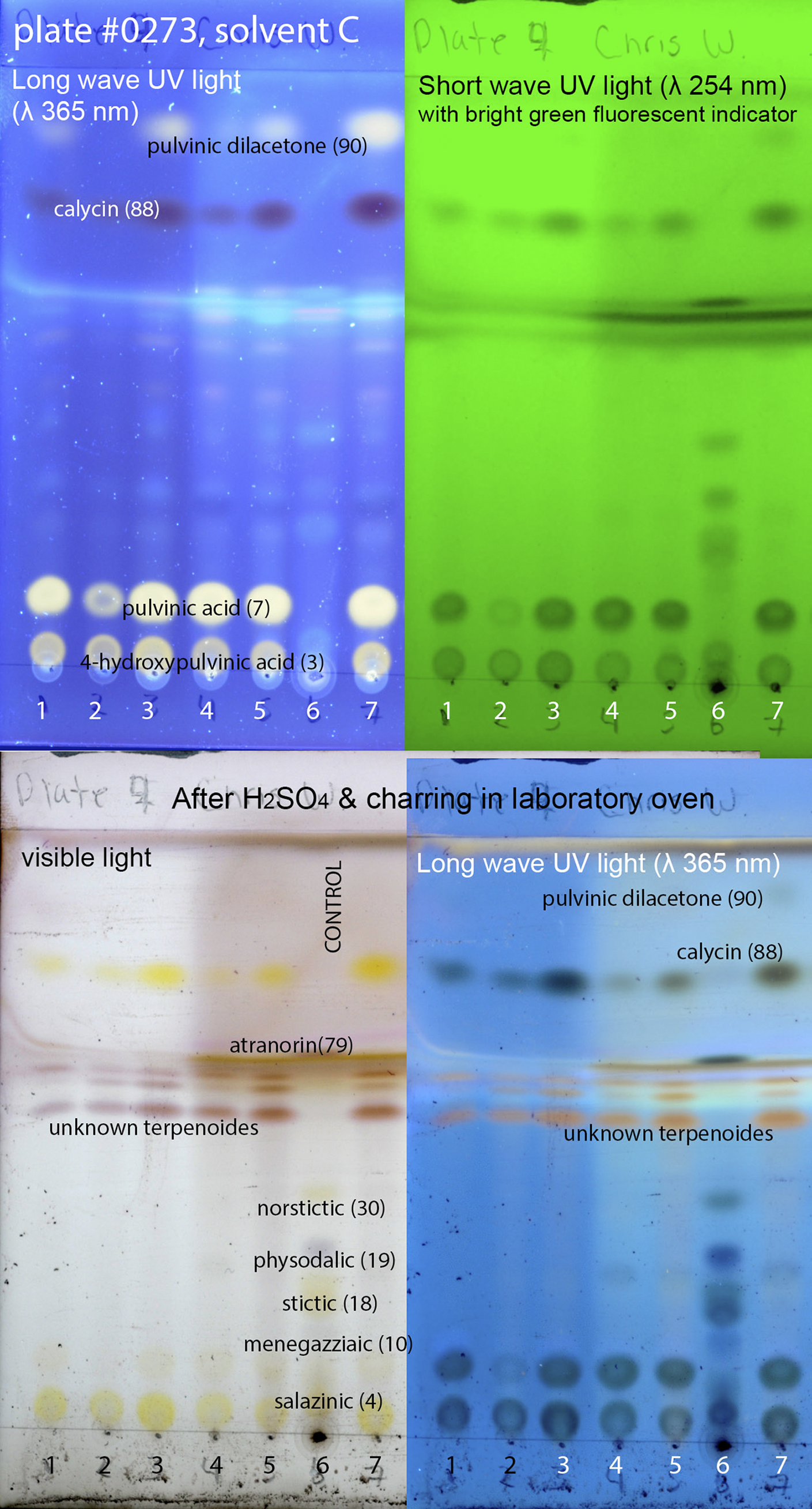
Figure 2. Thin-layer chromatography plate of selected specimens of Candelariaceae; numbers correspond to the following specimens: 1) Candelina submexicana (R. D. Worthington 31956 (ASUL024890)), 2) Candelina mexicana (C. Fox T120 (ASUL034888)), 3) Candelina submexicana (T. H. Nash 36550 (ASUL034815)), 4) Candelina mexicana (K. Kalb 24021 (WIS-L-0115751)), 5) Candelina mexicana (T. H. Nash 39918 (ASUL034850)), 6) Control (mixture of specimens with known secondary metabolites: Hypogymnia physodes, Hypotrachyna microblasta, Parmelia sulcata, Parmotrema crinitum, Physcia adscendens), 7) Candelina mexicana (K. Kalb 25843 (WIS-L-0141004)). Numbers 1, 3, 5 & 7 = chemotype A (pulvinic dilacetone, calycin, pulvinic acid, 4-hydroxypulvinic acid, unknown terpenoids); 2 & 4 = chemotype C (calycin, pulvinic acid, 4-hydroxypulvinic acid, unknown terpenoids). In colour online.
Among the material examined, all Candelariella rosulans and C. vitellina belong to chemotype B, reacting distinctly K+ red. Candelariella kansuensis is represented only by chemotype D, specimens reacting K− or K± weakly reddish. Most specimens of Candelina react distinctly K+ red, few K+ weakly reddish, and a small number are K−. Material of C. mexicana belongs to chemotypes A or C, and specimens of C. submexicana to chemotypes A or B; no specimens of Candelina were found that belong to chemotype D. Most specimens of Placomaronea belong to chemotype D (mostly reacting K± weakly reddish or K−, few K+ distinctly red); one specimen of P. candelarioides and one of P. mendozae were found to belong to chemotype B (both K+ distinctly red).
Anatomy
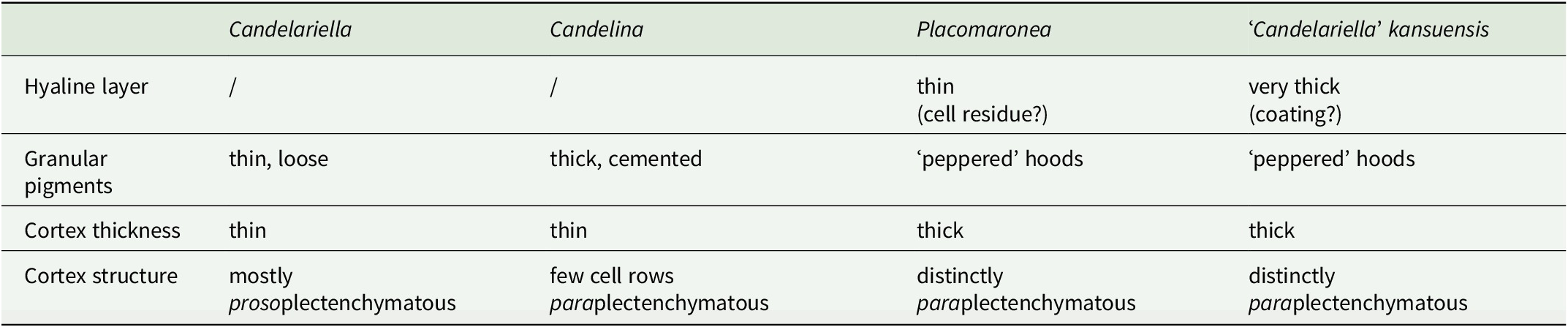
Most of the differences in cortex anatomy correspond with the three genera as currently recognized (Table 2): Placomaronea (thin colourless coating shedding off; peppered pigment hoods; thick cortex of cortical paraplectenchymatous hyphae), Candelina (no colourless coating; no hoods, but with a thick layer of closely cemented pigment granules; only the uppermost hyphae paraplectenchymatous), and Candelariella (no colourless coating; no hoods; thin layer of loose pigment granules; cortical hyphae mostly parallel, proso- to indistinctly paraplectenchymatous). Only in ‘Candelariella’ kansuensis can an almost identical cortical anatomy to Placomaronea be observed; the only difference is the extremely thick outermost colourless coating of C. kansuensis, causing a smooth, waxy, almost shiny thallus surface.
Table 2. Cortex anatomy in Candelariaceae.
Phylogeny
Our phylogenetic analyses strongly support the inclusion of the new species in Placomaronea (100% bootstrap; Fig. 3). Within Placomaronea, the two new species form a distinct, well-supported clade within P. mendozae (100% BS); together all three (P. fruticosa, P. placoidea and P. mendozae) form a well-supported clade (99% BS) that is sister to P. candelarioides and P. fuegiana.
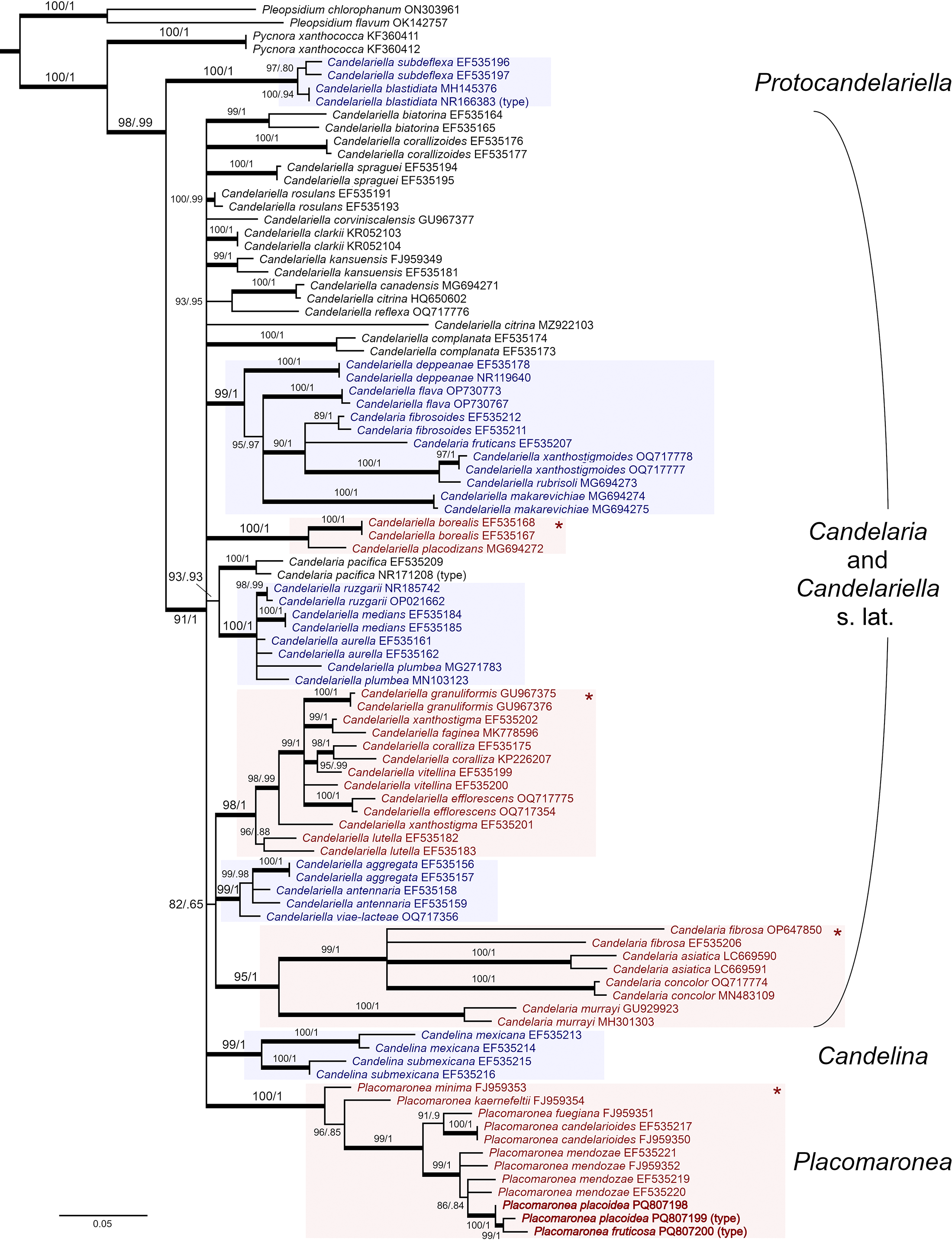
Figure 3. Phylogeny of the family Candelariaceae based on maximum likelihood analysis (ML) of ITS. Support is shown as ML bootstrap values (left)/Bayesian MCMC posterior probabilities (right); branches with strong support (bootstrap ≥ 95% and MCMC ≥ 0.99) are indicated with thick lines. Polysporous clades are indicated with an asterisk (*) and shaded red (colour version). Octosporous clades are shaded pale blue (colour version). GenBank Accession numbers or voucher information are provided after the taxon names. The new species are in bold at the bottom of the tree. In colour online.
The relationships between P. fruticosa, P. placoidea and P. mendozae remain poorly resolved. Placomaronea fruticosa is included in the same clade as P. placoidea, which in turn is part of the clade that forms P. mendozae. Both P. placoidea and P. mendozae are thus paraphyletic.
Our analysis recovers polysporous species of Candelariella into two separate but strongly supported, monophyletic clades: one with C. borealis M. Westb. and C. placodizans (Nyl.) H. Magn. (100% BS), the other one corresponding to Candelariella s. str., with C. coralliza (Nyl.) H. Magn., C. efflorescens R. C. Harris & W. R. Buck, C. faginea Nimis et al., C. granuliformis M. Westb., C. lutella (Vain.) Räsänen, C. vitellina and C. xanthostigma (Ach.) Lettau (98% BS). Polysporous species of Candelaria (C. asiatica D. Liu & J.S. Hur, C. concolor, C. fibrosa (Fr.) Müll. Arg. and C. murrayi Poelt) also form a well-supported, monophyletic clade (95% BS). In addition, our analysis confirmed two monophyletic clades of octosporous species of Candelariella: one that includes Candelariella aurella (Hoffm.) Zahlbr., C. medians (Nyl.) A.L. Sm., C. plumbea Poelt & Vězda and C. ruzgarii Halıcı et al. (100% BS), the other including Candelariella aggregata M. Westb., C. antennaria Räsänen and C. viae-lacteae G. Thor & V. Wirth (99% BS).
Morphology
Placomaronea fruticosa and P. placoidea are not easily categorized as crustose, foliose, or fruticose (Figs 4A–D & 5A–C). Both resemble crustose lichens closely, but their lobes radiating on the substrate surface have a distinctly corticate upper and lower side (Figs 4C & D, 5B). At least anatomically, these thalli are foliose.

Figure 4. Placomaronea fruticosa (Ramos 2946, HSP—holotype). A, surface view of the thallus, placodioid lobes spreading irregularly across the soil substrate, fruticose parts of thallus embedded in the soil. B–D, samples prepared by removing soil substrate to illustrate fruticose growth below horizontally spreading surface lobes. B, lateral view. C, view of the upper side. D, view of the lower side. E, section of an apothecium. F, polysporous ascus. G, hand-cut section of fruticose part of the thallus with medullary hyphae throughout the centre, surrounded by a photobiont layer and cortex. H, hand-cut section of the lateral part of a thallus lobe, showing the medulla, photobiont and cortical layers. I, close-up of the cortex, with a distinct layer of paraplectenchymatous cells, apically capped by cells with pigment hoods, peppered in pigment granules, covered in a thin, barely distinct coating of hyaline residue. Scales: A–D = 5 mm; E & G = 100 μm; F & I = 10 μm; H = 25 μm. In colour online.
Morphologically, these thalli cannot be distinguished reliably from species of Candelina, where Poelt (Reference Poelt1974) also described placodioid growth in combination with a lower cortex. Placomaronea placoidea is a close lookalike of Candelina submexicana and is easily confused. The lobes of P. placoidea are generally smaller, and more flattened and hollow inside; both are most reliably distinguished by their different cortex anatomy.
In the field, on the surface of the soil, thalli of P. fruticosa look almost identical to those of their saxicolous counterpart, P. placoidea, both having radiating placodioid lobes with an upper and lower cortex. This appearance is, however, deceiving. Below the surface, thalli of P. fruticosa are distinctly fruticose, extending upwards from deep within their substrate (Fig. 4B). Emerging from the soil, the coralloid branches spread laterally to mimic placodioid growth. Lobes of P. placoidea are hollow (Fig. 5D), whereas the centre of the lobes and coralloid branches of P. fruticosa contain hyphae, forming a lax medulla (Fig. 4G). No fertile material of P. placoidea has been found; only specimens of P. fruticosa were collected with apothecia (Fig. 4A).
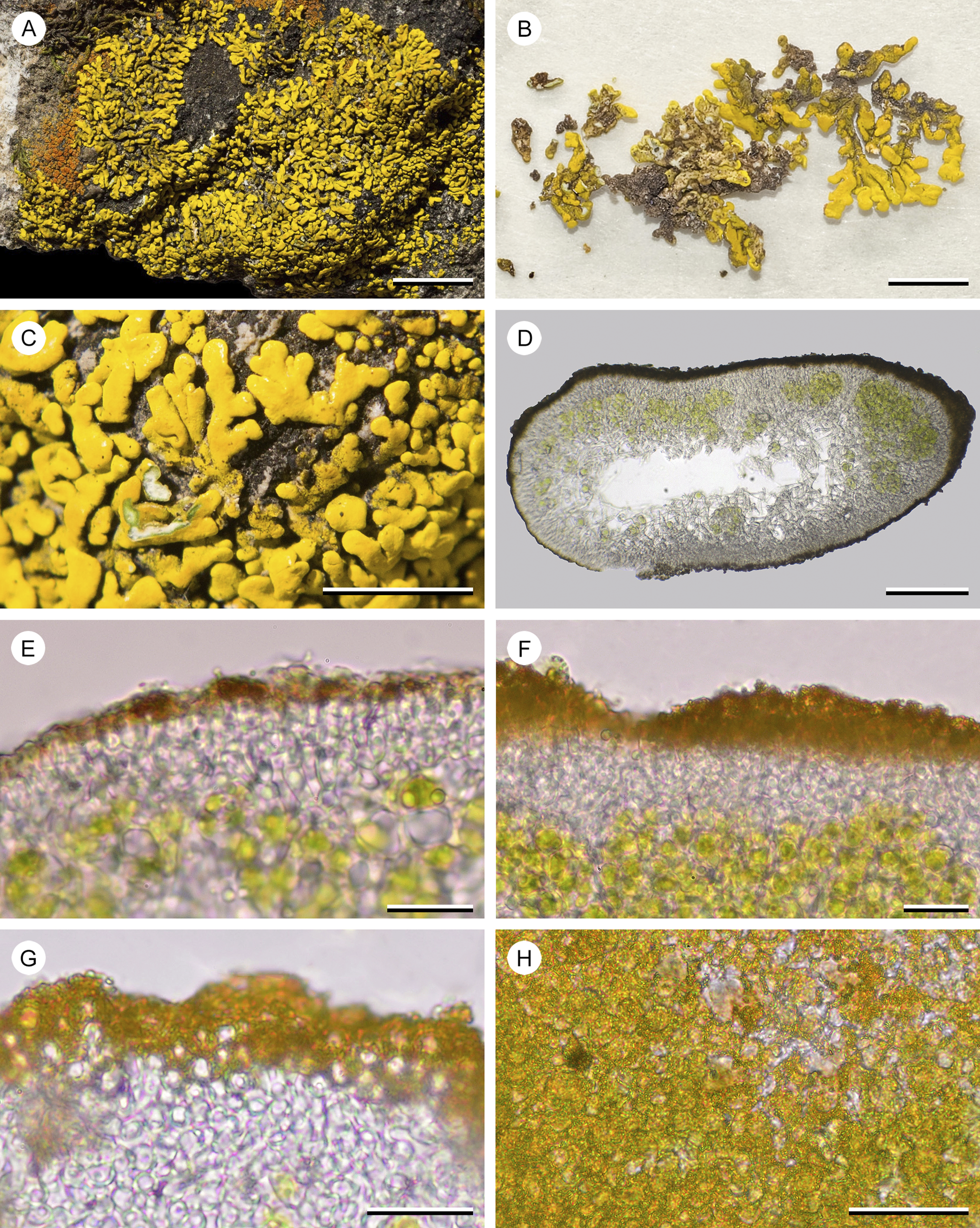
Figure 5. Placomaronea placoidea (Ramos 2908a, HSP—holotype). A, overview of the placodioid thallus covering a rock. B, thallus lobes removed from their substrate showing the upper and lower sides; upper cortex yellow, lower cortex brown, beige to ivory. C, close-up of lobes adhering to the rock substrate, cortex in part damaged showing the photobiont layer and white medulla. D, hand-cut section of hollow thallus lobe. E–G, hand-cut sections of photobiont layer and upper cortex. E, thin section, showing distinctly paraplectenchymatous hyaline cells, apically with a pigmented layer and a thin, barely distinct coating of hyaline residue. F, thick section with photobiont layer, hyaline and pigmented part of the cortex. G, thin section, showing distinctly paraplectenchymatous hyaline cells, apically capped by cells with pigment hoods peppered in pigment granules, covered in a thin, barely distinct coating of hyaline residue. H, squash preparation of inflated apical cortex cells with pigment hoods covered in pigment granules. Scales: A = 1 cm; B = 3 mm; C = 5 mm; D = 100 μm; E & F = 20 μm; G = 15 μm; H = 25 μm. In colour online.
Taxonomic Section
Placomaronea fruticosa Ramos, Hollinger & Bungartz sp. nov.
MycoBank No.: MB 857051
Differs from other species in the genus by a fruticose thallus part that is up to 8 mm deep and immersed in its soil substrate.
Type: Perú, Cusco, Condoroma, Carretera Condoroma-Oscollo, Roquedal rodeado por césped de puna, en suelo [along the road from Condoroma to Oscollo, rocky area surrounded by puna grass, within the soil], 15.2887°S, 71.1388°W, 4650 m alt., 6 May 2018, D. Ramos 2946 (HSP-17855—holotype!, GenBank Accession no. PQ807200; ASUL010486—isotype!).
(Fig. 4)
Thallus dimorphic, with a spread-out placodioid thallus on the substrate surface, and a subterranean fruticose part buried up to 8 mm within the soil, the placodioid part on the surface generally ±ellipsoid in outline, 1–3 cm diam., irregularly branched, towards the centre increasingly immersed in its substrate; the apical branches emerging from the soil horizontally spreading outwards, flattened, elongated, lobate, resembling placodioid crustose thalli, but the lobes corticate below, branch tips linear or spathulate, 2.6–5.6 mm long and 0.4–1.0 mm wide; basal, immersed thallus vertical, ±terete to distinctly cylindrical, solid, beige to violet (in parts), to 8 mm tall. Surface lacking pruina, the flattened placodioid part with a bright yellow to deep yellow upper side, the lower side whitish to beige; surface of the immersed cylindrical thallus beige to brown all around, in parts often violet. Cortex 14–30 μm thick, on the upper surface of the lobes differentiated into a hyaline, paraplectenchymatous layer 5–8 cells thick, capped by ±inflated terminal cells, their walls forming pigment hoods, ‘peppered’ on the outside with pigment granules, coated by a thin hyaline residue flaking off; lower cortex along the lobe edges a continuation of the upper one, becoming thinner, with less pigmentation on the lower site of the lobes and along the immersed, fruticose part of the thallus. Photobiont trebouxioid. Medulla white, lax.
Apothecia lecanorine, laminal or at the emerging apices of the cylindrical, immersed branches, 0.3–0.8 mm diam., thalline margin entire, thin, crenate, proper margin indistinct; disc plane, of the same colour or slightly darker than the thallus, without pruina. Hymenium 60–80 μm tall, inspersed with small oil droplets; paraphyses to 2 μm wide in the centre, straight, capitate, simple or subapically sparsely branched; epihymenium orange-brown to golden; subhymenium and hypothecium hyaline, also inspersed; proper exciple to 12 μm wide. Asci clavate 47–59 × 19–30 μm. Ascospores ellipsoid, (8–)11–13.5(–15) × 3–4.5 μm (n = 73), more than 20 per ascus, hyaline, simple or rarely with a central septum, most with two oil drops (bigutulate).
Pycnidia not observed.
Chemistry
Pulvinic acid (major) and 4-hydroxypulvinic acid (minor), no other secondary metabolites detected (chemotype D). Cortex K± very faintly reddish, C−, KC−, P−, UV−; medulla, all reactions negative; hymenium IKI+ blue.
Etymology
The name refers to its main characteristic, the subterranean fruticose growth.
Distribution
Terricolous, at elevations between 3800 and 4650 m, in exposed habitats among Andean grassland in the southern Andes of Peru (Cusco and Tacna), to Argentina.
Remarks
Although some species in Placomaronea show tendencies to form thalli ±elevated from their substrate (e.g. the ‘umbilicate’ lobes of P. candelarioides), P. fruticosa is the only species currently known where the major part of its thallus is fruticose (even though this fruticose part remains entirely immersed and only the placodioid lobes extend across the surface). Placomaronea fruticosa is also the only species known to grow on soil; all other species in the genus are saxicolous.
Additional specimens examined
Perú: Cusco: Condoroma, Carretera Condoroma-Oscollo, Roquedal rodeado por césped de puna, 15.2887°S, 71.1388°W, 4650 m alt., 2018, D. Ramos 2949 (HSP). Tacna: Candarave, Zona rocosa camino al poblado Yucamani, 17.2666°S, 70.2349°W, 3250 m alt., 2018, D. Ramos 2767 (HSP). Ticaco: Roquedal al lado de la carretera de Candarave a Ticaco, 17.2486°S, 70.0792°W, 3676 m alt., 2018, D. Ramos 2835 (HSP).—Argentina: Jujuy: 43 km E of La Quiaca, lower part of Sierra de Santa Victoria, along route 5 (dirt road to Santa Victoria), 22°08′S, 65°18′W, 3807 alt., alpine area, on soil, 1989, T. H. Nash 27947 (512253, ASUL010376).
Placomaronea placoidea Ramos, Hollinger & Bungartz sp. nov.
MycoBank No.: MB 857052
Differs from other species of Placomaronea by a tightly adnate thallus of placodioid radiating lobes with a distinct lower cortex, resembling species of Candelina but distinguished by a hollow medulla and a Placomaronea-type cortex (i.e. a paraplectenchymatous hyaline layer capped by pigment hoods, ‘peppered’ with pigment granules, coated in a thin hyaline residue flaking off).
Type: Perú, Arequipa, Cayarani, Roquedal al lado del camino de entrada al poblado de Cayarani, en rocas [rocky area next to the entrance road to the town of Cayarani, on rock], 14°40′10.8″S, 72°01′25.5″W, 3955 m alt., 5 May 2018, D. Ramos 2908a (HSP-17815—holotype!, GenBank Accession no. PQ807199; ASUL010485—isotype!).
(Fig. 5)
Thallus resembling placodioid crustose lichens, but the tightly adnate lobes with a lower cortex and thus foliose, irregularly branching lobes forming small rosettes, irregular to ±ellipsoid in outline, up to 6 cm diam.; lobes elongate, irregularly spreading from the thallus centre, simple to scarcely and irregularly branched, closely adjoining to ±imbricate, linear to apically barely spathulate, 2.0–4.9 mm long and 0.7–1.4 mm wide. Upper surface plane to undulate, mostly dull, but with some shiny areas, epruinose, bright yellow especially towards the lobe apices, often discoloured and darker yellow or even brownish in the thallus centre, lower surface beige, becoming paler towards the thallus centre, difficult to remove intact from its substrate. Cortex 10–30 μm thick, on the upper surface of the lobes differentiated into a hyaline, paraplectenchymatous layer 3–8 cells thick, capped by ±inflated terminal cells, their walls forming pigment hoods, ‘peppered’ on the outside with pigment granules, coated by a thin hyaline residue flaking off; lower cortex continuous with the upper cortex and of similar thickness along the lobe edges, but becoming thinner and almost devoid of pigments towards the thallus centre; lobes initially compact, but soon becoming hollow, the medulla at least in part not filling the interior of the thallus. Photobiont trebouxioid. Medulla white, composed of lax, occasionally anastomosing hyphae that are eventually confined to the upper and lower parts of the lobes, hollowed out in the centre.
Apothecia not observed.
Pycnidia scarce to abundant, inconspicuous, forming small depressions on the upper surface, ostioles concolorous with the surface; conidia ellipsoid, hyaline, (3.0–)3.5–4.5 × 1.2–1.9 μm.
Chemistry
Pulvinic acid (major) and 4-hydroxypulvinic acid (minor), no other secondary metabolites detected (chemotype D). Cortex K± very faintly reddish, C−, KC−, P−, UV−; medulla, all reactions negative.
Etymology
The name refers to the placodioid growth.
Distribution and ecology
Saxicolous, in exposed and sunny habitats, at elevations between 3000–4000 m, so far recorded only in the southern Andes of Peru (Cusco and Arequipa), but presumably with a wider distribution.
Remarks
The species is distinguished by its adnate, foliose-placodioid thallus resembling species of Candelina but distinctly different by its cortical anatomy and hollow medulla (see Discussion).
Additional specimens examined
Perú: Cusco: Santo Tomás, Roquedal en rodal de Puya raimondii, Carretera al borde del río Cayarani, 14.7771°S, 72.0412°W, 4082 m alt., 2018, D. Ramos 2899 (HSP, GenBank Accession no. PQ807198). Ocoruro: Carretera Ocoruro-Espinar, 14.9377°S, 71.1915°W, 3990 m alt., 2018, D. Ramos 2931 (HSP). Arequipa: Huaynacotas Rodal de Puya alrededores de la comunidad de Puyca, 14.9340°S, 72.7108°W, 4108 m alt., 2017, D. Ramos 2524 (HSP). Chuquibamba: Matorral en ladera, pasando el pueblo de Chuquibamba, 15.8295°S, 72.6615°W, 3004 m alt., 2017, D. Ramos 2502 (HSP).
Key to the genera of Candelariaceae

Key to the species of Placomaronea

Discussion
Secondary chemistry
Poelt (Reference Poelt1974, p. 190) suggested that among Candelariaceae ‘…Their chemistry is mostly uniform…’ (‘…Ihr Chemismus ist weitgehend einheitlich…’) and that the presence of ‘stictaurin’ (i.e. pulvinic dilacetone and calycin occurring together; Zopf Reference Zopf1907) was generally characteristic. In their revision of Placomaronea, Westberg et al. (Reference Westberg, Frödén and Wedin2009) list only secondary metabolites generally characteristic for the genus, not for individual species, reporting calycin, pulvinic dilacetone (= pulvic acid lactone), vulpinic acid and pulvinic acid. This agrees largely with our own analyses, although vulpinic acid could not be confirmed and, for the first time, 4-hydroxypulvinic acid is reported, present in virtually all specimens examined.
Overall, chemical variation in the material examined is low. Based on quantitative analyses using high performance liquid chromatography (HPLC), Westberg (Reference Westberg2005) distinguished only two chemosyndromes, ChA (with calycin as the dominant metabolite; characteristic for most species of Candelariella, Placomaronea, Candelina, and the 8-spored species of Candelaria) and ChB (with the secondary metabolites in approximately equal quantities; documented for polyspored species of Candelaria, Candelariella subdeflexa, and possibly Candelariella lutella).
We did not have access to HPLC and the chemotypes distinguished here are based on secondary metabolites detected by TLC. Here we document variation in whether terpenoids are present or not and if pulvinic dilacetone and/or calycin are absent, occur in minor concentration, or as a trace (Table 1). Whereas a much larger number of specimens and taxa needs to be analyzed to assess if any of this variation correlates with particular genera or species. This may seem surprising. In many groups of lichenized fungi, secondary chemistry is taxonomically relevant and chemotype patterns are often species-specific. Only recently, however, Díaz-Escandón et al. (Reference Díaz-Escandón, Tagirdzhanova, Vanderpool, Allen, Aptroot, Češka, Hawksworth, Huereca, Knudsen and Kocourková2022) suggested including Candelariaceae within Lichinomycetes, based on their much smaller genomes, arguing that these fungi are characterized by ‘reduced arsenals of carbohydrate-degrading enzymes and secondary metabolite gene clusters’ (Díaz-Escandón et al. Reference Díaz-Escandón, Tagirdzhanova, Vanderpool, Allen, Aptroot, Češka, Hawksworth, Huereca, Knudsen and Kocourková2022, p. 5209).
Most surprising in the material analyzed is the inconsistency of reddish spot test reactions in K. Zopf (Reference Zopf1895) reported that pure calycin reacts K+ red, but in the presence of pulvinic dilacetone (= pulvinic acid lactone) forms a complex called ‘stictaurin’, which no longer reacts with K. This observation cannot be confirmed. Virtually all specimens that contain both calycin and pulvinic dilacetone react distinctly K+ red, or at least K± weakly reddish (chemotypes A & B; Table 1), and specimens that contain only calycin and lack pulvinic dilacetone instead mostly react K− (chemotype C; Table 1). Curiously, even some specimens that contain neither pulvinic dilacetone nor calycin react distinctly K+ red, or K± weakly reddish.
Poelt (Reference Poelt1974) suggested, based on observations by Santesson, that polyporic acid might be responsible for a K+ weakly reddish spot test reaction in Candelina mexicana. For the material that we examined, this cannot be confirmed. Polyporic acid is a deep red pigment, whereas the medulla of C. mexicana is bright yellow, no different in colour than the cortex of that species (Fig. 7B). It seems unlikely that terphenylquinones would be present in a family of lichens generally characterized by pulvinic acid derivatives. Instead, in much of the material that we examined, we discovered a substance that most likely refers to 4-hydroxypulvinic acid, a pulvinic acid derivative (in solvent C it occurs at R f 3 as a distinctly pale yellow spot in λ 365 nm UV light before H2SO4 treatment; the same spot is pale yellow in visible light after H2SO4 treatment, and it turns dull greenish yellow in λ 365 nm UV light after H2SO4 treatment; Figs 1 & 2). This secondary metabolite was present in all chemotypes and for all chemotypes at least some specimens displayed a positive spot test K reaction. If 4-hydroxypulvinic acid is indeed responsible for this reaction, the strength of the reaction may be correlated with its concentration in the cortex. Comparing whether secondary metabolites show up on a thin-layer chromatography plate as saturated, pale or barely visible (major, minor or trace) depends on many factors and it is, at least to some extent, subject to experimental variation. More sensitive, quantitative analytical methods are therefore necessary to assess whether differences in concentration of 4-hydroxypulvinic acid might explain the variation in K reactions documented here.
Anatomy
In his revision of Candelariaceae, describing the genus Candelina, Poelt (Reference Poelt1974) was the first to recognize anatomical differences in the cortical structure of the family. He emphasized that Candelina, though appearing crustose-placodioid, is characterized by a well-defined lower cortex of few rows of closely aggregated cells, attached to its substrate with what he called ‘searching hyphae’, or ‘Suchhyphen’, whereas the lower cortex of Placomaronea, illustrating P. candelarioides as an example, has a lower cortex of several closely packed, dense cell layers (Poelt Reference Poelt1974, p. 190, fig. 1). Westberg et al. (Reference Westberg, Frödén and Wedin2009), distinguishing Placomaronea from all other genera of Candelariaceae, also emphasized cortex anatomy, which they described as ‘well-developed paraplectenchymatous…the pigments form[ing] characteristic hoods…usually also by the presence of a thin, gelatinous epicortex’ (Westberg et al. Reference Westberg, Frödén and Wedin2009, p. 526).
Closely comparing the cortical anatomy of Candelariella, Candelina and Placomaronea (Figs 4– 9), the distinction of the three genera at first seems less obvious than the assessment by Westberg et al. (Reference Westberg, Frödén and Wedin2009) suggests. All three genera show at least the tendency to form a paraplectenchymatous cortex. In all three, the yellow pigments are present as minute granules in the outer part of the cortex. A cursory comparison of, for example, the crustose-subsquamulose P. mendozae with Candelariella rosulans may not, at first, suggest conspicuous differences: both have a thin layer of unpigmented, somewhat paraplectenchymatous cells, apparently topped by yellow pigment granules. Only upon closer examination are the differences revealed. The outer cell layer of P. mendozae is distinctly paraplectenchymatous, and its cells have pigmented cell walls, the yellow granules adhering to these pigment caps. When these cells die off, they leave behind a residue of unpigmented organic material shedding off, a layer that Westberg et al. (Reference Westberg, Frödén and Wedin2009) referred to as ‘gelatinous epicortex’ (Fig. 6B & C). This is not the case for Candelariella rosulans; the species lacks this residue of organic material and the uppermost cells of the cortex are not distinctly paraplectenchymatous (Fig. 8C).

Figure 6. A–D, Placomaronea mendozae (T. H. Nash 25430 (ASUL010350)). A, squamulose thallus. B & C, paraplectenchymatous cortex with apically pigmented cells, covered by a hyaline coating, possibly the residue of dead cells. D, hooded pigment cells coated with pigment granules. E & F, Placomaronea lambii (I. M. Lamb 5413 (MSC0112896—isotype)). E, inflated erect thallus areoles. F, uppermost cortex with hooded pigment cells coated with pigment granules, covered by hyaline coating, possibly the residue of dead cells. G & H, Placomaronea candelarioides (T. H. Nash 28034 (ASUL010349)). G, upper and lower view of umbilicate thalli (some indistinctly attached by broadened multiple holdfasts). H, paraplectenchymatous cortex with apically pigmented cells, covered by a hyaline coating, possibly the residue of dead cells. Scales: A, E & G = 1 cm; B, C, D, F & H = 25 μm. In colour online.
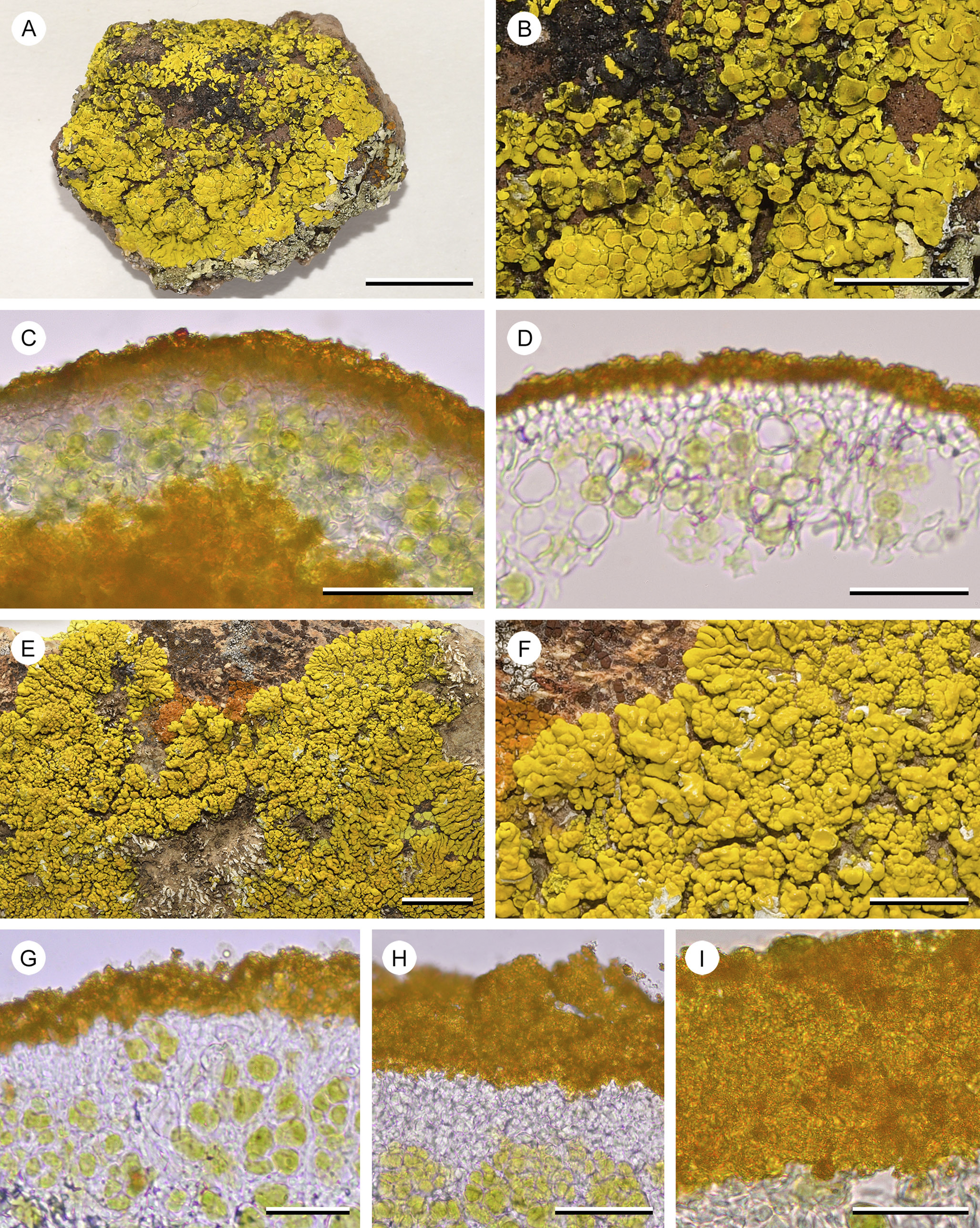
Figure 7. A–D, Candelina mexicana (T. H. Nash 39918 (ASUL034850)). A, overview of placodioid thallus. B, central part of the thallus, parts of the cortex damaged, the bright yellow medulla exposed. C, hand-cut section of the thallus, with a thin paraplectenchymatous cortex capped by a dense layer of pigment granules, green photobiont layer and yellow medulla below. D, thin section with two layers of paraplectencymatous cortical cells, capped by a dense layer of pigment granules. E–I, Candelina submexicana (R. D. Worthington 31956 (ASUL024890)). E, overview of placodioid thallus. F, detail of thallus lobes with sessile, adnate apothecia, parts of the cortex damaged, the white medulla exposed. G & H, hand-cut sections of the thallus showing the cortex; photobiont layer transitioning into a narrow layer of hyaline paraplectenchymatous cells, capped by a dense layer of pigment granules, hyaline coating absent. I, close-up of the dense layer of yellow pigment granules (squash preparation). Scales: A & E = 1 cm; B & F = 5 mm; C & H = 50 μm; D, G & I = 25 μm. In colour online.
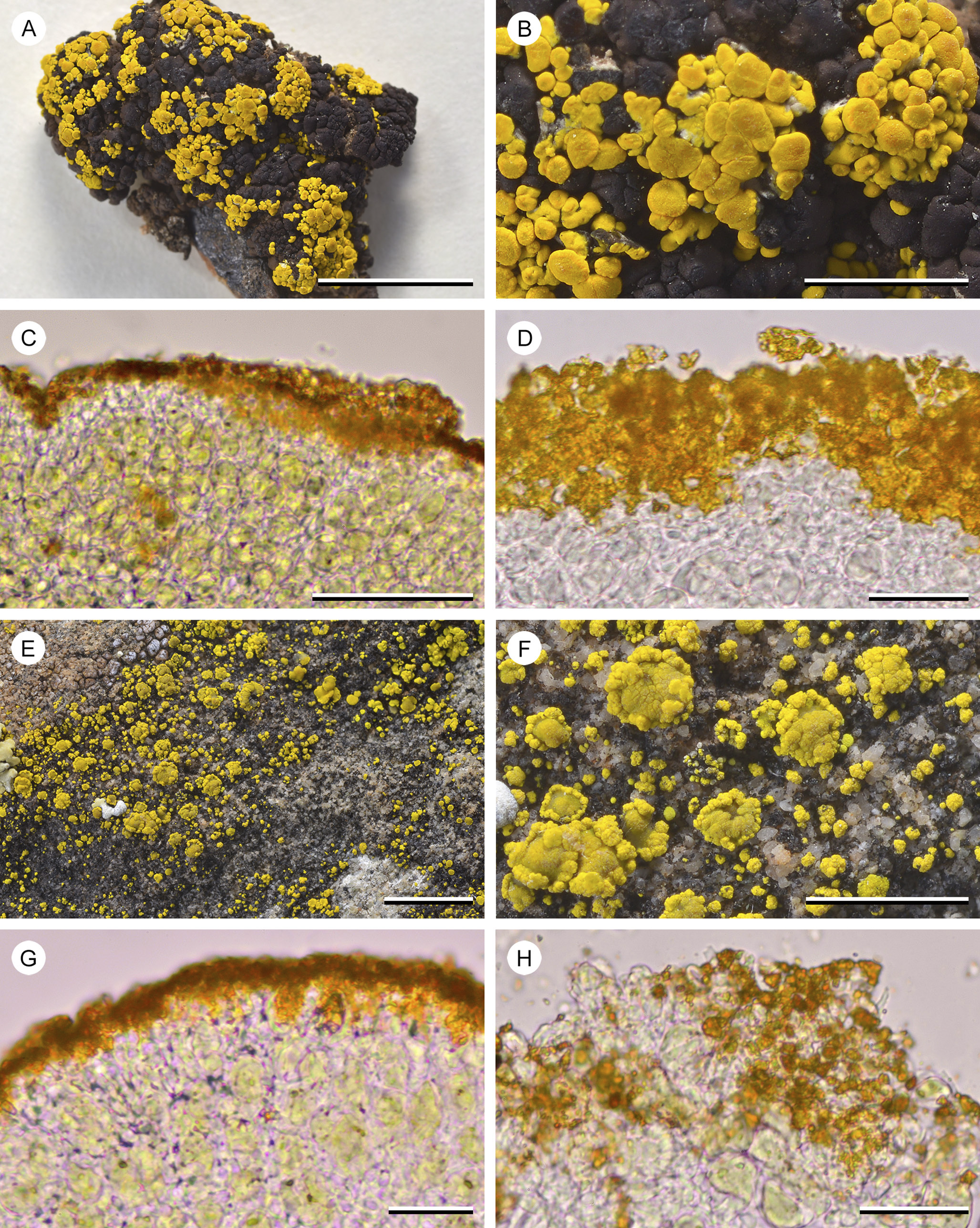
Figure 8. A–D, Candelariella rosulans (W. C. Davis 451 (ASUL011839)). A, overview of subsquamulose areoles with abundant apothecia. B, detail of the thallus with sessile, adnate apothecia. C, hand-cut section of the thallus, showing a thin layer of proso- to indistinctly paraplectenchymatous hyaline cells above the photobiont layer, apically covered by an accumulation of pigment granules. D, close-up of hyaline cortex cells and aggregation of pigment granules. E–H, Candelariella vitellina (W. C. Davis 617 (ASUL011791)). E, overview of thallus granules with scattered apothecia. F, detail of the thallus granules with scattered apothecia. G, hand-cut and somewhat squashed section of the thallus, showing a thin layer of proso- to indistinctly paraplectenchymatous hyaline cells above the photobiont layer, apically covered by an accumulation of pigment granules. H, close-up of the hyaline cortex cells and aggregation of pigment granules (squash preparation). Scales: A = 1 cm; B & F = 2 mm; E = 5 mm; C = 50 μm; D, G & H = 25 μm. In colour online.
Originally we assumed that the organic material was a residue of dead cortical cells, perhaps better called an epinecral layer, not an epicortex. However, the thick, hyaline, layered material covering the cortex of ‘Candelariella’ kansuensis shows the same ‘shedding’ at its surface. Thus, the cortex of Placomaronea and ‘Candelariella’ kansuensis appears structurally identical (compare Figs 4– 6 with Fig. 9). Since we do not understand its origin, we prefer to call it a ‘coating of layered organic material’.
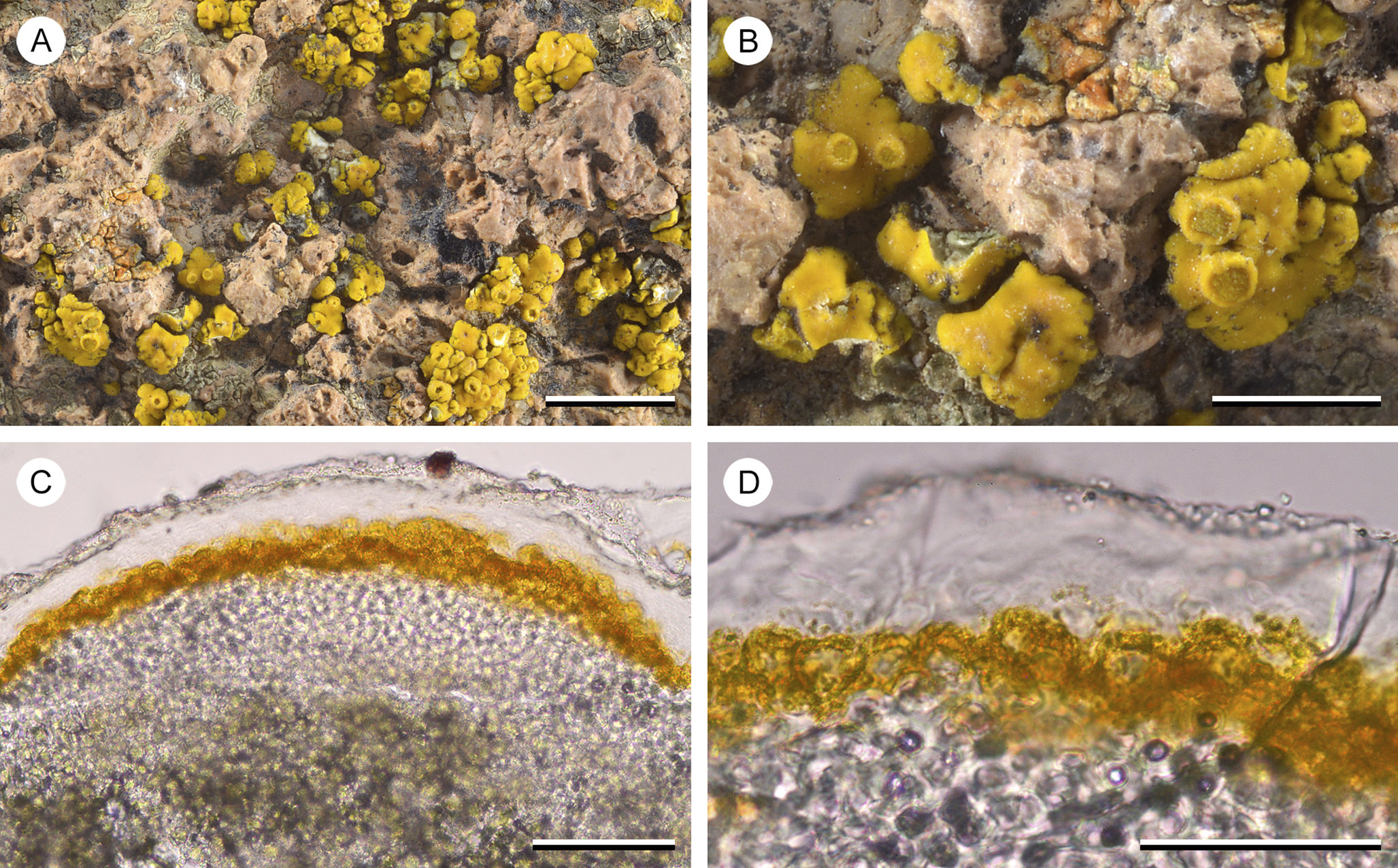
Figure 9. Candelariella kansuensis (C. M. Wetmore 55470 (ASUL011864)). A, overview of inflated, waxy thallus squamules. B, detail of squamules with few sessile, adnate apothecia. C, hand-cut section of the thallus, showing thick paraplectenchymatous layer of cortical cells, capped by swollen, hooded cells ‘peppered’ by pigment granules, covered by a thick hyaline, distinctly layered coating that apically erodes into minute, hyaline granules. D, close-up of the swollen, apical cells capped by yellow pigment hoods ‘peppered’ by pigment granules, covered in hyaline coating. Scales: A = 5 mm; B = 2 mm; C & D = 50 μm. In colour online.
Macroscopically, the genera also differ: the densely packed pigment granules on the surface of Candelariella rosulans cause a finely pruinose surface, whereas the surface of P. mendozae appears waxy, the coating of hyaline organic material smoothing the surface, covering pigment granules below. In Candelina the thallus surface appears dull, not pruinose but not smooth either, possibly a result of the much more densely packed pigment granules. The newly described Placomaronea fruticosa and P. placoidea not only appear similar macroscopically because of their placodioid growth form, but also from the outside their surface does not look different from the species of Candelina. Some material of P. fruticosa, a specimen at ASU from Argentina (T. H. Nash 27947, ASUL010376), was previously even annotated by Westberg as Candelina cf. submexicana. Entirely embedded in the substrate, the basal, fruticose growth of this specimen is easily overlooked. In addition, although the material has smaller, slightly narrower lobes, only careful examination of the cortex anatomy will reveal the distinct diagnostic differences of Placomaronea: the paraplectenchymatous cortex, apically with ‘peppered pigment hoods’, covered by a thin colourless coating sloughing off.
Phylogeny
Our trees are generally similar to those of Candelariaceae previously published (e.g. Westberg et al. Reference Westberg, Arup and Kärnefelt2007, Reference Westberg, Frödén and Wedin2009; Kondratyuk et al. Reference Kondratyuk, Lőkös, Jeong, Oh, Kondratyuk and Hur2020; Halıcı et al. Reference Halıcı, Kahraman Yiğit, Bölükbaşı and Güllü2023). All of the studies agree in several broad aspects: Candelariella blastidiata Yakovch. and C. subdeflexa (Nyl.) Lettau form a well-supported, monophyletic clade basal to the rest of the family (100% BS). Kondratyuk et al. (Reference Kondratyuk, Lőkös, Jeong, Oh, Kondratyuk and Hur2020) named this clade Protocandelariella. Also, Candelina and Placomaronea each form well-supported, monophyletic clades (99% and 100% BS, respectively). Polyspory must have evolved independently multiple times. Our phylogeny suggests that this occurred at least four times: three times in Candelaria and Candelariella s. lat., once when Placomaronea emerged.
Our phylogeny also agrees with Westberg et al. (Reference Westberg, Frödén and Wedin2009) in so far as the uniquely characteristic aspects of the cortex anatomy in Placomaronea must have evolved independently in Candelariella kansuensis: both are characterized by a distinctly paraplectenchymatous cortex with an outer layer of cells with granular-pigmented hoods. It remains unclear, however, whether the thick hyaline coating observed in C. kansuensis is analogous to what appears to be a residue of cells in Placomaronea.
Although several clades in our analysis received strong bootstrap support, the relationships between clades (and within clades) remains unresolved. Most clades formed a massive polytomy in the middle of the tree. The relationships between the clades in this polytomy were sensitive to the alignment and which particular ITS sequences we chose to include. For example, we were unable to determine with any support which clade might be sister to Placomaronea. Westberg et al. (Reference Westberg, Frödén and Wedin2009) placed it sister to Candelariella complanata, and Westberg et al. (Reference Westberg, Morse and Wedin2011) placed it in a polytomy similar to ours but with a few small changes: they included Candelariella aurella in the clade with Candelaria s. str., Candelariella s. str. and C. antennaria + C. aggregata (we found the position of the C. aurella + C. plumbea group to be unstable and left it in the polytomy), and they separated out a clade with C. corviniscalensis C.A. Morse & M. Westb., C. kansuensis and C. rosulans in a position basal to the polytomy (some versions of our tree had weak support for a similar basal clade which also included C. clarkii and C. spraguei (Tuck.) Zahlbr., but other versions did not, and so the conservative tree we present here leaves these three species unresolved).
Candelariella thus remains paraphyletic, and relationships between various clades of Candelaria, Candelariella, Candelina and Placomaronea remain for the most part unresolved. Notably, we were unable to confirm support for two of the three genera proposed by Kondratyuk et al. (Reference Kondratyuk, Lőkös, Jeong, Oh, Kondratyuk and Hur2020), namely Candelinella and Opeltiella. For example, one of the sequences available for Opeltiella fruticans (Poelt & Oberw.) S.Y. Kondr. (EF535207) appears to be nested within the same clade as Candelinella deppeanae (M. Westb.) S.Y. Kondr. and C. makarevichiae (S.Y. Kondr. et al.) S.Y. Kondr.
The phylogenies published so far (Westberg et al. Reference Westberg, Arup and Kärnefelt2007, Reference Westberg, Frödén and Wedin2009; Kondratyuk et al. Reference Kondratyuk, Lőkös, Jeong, Oh, Kondratyuk and Hur2020; Halıcı et al. Reference Halıcı, Kahraman Yiğit, Bölükbaşı and Güllü2023), although similar overall, nevertheless do not exactly correspond in their topologies, even though individual clades are supported by high bootstrap values. It is difficult to assess what causes these discrepancies. We had hoped that improving clade resolution and overall tree topology might be possible by adding additional loci. Unfortunately, all eight sequences newly generated by Kondratyuk et al. (Reference Kondratyuk, Lőkös, Jeong, Oh, Kondratyuk and Hur2020) remain unavailable. More importantly, of the sequences available in GenBank for different loci (303 ITS, 24 mtSSU and 21 nuLSU), not a single specimen had all three loci sampled. Constructing a multilocus ‘consensus’ tree based on such sparse and poorly overlapping data does not seem warranted, especially since some of the sequences appear to be of low quality. For example, a BLAST search with the mtSSU sequence of Candelariella terrigena (DQ986884) retrieves Porina. In addition, the mtSSU sequence for Candelaria concolor (MN508267) is missing numerous base pairs in nucleotide regions which are otherwise well conserved for other mtSSU sequences of Candelariaceae. This suggests that attempts to accurately resolve the overall phylogeny for the family remain premature without adding additional sequences from a broader array of loci. Whether any of the new genera proposed by Kondratyuk et al. (Reference Kondratyuk, Lőkös, Jeong, Oh, Kondratyuk and Hur2020) deserve recognition cannot be assessed without access to the specimens, sequences and alignments used in that study.
With regard to the species newly described here, our phylogenetic analysis suggests that they form a well-supported, monophyletic clade within Placomaronea. However, distinguishing the two from P. mendozae or recognizing P. fruticosa and P. placoidea as separate species is, unfortunately, not supported by the current topology. Not only are the two species part of a clade that includes P. mendozae, but P. fruticosa is also nested inside a clade that includes both P. placoidea and P. mendozae. Based on the molecular data, the hypothesis that the three taxa might be conspecific cannot therefore be rejected. Our phylogeny instead suggests that the conspicuously different growths forms could be interpreted as environmental modifications of a single, morphologically extremely plastic species: P. mendozae.
However, evolutionary processes such as introgression or incomplete lineage sorting could also explain this discrepancy, particularly in rapidly evolving lineages (Tremble et al. Reference Tremble, Suz and Dentinger2019). These processes are known to result in polyphyly within trees that are based on the analysis of a single gene only, even after speciation (Maddison & Knowles Reference Maddison and Knowles2006). Using a small number of, or even single, loci to construct a phylogeny can result in a topology where the gene tree is incongruent with the overall species tree (Górniak et al. Reference Górniak, Szlachetko, Olędrzyńska, Naczk, Mieszkowska, Boss and Ziętara2021). Such phenomena ultimately can be resolved only by expanding the scope of the molecular analysis to include additional loci and additional specimens, yielding a consensus tree which more accurately reflects the overall phylogeny of the species (Maddison & Knowles Reference Maddison and Knowles2006). Within a single gene tree, polymorphisms from an ancestor may persist in a new lineage for some time, even after species divergence (Maddison Reference Maddison1997). Relying on a single gene to construct a phylogeny, the parent population may then appear to be paraphyletic with respect to its descendants (Carstens & Knowles Reference Carstens and Knowles2007). Monophyly of the single gene tree may eventually be restored via lineage sorting and extinction, but typically only after sufficient time has passed. It has been argued that this depends on generation time and effective population size of the species involved (Knowles & Carstens Reference Knowles and Carstens2007). In such a situation, accurately inferring the species tree by molecular phylogenetic reconstruction requires increased sampling, adding both individuals and additional loci (Maddison & Knowles Reference Maddison and Knowles2006). It is not inconceivable that some of these processes are also responsible for the phylogeny inferred here.
Our analysis, where a phylogeny is based solely on ITS but is also conspicuously incongruent with distinct anatomical and morphological characters, suggests that ITS may not offer sufficient information to accurately resolve the correct phylogeny of these species. Even though nrITS is now widely accepted as the universal barcoding region for fungi (Nagy et al. Reference Nagy, Kocsubé, Csanádi, Kovács, Petkovits, Vágvölgyi and Papp2012; Schoch et al. Reference Schoch, Seifert, Huhndorf, Robert, Spouge, Levesque, Chen and Consortium2012; Xu Reference Xu2016), its effectiveness in reliably distinguishing some fungal lineages is increasingly being questioned (Badotti et al. Reference Badotti, de Oliveira, Garcia, Vaz, Fonseca, Nahum, Oliveira and Góes-Neto2017; Parks et al. Reference Parks, Kurylo, Batchelder, Vincent and Blanchard2019). Some authors suggest that this insufficient resolution can indeed be a result of incomplete lineage sorting or hybridization (Pino-Bodas et al. Reference Pino-Bodas, Martin, Burgaz and Lumbsch2013; Tremble et al. Reference Tremble, Suz and Dentinger2019).
Lack of sufficient ITS resolution has been documented for a variety of fungal lineages (O’Donnell & Cigelnik Reference O’Donnell and Cigelnik1997; Nilsson et al. Reference Nilsson, Kristiansson, Ryberg, Hallenberg and Larsson2008; Thiery et al. Reference Thiery, Vasar, Jairus, Davison, Roux, Kivistik, Metspalu, Milani, Saks and Moora2016; Hughes et al. Reference Hughes, Tulloss and Petersen2018; Kruse et al. Reference Kruse, Piątek, Lutz and Thines2018a, Reference Kruse, Dietrich, Zimmermann, Klenke, Richter, Richter and Thinesb ; Tremble et al. Reference Tremble, Suz and Dentinger2019; Stadler et al. Reference Stadler, Lambert, Wibberg, Kalinowski, Cox, Kolařík and Kuhnert2020), including some that are lichenized (Pino-Bodas et al. Reference Pino-Bodas, Martin, Burgaz and Lumbsch2013; Moncada et al. Reference Moncada, Reidy and Lücking2014; Steinová et al. Reference Steinová, Košuthová and Škaloud2022; Di Meglio & Goward Reference Di Meglio and Goward2023). It has been suggested that, even within groups of lichenized fungi where ITS generally offers good resolution (e.g. Magain & Sérusiaux Reference Magain and Sérusiaux2015; Moncada et al. Reference Moncada, Sipman and Lücking2020), augmenting the data with secondary barcodes will sometimes be necessary, particularly when dealing with rapidly evolving lineages (Lücking et al. Reference Lücking, Aime, Robbertse, Miller, Ariyawansa, Aoki, Cardinali, Crous, Druzhinina and Geiser2020).
Morphology
Despite inconclusive molecular data, accepting Placomaronea mendozae as one single, highly polymorphic species seems untenable. Both P. fruticosa and P. placoidea are characterized by a thallus morphology conspicuously different from P. mendozae.
It could be argued, of course, that the two new species are simply environmentally induced morphotypes. Countless examples of dimorphic lichens are known where a basal crustose or squamulose primary thallus gives rise to a secondary fruticose thallus. However, these transitions are easily recognized when studying specimens and no such transitional morphotypes could be observed here: Placomaronea mendozae is composed of minute, scattered subsquamulose to barely squamulose, broadly attached, gomphate areoles. Even exuberant specimens are barely squamulose, not closely aggregated, the squamules broadly attached to their substrate. The thalli are not hollow like the placodioid lobes of P. placoidea, and unlike P. fruticosa, subsquamulose areoles of P. mendozae do not show any tendency to become fruticose.
Crustose, placodioid lichens transitioning into coralloid fruticose lichens have been described from distantly related species in Caloplaca s. lat. Along the Pacific coast of Baja California and Baja California Sur in Mexico, Polycauliona thamnodes (Poelt) Arup et al. forms placodioid thalli in less exposed microhabitats, but transitions into distinctly coralloid fruticose outgrowths at more exposed sites. In the Atacama of southern Peru and northern Chile, Follmannia orthoclada (Zahlbr.) Frödén et al. also transitions from distinctly placodioid into entirely fruticose specimens. The crustose thalli are found at the most sheltered sites, and the fruticose ones at the most exposed habitat sites. Obviously, the morphology of these two not closely related species is highly plastic; it can be interpreted as fog-induced and is clearly an adaptation of the morphology to microhabitat variation at the sites where these species grow.
Interpreting the different morphologies of P. fruticosa and P. placoidea as also being environmentally induced, however, ignores how these thalli presumably arose. Both species have been found in ecologically similar areas; Placomaronea placoidea grows on the surface of rocks embedded in soil, and these surrounding soils are inhabited by thalli of P. fruticosa, which develop as an adaptation to the soil in which the species grows. At these sites the loamy soil is hardened and compact and if the lichen thalli were not ‘rooted’ deeply inside their substrate these lichens could not compete with grasses. Thalli of P. placoidea growing nearby obviously cannot penetrate the rock they grow on and any tendencies to develop a fruticose growth form would necessarily result in erect thalli emerging from the rock surface. Such thalli have, however, not been observed. Transitional forms, where lobes of P. fruticosa creep onto rocks from the surrounding soil, have also not been found. Both species thus maintain their distinct morphologies, even when growing side by side. Both also maintain distinct anatomical differences. The lobes of P. placoidea consistently have a hollow medulla, whereas the fruticose stalks of P. fruticosa as well as its placodioid lobes on the substrate surface are solid.
Conclusion
Our decision to describe Placomaronea placoidea and P. fruticosa at species level follows recent guidelines published by Lücking et al. (Reference Lücking, Leavitt and Hawksworth2021). Both form nested (paraphyletic residual) clades that are morphologically and anatomically distinct. The geographical distributions of the new species overlap with one another (Fig. 10) and with that of P. mendozae. At this stage, with only a small number of specimens known, it is possible that all three are either fully sympatric or, more likely, P. placoidea and P. fruticosa have a nested, more limited distribution within the broader range of one another and, by comparison, also within the range of the much more widely distributed P. mendozae. In any case, the three species are clearly not allo-, para- or peripatric. According to the categorization schema distinguishing main patterns of phylogenetic topologies in Lücking et al. (Reference Lücking, Leavitt and Hawksworth2021, p. 130, fig. 10), Placomaronea placoidea and P. fruticosa should therefore be recognized not as subspecies, but as species.
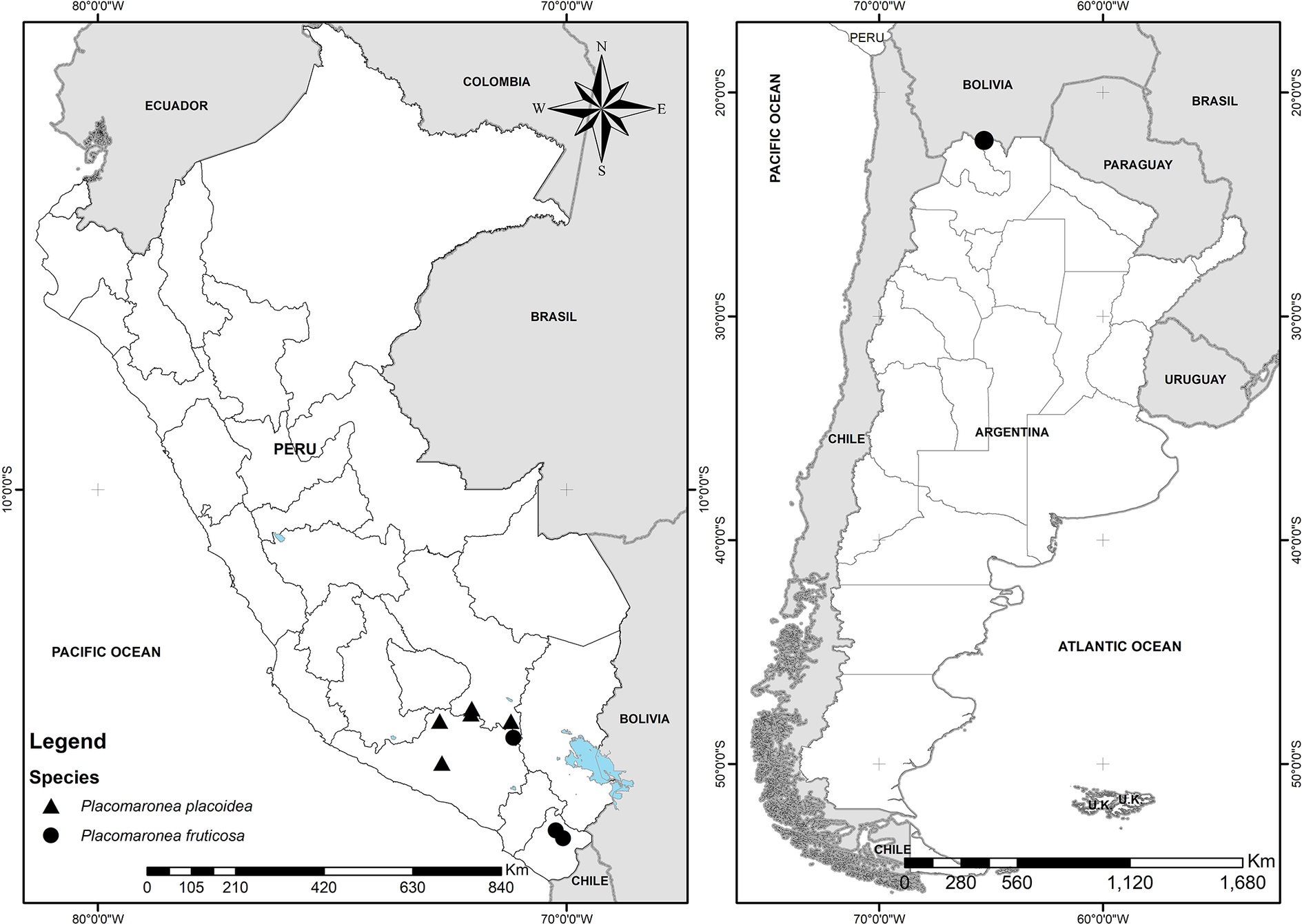
Figure 10. Known distribution of Placomaronea fruticosa (circles) and P. placoidea (triangles) in Peru and Argentina. In colour online.
Supplementary Material
The Supplementary Material for this article can be found at http://doi.org/10.1017/S0024282925000040.
Acknowledgements
We appreciate the help of our colleagues in collecting specimens in the field, especially Melvi Larico, Claudia Sanz and Martín Flores, as well as Dr Victor Quipuscoa (Instituto IMOD, Perú) for providing facilities both during fieldwork and at the Institute. Dr Mónica Arakaki (Herbario USM, Perú) helped in the laboratory with extracting and sequencing DNA; without her guidance the first author would not have learned the techniques required to obtain the molecular sequences generated in this study. Martin Wojciechowski (ASU) provided very helpful advice on the phylogenetic analyses. Riley McInnis and Alexandra Dubé (both ASU) ran the thin-layer chromatography plates of the specimens studied here. John A. Elix, Canberra, suggested that the unidentified pulvinic acid derivative observed at R f 3 in solvent C most likely corresponds to 4-hydroxypulvinic acid. Based on a catalogue of lichen secondary metabolites provided by John A. Elix, Daryl Lafferty built the software program Mytabolites, used here for the interpretation of thin-layer chromatography plates. We are grateful to the curators and collection managers of COLO, LSU, MSC, NY and WIS for providing loans of Candelariaceae specimens for comparison with the material collected by the first author in Peru. We thank Martin Westberg (Uppsala) and two anonymous reviewers for their constructive criticism helping to improve the original manuscript.
Author ORCIDs
Daniel Ramos, 0000-0003-4890-3180; Jason Hollinger, 0000-0003-2465-2487; Frank Bungartz, 0000-0002-0717-9264.














