Introduction
Sterile oat [Avena sterilis L. ssp. ludoviciana (Durieu) Gillet & Magne], a member of the Poaceae family, is a fast-growing annual grass commonly found in disturbed environments such as agricultural fields, roadsides, and grasslands (Loskutov et al. Reference Loskutov, Gnutikov, Blinova and Rodionov2023). Like other weeds in the Poaceae family, A. sterilis is a strong competitor for water, nutrients, and light due to its dense leaf cover; robust tillering ability; tall growth habit; allelopathic properties; and ecological, biological, and botanical similarities to cereals (Hasanfard et al. Reference Hasanfard, Rastgoo, Darbandi, Nezami and Chauhan2022). This species can tolerate high temperatures and arid conditions, making it a formidable competitor in hot, dry environments. In winter wheat (Triticum aestivum L.) fields across Iran, A. sterilis is considered a problematic weed that competes with wheat for essential resources, thereby reducing crop yields—particularly in regions with limited irrigation (Hasanfard et al. Reference Hasanfard, Rastgoo, Darbandi, Nezami and Chauhan2022; Nosratti et al. Reference Nosratti, Sabeti, Chaghamirzaee and Heidari2020). Armin and Asghripour (Reference Armin and Asghripour2011) demonstrated that varying densities of A. sterilis significantly reduced wheat yields in Iran. For instance, densities of 25, 50, and 75 plants m−2 resulted in yield reductions of 10%, 19%, and 20%, respectively. The species’ ability to withstand both drought and high temperatures further complicates weed management in these agricultural systems. Moreover, its capacity for rapid germination and growth enables A. sterilis to outcompete wheat, especially under resource-limited conditions, exacerbating the challenges faced by farmers.
Understanding the germination behavior of weed seeds in response to temperature and drought is essential for developing effective weed management strategies in both agricultural and natural ecosystems (Anwar et al. Reference Anwar, Islam, Yeasmin, Rashid, Juraimi, Ahmed and Shrestha2021; Toscano et al. Reference Toscano, Romano, Tribulato and Patanè2017). Temperature and moisture are critical environmental factors that influence seed germination (Dürr et al. Reference Dürr, Dickie, Yang and Pritchard2015). Different weed species exhibit varying levels of sensitivity to these conditions; some may require specific temperature ranges to initiate germination, while others may be more resilient to drought stress. By exploring how these factors interact, land managers can devise targeted approaches to control weed populations, thereby minimizing their impact on crop yields and native plant communities. This knowledge enables timely interventions, such as adjusting planting schedules or employing specific irrigation techniques to reduce competition from weeds. Furthermore, understanding the dynamics of seed germination can help predict weed emergence patterns, allowing for proactive management before weed populations become problematic (Ramesh Reference Ramesh2015). Overall, integrating this knowledge into management practices not only enhances agricultural productivity but also supports biodiversity conservation in natural ecosystems.
The geographic origin of weed seeds such as A. sterilis significantly influences their germination characteristics, including germination percentage and rate. Seeds from different regions may adapt to local environmental conditions, such as temperature, moisture, and seasonal variations. For instance, seeds from colder climates might exhibit a longer dormancy period to prevent premature germination, while those from warmer or more stable climates may germinate more quickly and uniformly. In a study assessing nine common weed species through temperature gradient experiments, it was observed that summer annuals exhibited higher base temperatures for germination compared with winter annuals (Steinmaus et al. Reference Steinmaus, Prather and Holt2000).
Salinity is one of the major abiotic stresses that adversely affects seed germination by reducing water uptake, lowering osmotic potential, and causing ion toxicity. These effects can lead to a decline in both the rate and percentage of germination through cellular damage and metabolic disruption. Amini et al. (Reference Amini, Hasanfard, Ahmadian and Yuzband2024) found that increasing NaCl concentration significantly reduced the germination percentage of Cichorium glandulosum Boiss. et Huet seeds in both the Tabriz and Marand populations. In Tabriz, germination remained around 90% up to 2 dS m−1 but decreased as salinity levels exceeded this threshold. In contrast, Marand seeds exhibited 95% germination under nonsaline conditions, with a marked decline when salinity surpassed 1 dS m−1. Both populations demonstrated nearly zero germination at 10 and 12 dS m−1. Factors such as soil type, photoperiod, and rainfall patterns can shape the germination traits of A. sterilis, leading to variations in seed viability and overall germination behavior based on their geographic origin. Therefore, examining the germination responses of A. sterilis populations from different regions can help identify how these populations may vary under diverse environmental conditions and predict their germination patterns. The primary objective of this study is to examine the germination responses of two A. sterilis populations originating from different geographic regions (Mashhad and Tabriz) under various environmental conditions, including temperature, humidity, salinity, and light requirement. By testing the hypothesis that both environmental factors and regional origin significantly influence germination behavior, this research aims to provide insights into the role of local adaptation in seed responses to abiotic stressors.
Materials and Methods
Study Design, Seed Population, and Climate
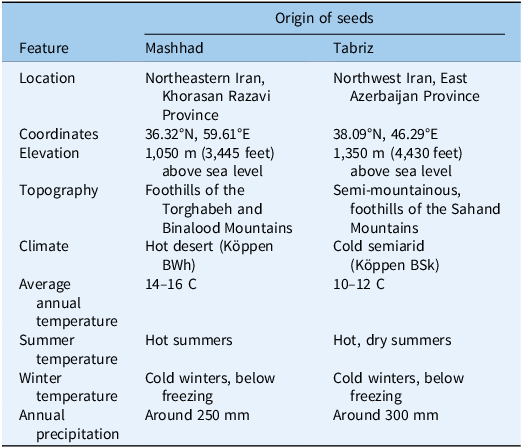
This study was conducted twice, in 2020 and 2021, at the greenhouse research laboratory of the Faculty of Agriculture at Ferdowsi University of Mashhad in Iran. It was designed as a factorial experiment utilizing a completely randomized design with six replications. The study employed two populations of A. sterilis collected from the margins of wheat fields in northeastern Iran (Mashhad) and northwestern Iran (Tabriz) (Table 1). Mashhad, located in Razavi Khorasan, has a semiarid climate with hot summers and cold winters, receiving approximately 250 mm of rainfall annually. This region is known for cultivating wheat, barley (Hordeum vulgare L.), and saffron (Crocus sativus L.). Due to the cold and dry winters, wild oats (Avena sterilis), a winter weed, particularly thrives in cereal fields, such as wheat and barley, in this area. The autumn and winter rainfall in Mashhad, combined with suitable temperatures, provides ideal conditions for the growth and propagation of A. sterilis. This weed, which can grow well under drought and cold winter conditions, can pose strong competition to agricultural crops, negatively affecting wheat and barley yields. In contrast, Tabriz, in East Azerbaijan Province, has a continental climate with cold winters and hot summers, receiving about 300 mm of rainfall annually. While the autumn and winter rainfall in Tabriz is higher than in Mashhad, the severe cold and specific climatic conditions prevent A. sterilis from spreading as extensively as in Mashhad, resulting in a more limited competition with agricultural products. Given that the interaction between temperature and drought is particularly common, especially in Iran’s climate (Rastgoo and Hasanfard Reference Rastgoo and Hasanfard2021), we specifically focused on the interaction between these two factors.
Table 1. Geographic locations and climatic characteristics of seed collection origins.
Seed Preparation and Dormancy-breaking Treatment
In this study, the preparation of A. sterilis seeds involved the careful removal of lemma and palea, followed by the elimination of empty and damaged seeds. To break seed dormancy, a stratification treatment was employed through a moist-chilling process. Over a period of 3 d, the seeds were subjected to a constant temperature of 5 C in a dark environment, effectively mimicking their natural winter conditions. This treatment successfully facilitated germination and ensured optimal growth in the subsequent stages.
Seed Disinfection and Germination
For the experimental setup, 10 viable seeds were selected and placed in 9-cm-diameter petri dishes lined with Whatman No. 1 Filter Paper. Before the experiments, seeds were disinfected using a 5% sodium hypochlorite solution for 3 to 5 min at room temperature, followed by thorough rinsing with distilled water. Each petri dish was supplied with 10 ml of a prepared solution corresponding to the designated drought level (Rastgoo et al. Reference Rastgoo, Izadi Darbandi, Hasanfard and Mijani2023). To maintain precise temperature control, the germinator was pre-adjusted to the required temperature settings 1 d before the initiation of the thermal cycle. Once the temperature fluctuations were confirmed to be within ±1 C, the petri dishes were transferred into the germinator (Grouc Engineering Design, Tehran, Iran), ensuring exposure to the desired temperature in dark conditions throughout the experiment.
Temperature and Osmotic Potential Levels
The experiment was conducted separately for each of the factors: temperature by osmotic potential, salinity, and light/dark regimes. The selected levels of temperature, osmotic potential, and salinity were determined based on regional climate characteristics and soil conditions, whereas the light/dark regimes were informed by the general daylength patterns observed in the native habitats of the studied populations. In this study, we focused on the interaction between temperature and osmotic potential, as these two factors play a fundamental role in seed germination, particularly under conditions of environmental stress. Given their strong interdependence, a more detailed analysis was necessary to understand their combined effects on the germination of A. sterilis. The experimental treatments consisted of six temperature levels (5, 10, 15, 20, 25, and 30 C) and five levels of osmotic potential, which were established using polyethylene glycol 6000 (PEG 6000) (Rastgoo et al. Reference Rastgoo, Izadi Darbandi, Hasanfard and Mijani2023). PEG is widely used to create controlled osmotic potential in experiments, as it effectively reduces water availability in a solution without interfering with the plants’ nutrient uptake. The osmotic potential levels were set at 0, −0.3, −0.6, −0.9, and −1.2 MPa by dissolving varying concentrations of PEG in the solution.
Salinity Stress
Sodium chloride (NaCl) solutions with salinity concentrations of 0 (control), 1, 2, 3, 4, 5, 6, 8, 10, and 12 dS m−1 were prepared to establish 10 distinct levels of salinity stress (Poljakoff-Mayber et al. Reference Poljakoff-Mayber, Somers, Werker and Gallagher1994).
Light/Dark Regimes
The effect of various light/dark cycles, including 24/0, 12/12, 10/14, 8/16, and 0/24 h at 20/10 C, on the germination of A. sterilis seeds was monitored daily for 18 d following treatment. The light intensity of 156 μmol m−2 s−1 was supplied by fluorescent lamps in a growth chamber. To assess germination in the absence of light, the petri dishes were covered with two layers of aluminum foil (Baskin and Baskin Reference Baskin and Baskin1998).
Calculations and Statistical Analysis
The counting of germinated seeds continued for 23 d, until the number of germinating seeds became constant. Subsequently, the percentage and rate of germination were calculated using Equations 1 and 2. Additionally, the time needed to achieve 50% germination was determined by fitting a logistic model of cumulative germination percentage over time. Statistical analysis of the data was conducted using SAS v. 9.4 (SAS Institute, Cary, NC, USA) and GraphPad Prism software (version 8.0.1, La Jolla, CA, USA.
where GP represents the percentage of germination, n stands for the number of germinated seeds, and N denotes the total number of seeds.
In this equation, R50 represents the germination rate, and D50 indicates the time needed to achieve 50% of the final germination.
Cumulative germination graphs, along with parameters for maximum germination and the time required to reach 50% germination at various temperatures and osmotic potentials, were obtained through nonlinear regression analysis using a three-parameter log-logistic equation (Equation 3).
where Y represents the response rate (cumulative germination) at time x, d denotes the upper limit for Y, b indicates the slope of the curve at point e, and e is the time required to achieve 50% germination (in days).
For germination data analysis, an ANOVA was performed using SAS v. 9.4. Differences between treatments (temperature, osmotic potential, salinity, and light/dark regimes) were assessed using the standard error of the mean (SEM) values. Data from both runs were pooled, as no significant differences were observed between runs (P > 0.05). Cumulative germination graphs were generated using GraphPad Prism software (v. 8.0.1, La Jolla, CA, USA).
Results and Discussion
Temperature and Osmotic Potential
Cumulative Germination
The interaction among population, temperature, and osmotic potential significantly affected the germination percentage (P < 0.01). As temperature increased and osmotic potential decreased (indicating increased drought stress), the germination percentage of both populations decreased (Figures 1 and 2). The highest germination percentages (99% and 100%, respectively) were observed in the Mashhad population at 10 and 15 C and 0 MPa osmotic potential (Figure 1; Table 2). The Tabriz population exhibited the highest germination percentages (97% and 96%, respectively) at 15 and 20 C and 0 MPa osmotic potential (Figure 2; Table 3).
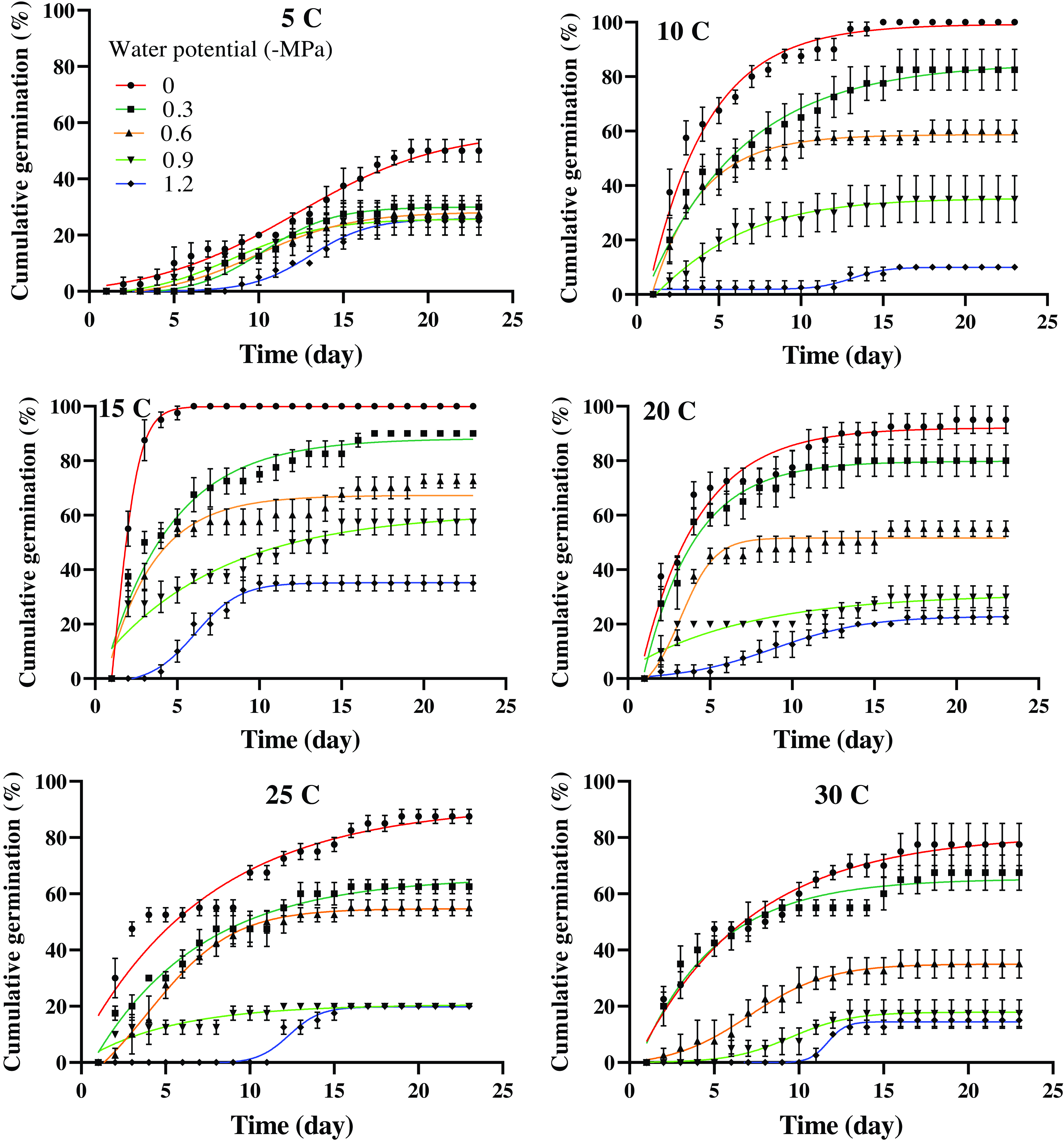
Figure 1. Cumulative germination of Avena sterilis in the Mashhad population over 23 d in response to temperature and osmotic potential. The vertical bars represent the standard error (±SE). The parameter estimates for the model are presented in Table 2.
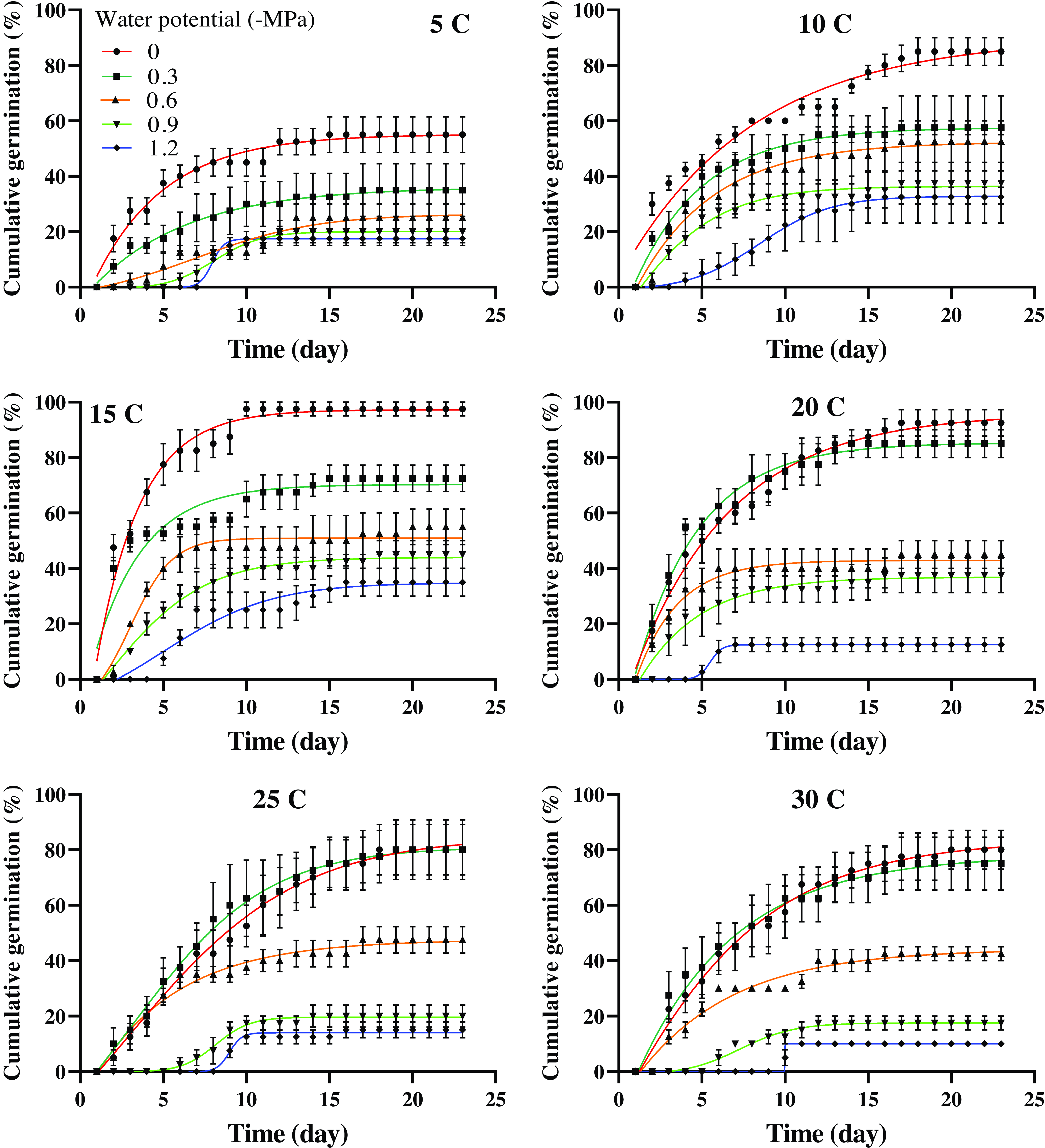
Figure 2. Cumulative germination of Avena sterilis in the Tabriz population over 23 d in response to temperature and osmotic potential. The vertical bars represent the standard error (±SE). The parameter estimates for the model are presented in Table 3.
Table 2. Nonlinear regression analysis for water potential and temperature in the Avena sterilis population of Mashhad a .
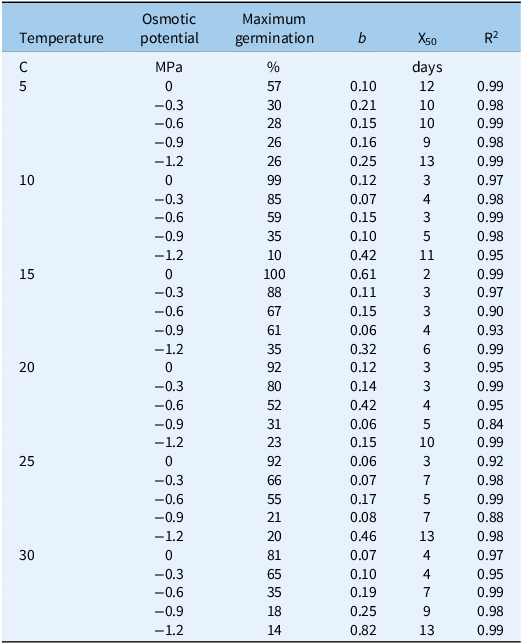
a Abbreviations: b, slope; X50, the time required to reach 50% germination; R2, the coefficient of determination.
Table 3. Nonlinear regression analysis for water potential and temperature in the Avena sterilis population of Tabriz a .
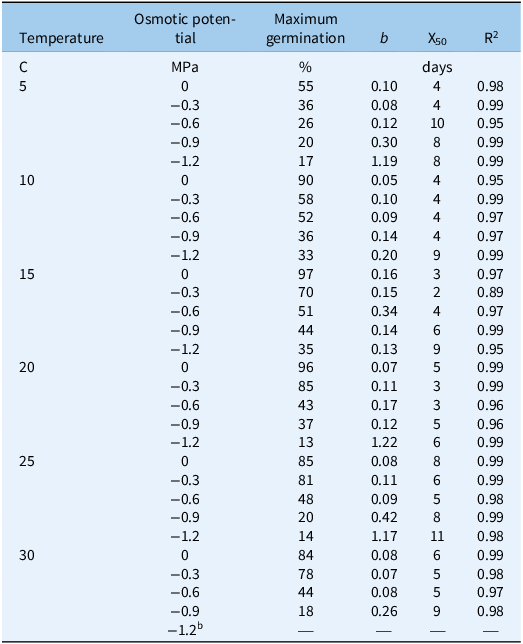
a Abbreviations: b, slope; X50, the time required to reach 50% germination; R2, the coefficient of determination.
b Lack of proper fit.
The observed decline in germination percentages as drought stress and temperatures increased highlights the significant impact of abiotic stress on seed germination, an essential phase in the life cycle of plants. Both drought stress and elevated temperatures are known to negatively affect seed viability and germination rates, with water scarcity hindering seed hydration and temperature extremes potentially disrupting enzymatic activity and cellular functions necessary for germination (Abdellaoui et al. Reference Abdellaoui, Boughalleb, Zayoud, Neffati and Bakhshandeh2019; Bewley et al. Reference Bewley, Bradford, Hilhorst and Nonogaki2013).
The comparison between the Mashhad and Tabriz populations suggests that these populations have different adaptive strategies or tolerance levels to these abiotic stress factors. The Mashhad population exhibited the highest germination percentage under optimal conditions of 0 MPa osmotic potential and cooler temperatures. This indicates that the Mashhad population might be adapted to a more temperate or moisture-rich environment, where lower temperatures and higher water availability are common. Conversely, the Tabriz population exhibited a similar decline in germination as drought stress and temperature increased. However, the Tabriz population occasionally demonstrated the ability to tolerate slightly higher temperatures, suggesting that the Tabriz population may be better adapted to a warmer, potentially more arid environment.
This population-level variation in germination traits likely reflects local adaptation to contrasting environmental conditions. In Mashhad, cooler temperatures and more consistent moisture availability may have selected for traits that enhance germination under lower temperature and higher water conditions. In contrast, the Tabriz population may have experienced stronger selective pressures related to heat and water scarcity, possibly including episodic drought and increased salinity, which are common in semiarid and arid regions. These environmental factors may have driven the evolution of increased thermal tolerance or more conservative germination strategies in this population. These findings imply that local environmental conditions such as temperature and water availability may drive the evolution of specific stress-tolerance mechanisms in plant populations. The Mashhad population might have evolved mechanisms that prioritize survival in cooler, wetter climates, while the Tabriz population could be better suited to withstand the higher temperatures and potentially drier conditions typical of its native environment. Thus, the observed differentiation in germination behavior may be a product of ecological divergence driven by climate variability, water availability, and, potentially, soil salinity. In various regions, distinct microclimates may exist, each influencing environmental effects (Wong et al. Reference Wong, Lai, Low, Chen and Hart2016). This suggests that the average temperature and humidity conditions in cities such as Tabriz or Mashhad may vary across specific areas within these regions. For instance, while Tabriz is generally cooler and more humid, some parts of the region might experience higher temperatures and increased dryness during the summer, to which the seeds of the Tabriz population may have specifically adapted. Future studies could further explore these patterns by linking environmental data from the native habitats of each population with trait-based responses in controlled experiments.
Such variations in stress tolerance are important for understanding how populations might respond to climate change, which is expected to intensify both drought conditions and temperature extremes. They also emphasize the importance of considering population-specific responses to environmental stress when designing conservation strategies or when assessing the ecological resilience of species in changing climates. The study by Saburi-Rad et al. (Reference Saburi-Rad, Kafi, Nezami and Bannayan Aval2012) demonstrated that the highest germination percentage in kochia [Bassia scoparia (L.) A.J. Scott] seeds occurred at temperatures between 20 and 25 C and at osmotic potentials of −0.25, −0.50, −0.75, and −1 MPa.
Time Required to Reach 50% Germination
The shortest time required to achieve 50% germination was observed in the Mashhad population at 15 C and 0 MPa osmotic potential (2 d) (Figure 1; Table 2), as well as in the Tabriz population at 15 C and −0.3 MPa osmotic potential (2 d) (Figure 2; Table 3). According to Figure 1, the time required to achieve 50% germination in the Mashhad population at 5 C ranged from 9 to 13 d, which was the longest duration compared with other temperatures. In the Tabriz population at 25 C, this duration ranged from 5 to 11 d (Figure 2).
The geographic distribution of the Mashhad and Tabriz populations is closely linked to their germination characteristics, particularly their responses to water availability. The Tabriz population, which shows rapid germination at slightly negative osmotic potentials (−0.3 MPa), may be better suited to drier, more arid regions with limited water resources. This ability to tolerate low water availability could increase its geographic spread in such environments. On the other hand, the Mashhad population, which germinates efficiently at 0 MPa, may thrive in regions with intermittent or seasonal droughts. The differing drought-tolerance strategies of these populations highlight how local environmental conditions influence seed germination, survival, and, ultimately, the distribution of these populations. The ability to withstand dry conditions could allow these populations to colonize a wider range of habitats, driving their geographic expansion.
Germination Rate
The interaction among population, osmotic potential, and temperature significantly affected the germination rate (P < 0.05). The highest germination rate was observed in the seeds of Mashhad and Tabriz populations at 15 C and at osmotic potentials of 0 MPa and −0.3 MPa, respectively (0.52 and 0.48 seeds d−1, respectively) (Table 4).
Table 4. The germination rates of two Avena sterilis populations from Mashhad and Tabriz.
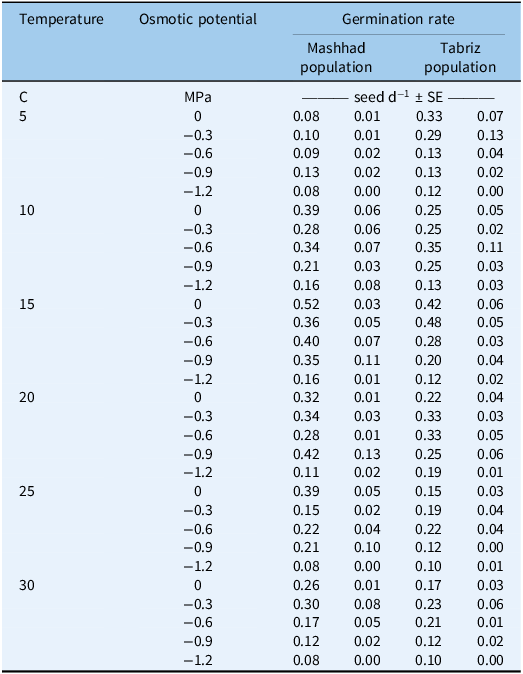
These findings suggest that the Mashhad population may be more sensitive to drought stress during germination than the Tabriz population. In a similar study, the Turkish red pine (Pinus brutia Ten.) provenances (Burdur/Bucak) exhibited the highest germination rate under an osmotic potential of −0.2 MPa (Koç Reference Koç2021).
Salt Stress
The germination rate was significantly influenced by the interaction between population and NaCl concentration (P < 0.01). Increasing the NaCl concentration decreased the germination percentage of A. sterilis seeds in both the Mashhad and Tabriz populations (Figure 3). The rate of germination reduction was steeper for the Tabriz population (Hill slope = −0.36) than for the Mashhad population (Hill slope = −0.34). At all NaCl concentrations, the germination rates in the Mashhad population were consistently higher than those in the Tabriz population. The NaCl concentration required for 50% inhibition of seed germination was 4.76 dS m−1 for the Mashhad population and 3.90 dS m−1 for the Tabriz population (Figure 3).
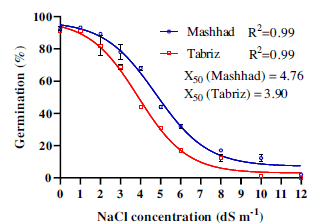
Figure 3. Effect of sodium chloride (NaCl) concentration on the germination percentage of Avena sterilis populations from Mashhad and Tabriz. X50 indicates the concentration of NaCl required to inhibit 50% of germination. The vertical bars represent the standard error (±SE). The equations (four-parameter log-logistic) for the populations of Mashhad and Tabriz are:
The observed decrease in germination percentage with increasing NaCl concentration aligns with previous studies highlighting the detrimental effects of salinity on seed germination (Nikolić et al. Reference Nikolić, Ghirardelli, Schiavon and Masin2023). However, the more pronounced reduction in germination rates for the Tabriz population suggests a differential tolerance to salinity between the two populations. The steeper slope observed in the Tabriz population indicates a heightened sensitivity to salt stress, meaning that even at lower NaCl concentrations, Tabriz seeds experience a more rapid decline in germination compared with Mashhad seeds. The population from Mashhad exhibited relatively higher germination rates across all salinity levels, indicating better adaptation or greater intrinsic tolerance to salt stress. The lower concentration of NaCl required to achieve 50% inhibition of germination in the Tabriz population further supports the notion that this population is more sensitive to salt. These findings suggest local adaptation to environmental conditions, where the Mashhad population may be more frequently exposed to saline environments, either naturally or through agricultural practices, resulting in enhanced salt tolerance. In contrast, the Tabriz population may be adapted to less-saline conditions, which accounts for its increased sensitivity to salt. This underscores the importance of understanding population-level variations in salinity tolerance for improved crop management and breeding strategies in areas susceptible to salinity (Amini et al. Reference Amini, Hasanfard, Ahmadian and Yuzband2024).
Light/Dark Regimes
The germination rate was significantly affected by the interaction between population and light/dark regime (P < 0.01). In both populations, the highest germination percentage was observed under a light/dark regime of 10/14 h (Figure 4). The lowest germination percentages were recorded in the Mashhad (13%) and Tabriz (10%) populations under continuous darkness (0/24-h light/dark) (Figure 4). Germination rates under alternating light/dark regimes ranged from 64% to 93% in the Mashhad population and from 35% to 91% in the Tabriz population (Figure 4).
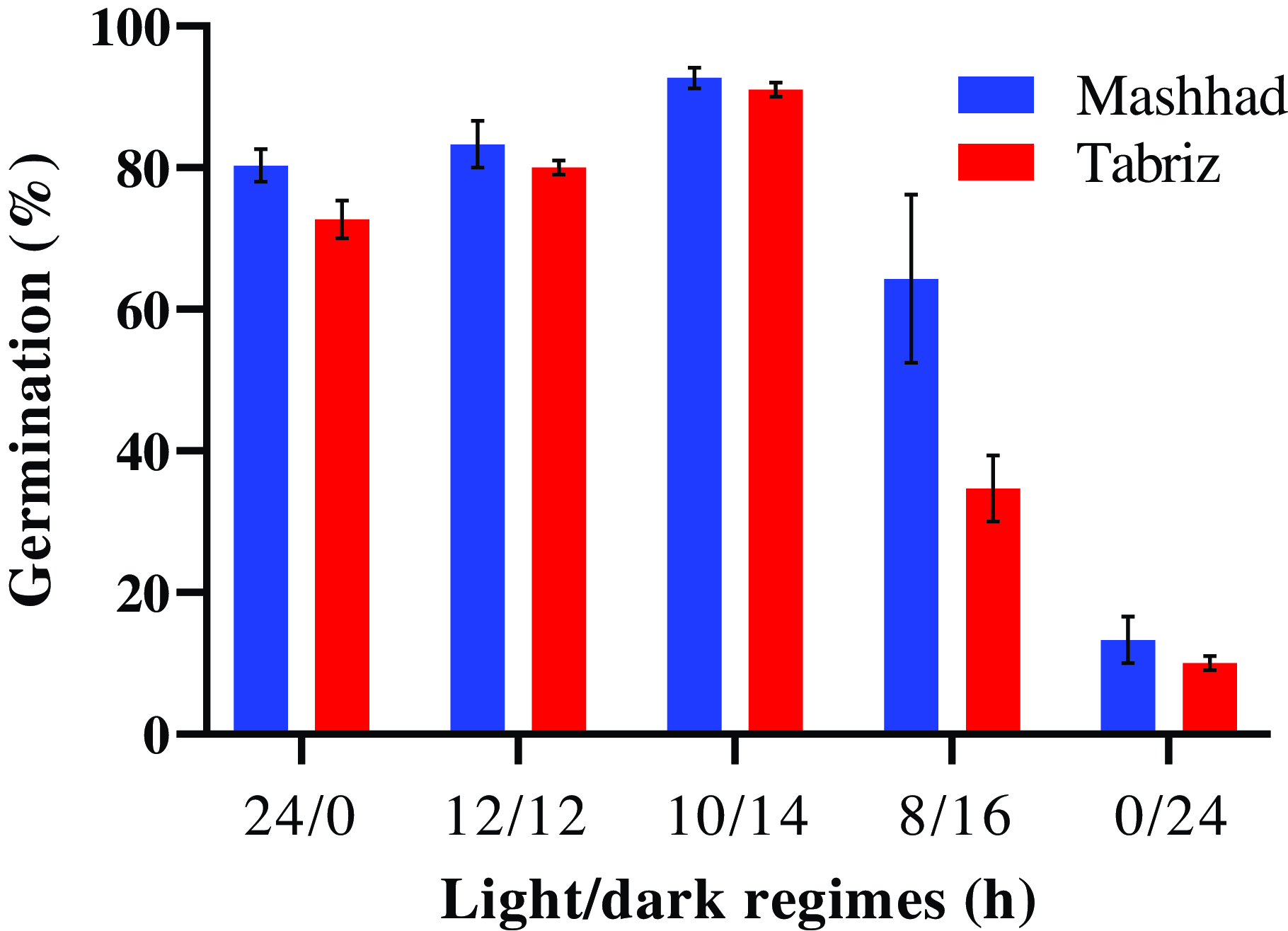
Figure 4. Effect of light/dark regimes on the germination percentage of Avena sterilis populations from Mashhad and Tabriz. The vertical bars represent the standard error (±SE).
These results indicate that a period of darkness followed by light exposure, which simulates natural environmental conditions, is optimal for seed germination. Light plays a crucial role in regulating seed dormancy and germination through phytochromes, which are sensitive to light (Amini et al. Reference Amini, Gholami and Ghanepour2017; Yang et al. Reference Yang, Liu and Lin2020). The lowest germination percentages observed in both populations under continuous darkness (0/24-h light/dark) underscore the essential role of light in the germination process. Seeds kept in prolonged darkness may fail to undergo critical physiological changes, such as the breakdown of stored reserves and enzyme activation, which often depend on light. This suggests that both populations are adapted to environments where light significantly influences seed germination, with the Mashhad population potentially demonstrating greater resilience or adaptability to light/dark cycles. The study conducted by Chauhan et al. (Reference Chauhan, Manalil, Florentine and Jha2018) demonstrates that windmill grass (Chloris truncata R. Br.) seeds placed on the soil surface exhibit a higher germination percentage compared with those buried in the soil. This phenomenon can be attributed to the increased availability of light and oxygen during the germination process, which are more accessible at the soil surface.
Although there were differences in the germination ecology of A. sterilis populations between Mashhad and Tabriz, both populations exhibited a strong response to various temperatures, humidity levels, light conditions, and salinity. The variations in how these populations respond to environmental factors may be attributed to local adaptations to the distinct climatic and soil conditions in each region. Factors such as temperature fluctuations, precipitation, and soil composition significantly influence seed dormancy and germination, driving adaptations that enable each population to survive and establish itself in its specific environment. Understanding these germination patterns is essential for developing targeted weed management strategies. For Mashhad, where conditions favor broader germination, early-season herbicide applications, coupled with precise irrigation management to avoid excess moisture, can prevent premature germination. Additionally, implementing crop rotation as a long-term strategy will help reduce A. sterilis proliferation. In Tabriz, where the germination window may be narrower, a more targeted approach utilizing postemergence herbicides, along with mechanical controls such as mowing or plowing, would be more effective in managing established plants. Given the favorable conditions for A. sterilis germination across Iran, its unchecked spread remains a significant concern. Therefore, the integration of these tailored management strategies—incorporating predictive emergence models and optimal herbicide timing—will be crucial to prevent the spread of A. sterilis and minimize its impact on cropping systems and pastures.
Funding statement
This research received no specific grant from any funding agency or the commercial or not-for-profit sectors.
Competing interests
The authors have no conflicts of interest to declare.










