Community-based participatory research (CBPR) is a collaborative model of translational research [Reference Fort, Herr, Shaw, Gutzman and Starren1] that engages community members and researchers as equal partners, focused on addressing community-identified needs to drive policy change.[Reference Lacombe, Bogaerts and Tombal2–Reference Tariq, Grewal and Booth4] Increasingly, early-stage translational researchers partner with affected populations, including community-based organizations (CBOs), to identify and overcome barriers to recruitment and retention [Reference Georas, Wright and Ivanova5,Reference Reyfman, Sugar and Hazucha6]. While such a role is critical, limiting CBOs involvement to recruitment and retention views these organizations’ primary function as serving as an intermediary between researchers and participants and fails to appreciate the full scope of what partner organizations can offer to research collaborations.
We advocate for expanded inclusion of CBOs in designing early-stage translational research as a part of a paradigm shift to designing for dissemination and sustainability (D4DS) [Reference Kwan, Brownson, Glasgow, Morrato and Luke7]. D4DS refers to principles and methods for ensuring the fit between a health innovation and the context before adoption. A co-design process including researchers and CBOs can ensure that innovations are usable by the communities which are the target of the intervention [Reference Kwan, Brownson, Glasgow, Morrato and Luke7–Reference Stock, Ceïde, Lounsbury and Zwerling11]. This report describes a unique example of a community-led summit which brought together patients, community organizations, federal and state organizations, and state legislators to address community concerns regarding Long COVID. To our knowledge, this is the first example of community involvement on a national scale for a study that spans multiple methodologies including pathogenesis, epidemiology, and clinical trials. The NIH Researching COVID to Enhance Recover (RECOVER) Initiative aims to deepen our understanding of Long COVID’s epidemiology and develop effective treatments for affected individuals [Reference Marrazzo, Gibbons and Koroshetz12]. RECOVER is one of the largest studies ever funded by the federal government representing $1.7 billion in investment since its inception in 2021. Here, we highlight how CBOs leveraged their access to scientific experts and healthcare providers to elevate awareness of affected communities’ needs, organizing a national meeting with local, state, and federal policymakers.
The Illinois Research Network (ILLInet) Hub, a collaborator in the RECOVER Adult Cohort Study, operates across five Illinois sites, including two operated by CBOs (Illinois Unidos and Bright Star Community Outreach) [13]. ILLInet includes seven additional CBOs (ASI Home Care Services, Envision Community Service, Teamwork Englewood, Tri-County Urban League, Peoria Friendship House, Chicago Urban League, Central Illinois Friends) who contribute by co-designing and disseminating educational materials on COVID-19 and Long COVID, providing feedback on community-based research activities, and identifying effective communication channels for outreach. These partnerships were essential to ILLInet’s enrolling and conducting follow-up visits in 900 diverse participants (49% non-Hispanic white, 22% non-Hispanic Black, 14% Hispanic, 6% multiple, 5% Asian, 3% unknown).
In the study’s fourth year, three CBOs (ASI Home Care Service, Bright Star Community Outreach, and Illinois Unidos) organized a summit to learn about research findings and engage state policymakers. The summit featured 61 in-person and 367 virtual attendees (Table 1) and included live Spanish interpretation. People with Long COVID, representatives of community organizations, and federal and state government officials served as speakers and discussants on various topics, including the 2024 National Academies’ definition of Long COVID [Reference Ely, Brown and Fineberg14], the NIH RECOVER Initiative (observational studies and clinical trials) [Reference Marrazzo, Gibbons and Koroshetz12], strategies for stakeholder engagement and policy advocacy[Reference Sprague Martinez, Sharma and John15], barriers to Long COVID care[Reference Macpherson, Cooper, Harbour, Mahal, Miller and Nairn16], and the AHRQ Long COVID Care Network [17].
Table 1. Stakeholder groups participating in the long COVID summit. 1
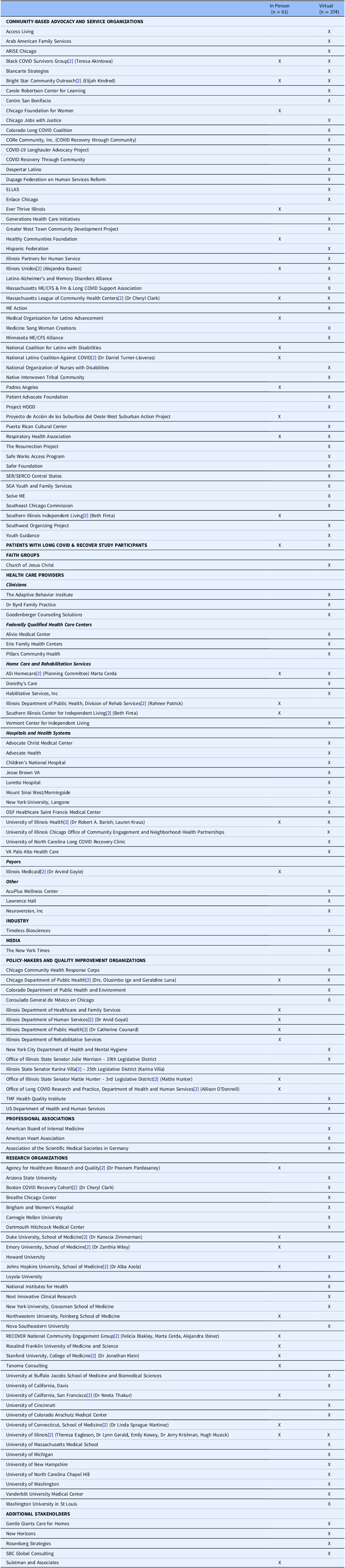
1 Organizations that registered for the Summit are grouped into categories to show the breadth and diversity of participants, but organizations may fit into more than one category. [Reference Lacombe, Bogaerts and Tombal2] Indicates speaker, panelist, or moderator at the Summit.
After the didactic portions of the meeting, in-person summit participants were divided into work groups for in-depth discussion to determine recommendations for actions to improve Long COVID care. The following actions were recommended:
-
1. Engage CBOs or other trusted representatives of affected populations as research partners from the start of studies involving human volunteers to ensure study activities are culturally sensitive, linguistically accessible, and address systemic barriers (e.g., mistrust in medical institutions among people of color).
-
2. Fund CBOs or other trusted representatives to co-design outreach and support (e.g., education, mask distribution), provide navigation services (e.g., disability applications, provider referrals), and share information about research opportunities, including clinical trials.
-
3. Expand the AHRQ Long COVID Care Network and increase funding for training healthcare professionals to effectively diagnose, treat, and advocate for Long COVID patients.
-
4. Support researcher-CBO collaborations to drive policy change and combat misinformation, enhancing trust and promoting health equity.
CBO partners are presenting these recommendations to the Illinois State Legislature which are more likely to be adopted because CBOs and researchers worked together. The recommendations are based on science but also consider the needs and desires of the communities most affected by Long COVID. CBOs have substantial influence in lobbying legislators and public health officials to adopt and sustain programs and policies.
We conclude that the current model of CBPR should be expanded to support greater reciprocity between early-stage translational research and affected populations. By collaborating closely with CBOs and other representatives, researchers can go beyond traditional recruitment and retention goals to conduct studies that address knowledge gaps and are designed to promote trustworthiness in both the research process and study results. CBOs can be valuable collaborators in co-designing research questions and implementing studies. These partnerships enable researchers to gain valuable insight into community needs, ensure culturally and contextually appropriate study designs, provide mechanisms for communities to engage meaningfully in research, and enhance adoption potential. Moving toward a D4DS approach enhances the public health impact of research by ensuring effective dissemination such as policy changes that benefit all populations.
Acknowledgements
We would like to acknowledge all summit participants, including the moderators and speakers at our Long COVID Summit. Teresa Akintowa, Health Equity Consultant, Long COVID Patient Advocate; Alba Azola, MD, Assistant Professor at Johns Hopkins University School of Medicine; Robert A. Barish, MD, MBA, Vice Chancellor for Health Affairs, University of Illinois Chicago; Cheryl Clark, MD, ScD, Executive Director & Senior Vice President, Massachusetts League of Community Health Centers; Catherine Counard, MD, MPH, State Medical Officer, Illinois Department of Public Health; Felicia Davis-Blakley, CEO of Primo Center; Theresa Eagleson, Executive Director, Healthcare Strategy, University of Illinois Chicago; Beth Finta, advocate from Southern Illinois Center for Independent Living; Arvind Goyal, MD, MPH, MBA, Medical Director at Illinois Medicaid; Olusimbo Ige, MD, MS, MPH, Commissioner of the Chicago Department of Public Health; Jonathan Klein, MD, MPH, Professor and Chief, Division of Adolescent Medicine, Stanford University; Lauren Krause, Director of Government Relations; Geraldine Luna, MD, MPH, Medical Director COVID-19 Response Chicago Department of Public Health; Allison O’Donnell, MPH, Deputy Director of the Office of Long COVID Research and Practice, Department of Health and Human Services; Poonam Pardasaney, ScD, DPT, Staff Service Fellow at the Agency for Healthcare Research and Quality; Rahnee Patrick, Director of Rehabilitation Services, Illinois Department of Human Services; Linda Sprague-Martinez, PhD, Professor, University of Connecticut School of Medicine; Neeta Thakur, Associate Professor in Residence, University of California San Francisco; Daniel Turner-Lloveras, MD, Executive Director, The Latino Coalition Against COVID-19; Senator Karina Villa, 25th District, Illinois General Assembly; Zanthia Wiley, MD, Associate Professor, Emory University School of Medicine; Kanecia Zimmerman, MD, PhD, MPH, Professor, Duke University Medical Center.
Author contributions
Elijah Kindred: Conceptualization, Data curation, Formal analysis, Methodology, Writing-original draft, Writing-review & editing; Alejandra Ibanez: Conceptualization, Data curation, Formal analysis, Project administration, Writing-review & editing; Marta Cerda: Conceptualization, Data curation, Formal analysis, Project administration, Writing-review & editing; Emily Kowey: Conceptualization, Data curation, Project administration, Writing-review & editing; Hugh Musick: Conceptualization, Data curation, Formal analysis, Writing-review & editing; Robin Mermelstein: Conceptualization, Formal analysis; Lynn Gerald: Conceptualization, Formal analysis, Project administration, Writing-original draft, Writing-review & editing; Jerry Krishnan: Conceptualization, Data curation, Formal analysis, Funding acquisition, Project administration, Writing-review & editing.
Funding statement
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Competing interests
The authors have no conflicts of interest to report.


