Management Implications
Managing Anthriscus sylvestris (wild chervil) and Myrrhis odorata (anise) in subarctic urban areas will depend on their distribution pattern and preferred habitat type. It is important to first map their distribution, identify hotspots, and prioritize management areas. Adopting a long-term adaptive management approach will help test and determine the best localized actions ranging from eradication to control of further range expansion. Because M. odorata is not as widespread in Reykjavík, monitoring and assessing potential negative impacts is recommended. In addition, proactively testing eradication and control methods locally increases the success of managing M. odorata if it becomes invasive in the future. In green spaces that are managed by the city with individual M. odorata and A. sylvestris seedlings, digging them out with their taproots before they bloom is a feasible approach to control their spread. Given that A. sylvestris thrives well in nutrient-rich soil, we also recommend monitoring soil nitrogen levels and monoculture stands of Lupinus nootkatensis (Nootka lupine), a widespread alien invasive plant in Iceland that fixes nitrogen and facilitates the invasion of A. sylvestris. In places with long summer daylight hours, such as Iceland, effective mowing frequency needs to be tested locally. When large monocultures of A. sylvestris are present, integrated control strategies are likely needed. For example, mowing frequently (≥4 times) in 2-wk intervals, starting before or at peak flowering and continuing throughout the growing season; applying herbicides (e.g., glyphosate) three to four times at least for two consecutive years when feasible; tillage and native grass seeding; and/or controlled grazing. Finally, public outreach through social media and harvesting events focusing on the edible uses of M. odorata, A. sylvestris, and other edible problematic alien plants will increase public participation in management efforts.
Introduction
Urban biodiversity conservation is high on the agenda for cities around the globe as alien plant invasions are diminishing local plant diversity (Keller et al. Reference Keller, Geist, Jeschke and Kühn2011; Nilon et al. Reference Nilon, Aronson, Cilliers, Dobbs, Frazee, Goddard, O’Neill, Roberts, Stander, Werner, Winter and Yocom2017; Pimentel et al. Reference Pimentel, Zuniga and Morrison2005). Urban areas tend to be hotspots for introductions and invasions of alien plants, while hosting high plant richness (Gaertner et al. Reference Gaertner, Wilson, Cadotte, MacIvor, Zenni and Richardson2017; Shochat et al. Reference Shochat, Lerman, Anderies, Warren, Faeth and Nilon2010). Alien plants are becoming an increasing problem due to global economies, tourism and trade, and the risk of invasions to disturbed ecosystems (Chytrý et al. Reference Chytrý, Jarosik, Pysek, Hájek, Knollová, Tichý and Danihelka2008; Clark and Johnston Reference Clark and Johnston2011; EEA 2016; Walker and Steffen Reference Walker and Steffen1997). In fact, Target 6 of the Kunming-Montreal Global Biodiversity Framework aims to reduce and manage the spread and impacts of invasive alien species on biodiversity (UNCBD 2023). Moreover, addressing invasive alien plants is necessary to meet UN Sustainable Development Goal 15 (Life on Land) (UN Department of Economic and Social Affairs 2015).
The introduction of alien plants poses multiple threats to ecosystem functions and services, native biodiversity, landscape aesthetics, and agriculture (Pejchar and Mooney Reference Pejchar and Mooney2009; Pimentel et al. Reference Pimentel, Zuniga and Morrison2005; Simberloff et al. Reference Simberloff, Martin, Genovesi, Maris, Wardle, Aronson, Courchamp, Galil, García-Berthou, Pascal, Pyšek, Sousa, Tabacchi and Vilá2013). This is worrisome, as the negative effects of invasive alien plants can be gradual but end up having large-scale, long-lasting, and often irreversible consequences (EEA 2016; Luoma Reference Luoma2019; Simberloff et al. Reference Simberloff, Martin, Genovesi, Maris, Wardle, Aronson, Courchamp, Galil, García-Berthou, Pascal, Pyšek, Sousa, Tabacchi and Vilá2013). Urban areas can be larger in size and have high habitat heterogeneity, leading to greater plant richness (native and alien) than the neighboring countryside (Kühn et al. Reference Kühn, Brandl and Klotz2004; Lososová et al. Reference Lososová, Chytrý, Tichý, Danihelka, Fajmon, Hájek, Kintrová, Kühn, Láníková, Otýpková and Řehořek2012a; Pyšek, Reference Pyšek1998; Wania et al. Reference Wania, Kühn and Klotz2006). The invasibility of habitats varies depending on the fluctuation of available resources, especially nutrients, and the frequency and level of habitat disturbance (Pyšek et al. Reference Pyšek, Chytrý, Jarošík, Perrings, Mooney and Williamson2009). Cadotte et al. (Reference Cadotte, Yasui, Livingstone and MacIvor2017) generated categories that make alien species thrive in urban areas such as propagule pressure, reduced negative interactions, resource supply, and the combination of altered and unique environmental conditions (Luoma Reference Luoma2019). For example, plant invasions in urban areas can start from the spread of ornamental plantings (propagule pressure) or where suitable habitat conditions exist (resource supply) (Cadotte et al. Reference Cadotte, Yasui, Livingstone and MacIvor2017; Lee et al. Reference Lee, Perkins, Campbell, Passero, Roe, Shaw and Congalton2015; Mayer et al. Reference Mayer, Haeuser, Dawson, Essl, Kreft, Pergl, Pyšek, Weigelt, Winter, Lenzner and van Kleunen2017).
The Arctic provides an interesting area to explore problematic alien plants, because until recently it has experienced relatively limited plant introductions (Lassuy and Lewis Reference Lassuy, Lewis, Meltofte, Josefson and Payer2013). This is due to the remoteness of the region, challenging climatic and growing conditions, low number of inhabitants, and limited trade (Alsos et al. Reference Alsos, Eidesen, Ehrich, Skrede, Westergaard, Jacobsen, Landvik, Taberlet and Brochmann2007). Researchers have found that alien plant invasions in the Arctic are mostly local, with no invasive plants occurring in multiple Arctic regions (Wasowicz et al. Reference Wasowicz, Sennikov, Westergaard, Spellman, Carlson, Gillespie, Saarela, Seefeldt, Bennett, Bay, Ickert-Bond and Väre2019). Furthermore, it seems that Arctic areas with larger human populations and older settlements are impacted more by alien plants. The Arctic is warming three times faster than other areas, and with commerce increasing in the area, it is likely that alien plant distribution and invasions will expand (Rantanen Reference Rantanen2024; Rantanen et al. Reference Rantanen, Karpechko, Lipponen, Nordling, Hyvärinen, Ruosteenoja, Vihma and Laaksonen2022; Wasowicz et al. Reference Wasowicz, Sennikov, Westergaard, Spellman, Carlson, Gillespie, Saarela, Seefeldt, Bennett, Bay, Ickert-Bond and Väre2019; Zhou et al. Reference Zhou, Leung and Lu2024). Warming is also apparent in subarctic areas; for example, in northwestern Iceland, between 1981 and 2020, mean temperatures rose 2.6 times more per decade than the global average (Bannan et al. Reference Bannan, Ólafsdóttir and Hennig2022). Currently, 341 alien vascular plant taxa have been reported in the Arctic (including Iceland), of which 11 are classified as invasive (Wasowicz et al. Reference Wasowicz, Sennikov, Westergaard, Spellman, Carlson, Gillespie, Saarela, Seefeldt, Bennett, Bay, Ickert-Bond and Väre2019). Although most alien plants in the Arctic are found in urban areas, there is limited knowledge about their distribution and impact on native plants.
The city of Reykjavík is an ideal location for a case study to research the invasion process of alien plants in cities that are remote and at a high latitude (≥63° N) and where biodiversity and growing season are limited by challenging weather conditions (Gretarsdottir et al. Reference Gretarsdottir, Aradottir, Vandvik, Heegaard and Birks2004; Luoma Reference Luoma2019). Iceland has only 45% vegetation cover, the lowest in Europe, and >40% of the land is deserts, making it a priority to conserve native plant communities (Arnalds Reference Arnalds2015). Currently, there are 282 casual alien vascular plants (no self-sustaining populations) and 65 naturalized plants, including two that are classified as invasive in Iceland (Wasowicz Reference Wąsowicz2020). Most of these naturalized plants are present in urban areas like Reykjavík. Recently, the total cost of invasive alien species (plants and animals) in Iceland was reported as U$25.45 million; however, this is likely underestimated (Kourantidou et al. Reference Kourantidou, Verbrugge, Haubrock, Cuthbert, Angulo, Ahonen, Cleary, Falk-Andersson, Granhag, Gíslason, Kaiser, Kosenius, Lange, Lehtiniemi and Mangussen2022).
Invasive species are recognized as an area of focus in Reykjavík’s biodiversity policy (Garðarsson et al. Reference Garðarsson, Þorvaldsdóttir, Guðmundsdóttir, Jónsson, Haradsdóttir, Hrafnsdóttir and Sigurðsson2016). Its biodiversity strategy includes actions such as mapping and identifying invasive plants, public engagement, and implementing management actions (Garðarsson et al. Reference Garðarsson, Þorvaldsdóttir, Guðmundsdóttir, Jónsson, Haradsdóttir, Hrafnsdóttir and Sigurðsson2016; Luoma Reference Luoma2019). Moreover, the city has published a strategic plan, the Green Deal, which mentions its vision of maintaining and managing biodiversity and addressing the costs of invasive species (Anonymous 2022). Alien plants, like giant hogweed (Heracleum mantegazzianum Sommier and Levier) already threaten public health locally (Anonymous 2016b; Sigurgeirsdóttir and Hilmarsdóttir 2017), and Nootka lupine (Lupinus nootkatensis Donn ex Sims) may impact insect pollinators in urban areas (Willow et al. Reference Willow, Tamayo and Jóhannsson2017). Other plants of concern are wild chervil [Anthriscus sylvestris (L.) Hoffm.] and anise [Myrrhis odorata (L.) Scop.], which have been spreading throughout Iceland, but their distribution in Reykjavík’s green spaces is not well understood (IINH 2024; Luoma Reference Luoma2019; Magnússon Reference Magnússon2011; Wasowicz et al. Reference Wasowicz, Przedpelska-Wasowicz and Kristinsson2013).
Green spaces can vary in size and shape, ranging from wetlands and narrow coastal grassy pathways to large forested outdoor areas, creating an urban matrix with various green segments (Lepczyk et al. Reference Lepczyk, Aronson, Evans, Goddard, Lerman and MacIvor2017). Data on the distribution patterns of alien plants in Reykjavík’s green areas are limited, making it difficult to determine potential impacts, develop management strategies, and meet biodiversity goals. Given these issues, the goal of our study was to assess the distribution of A. sylvestris and M. odorata in four popular and diverse green spaces in Reykjavík. We focused on characterizing the growth patterns (scattered plants vs. dense stands), distribution, and habitats (e.g., wetland, riversides, and grasslands) of these two plants. Another goal was to create a baseline for tracking A. sylvestris and M. odorata within the city. This baseline helps to generate management recommendations for land planning actions, fostering urban biodiversity, and tracking other emerging problematic plants. Although we focus on Reykjavík, our study provides insight into potential impacts and risks of emerging alien plant invasions to urban green spaces that are applicable to other subarctic and Arctic cities.
Materials and Methods
Target Species
Anthriscus sylvestris
This vascular plant normally grows 0.3- to 1.5-m tall and is either a short-lived perennial or herbaceous biennial plant (Darbyshire et al. Reference Darbyshire, Hoeg and Haverkort1999; Magnússon Reference Magnússon2011); but it can reach 2 m in height in Iceland (MOL, unpublished data). Anthriscus sylvestris blooming peaks by mid-June in Iceland, with most seeds produced in early August (Luoma Reference Luoma2019; Magnússon Reference Magnússon2011; Figure 1). This plant can reproduce asexually from the root buds, but also sexually by producing ≤10,000 seeds (Darbyshire et al. Reference Darbyshire, Hoeg and Haverkort1999). Seed dispersal is via human activities (e.g., agriculture), water, and wind (Hansson and Persson Reference Hansson and Persson1994; Magnússon Reference Magnússon2011). Anthriscus sylvestris is native to Eurasia (Magnússon Reference Magnússon2011; Wasowicz et al. Reference Wasowicz, Przedpelska-Wasowicz and Kristinsson2013) but alien to Iceland, Svalbard (Alsos et al. Reference Alsos, Ware and Elven2015; Gederaas et al. Reference Gederaas, Moen, Skjelseth and Larsen2012), Greenland, the Faroe Islands, the United States (USDA 2024a), Canada (Darbyshire et al. Reference Darbyshire, Hoeg and Haverkort1999), central and southern Africa, New Zealand, and south Georgia (Magnússon Reference Magnússon2011). The first records of A. sylvestris in Iceland are from the city of Akureyri in 1927 (Óskarsson Reference Óskarsson1932), and the plant was not considered invasive in Iceland until the last two decades, when it became evident that its distribution and abundance had increased across the country (Magnússon Reference Magnússon2011). Researchers suspect that A. sylvestris began spreading in urban areas in Iceland as a garden escape (Magnússon Reference Magnússon2011; von Schmalensee and Stefánsson Reference von Schmalensee and Stefánsson2009).

Figure 1. Anthriscus sylvestris blooming in Iceland. Photo by MT.
Anthriscus sylvestris is forming dense monoculture stands along waterways and roads (Luoma Reference Luoma2019; Magnússon Reference Magnússon2011; von Schmalensee and Stefánsson Reference von Schmalensee and Stefánsson2009). It spreads rapidly, replaces other plant species, facilitates alien plant invasions, increases soil erosion, degrades cultural landscapes, and is difficult to eradicate once it becomes established (Darbyshire et al. Reference Darbyshire, Hoeg and Haverkort1999; Førde and Magnussen Reference Førde and Magnussen2015; Hansson and Persson Reference Hansson and Persson1994; Jørgensen et.al. Reference Jørgensen, Tørresen, Dyrhaug, Myrstad, Svendsen, Magnussen, Førde and DiTommaso2013; Magnússon Reference Magnússon2011). Anthriscus sylvestris often spreads in former pasturelands, but urban areas are also vulnerable to the negative impacts of this plant (Luoma Reference Luoma2019; Magnússon Reference Magnússon2011; Pilto Reference Pilto2012).
Myrrhis odorata
Myrrhis odorata is a perennial plant that looks very similar to A. sylvestris, but it has several features that differ from A. sylvestris, such as a strong anise-like odor, larger seeds, and lighter green leaves (Kristinsson Reference Kristinsson2013; Luoma Reference Luoma2019; Figure 2). Myrrhis odorata’s native range covers central and southern Europe (Kew Science 2024). The alien distribution of M. odorata includes the Nordic countries, the Baltic States, northern Russia, the United Kingdom, Ireland, Belgium, the Netherlands, Poland, the Czech Republic, Ukraine, North America, and New Zealand (Biological Records Centre 2018) iNaturalist-NZ 2024; Kew Science 2024; Native Plant Trust 2024). Myrrhis odorata is an ornamental plant used for medicinal purposes and food consumption (Penny Reference Penny2024; Petřík et al. Reference Petřík, Sádlo, Hejda, Štajerová, Pyšek and Pergl2019; Rančić et al. Reference Rančić, Soković, Vukojević, Simić, Marin, Duletić-Laušević and Djoković2005), making garden escape a likely dispersal pathway. In Iceland, M. odorata is classified as an alien plant and is yet to be evaluated for invasiveness (von Schmalensee Reference von Schmalensee2010). The first known record is from 1936, and this plant has been expanding its distribution in Iceland, especially in urban areas (IINH 2024; Seebens et al. Reference Seebens, Blackburn, Dyer, Genovesi, Hulme, Jeschke, Pagad, Pyšek, Winter, Arianoutsou, Bacher, Blasius, Brundu, Capinha and Celesti-Grapow2017). Reproduction of M. odorata is by taproot and seeds, similar to A. sylvestris (Penny Reference Penny2024). Myrrhis odorata is present in farms, woodlands, and along streams, as well as in urban gardens throughout Iceland (IINH 2024).

Figure 2. Myrrhis odorata blooming in Iceland. Photo by MOL.
Study Areas
We assessed the distribution of A. sylvestris and M. odorata in four green spaces in Reykjavík, located in Laugarnes (64.15°N, 21.88°W), Vatnsmýri (64.14°N, 21.94°W), Elliðaárdalur (64.12°N, 21.85°W), and Ægisíða (64.14°N, 21.96°W) (Figure 3). These areas represent a heterogeneity of green spaces in the city, providing a gradient of urban habitats. Together these four areas include five of the seven urban habitat types described by Lososová et al. (Reference Lososová, Chytrý, Tichý, Danihelka, Fajmon, Hájek, Kintrová, Kühn, Láníková, Otýpková and Řehořek2012a) for central European cities, ranging from densely built residential areas to recently disturbed sites. These sites will also act as a foundation to build a database for local green spaces and their biodiversity.

Figure 3. Study areas in Reykjavík are highlighted in red: (1) Laugarnes, (2) Vatnsmýri, (3) Elliðaárdalur, and (4) Ægisíða. Scale 1:24,000.
Laugarnes is a popular outdoor coastal area with a museum and some residential buildings, located in north-central Reykjavík (Figure 3). Many archaeological sites are found in this area, some dating back to the settlement period (ca. 870 to 930), and the area also hosted a military community during World War II and was later used for pastureland (Guðmundsdóttir Reference Guðmundsdóttir2003; Hallgrímsdóttir Reference Hallgrímsdóttir1996). Our study area in Laugarnes is grassland habitat, where invasive and other alien plants of concern such as H. mantegazzianum and L. nootkatensis are also present. Laugarnes fits the urban habitat type 7, a mid-successional site, from Lososová et al. (Reference Lososová, Chytrý, Tichý, Danihelka, Fajmon, Hájek, Kintrová, Kühn, Láníková, Otýpková and Řehořek2012a), with the exception that our study site has been abandoned for more than 15 yr and some sections are mowed by the city.
Vatnsmýri is located next to the University of Iceland and the Reykjavík Airport in the western part of Reykjavík (Figure 3). This wildlife nature reserve is large, approximately 3.7 ha in size, and is mostly wetland habitat. We only surveyed the outer grassland area by the wetland, because the inner part of the nature reserve is closed in the summer during the bird nesting season (Luoma Reference Luoma2019; Pálsson Reference Pálsson2003; Sigurðsson Reference Sigurðsson2015). Vatnsmýri hosts 83 vascular plants, 65 of which are native to Iceland (Pálsson Reference Pálsson2003). This area has been experiencing major urban development, impacting the reserve (Garðarsson et al. Reference Garðarsson, Þorvaldsdóttir, Guðmundsdóttir, Jónsson, Haradsdóttir, Hrafnsdóttir and Sigurðsson2016). In 2013 and 2014, improvements to the bird nesting habitat were implemented, which included increasing the water level and decreasing the elevation of wetland islands via soil and plant removal to manage alien plants such as A. sylvestris (Luoma Reference Luoma2019). Vatnsmýri is an example of an urban habitat type 6, an early successional and recently disturbed site, as described by Lososová et al. (Reference Lososová, Chytrý, Tichý, Danihelka, Fajmon, Hájek, Kintrová, Kühn, Láníková, Otýpková and Řehořek2012a).
Elliðaárdalur, a large green space, is a popular biking, hiking, and fishing area. This area in the eastern part of Reykjavík (Figure 3) was important for forestry and had summer homes and sheep farms (Anonymous 2016a; Luoma Reference Luoma2019). Today, Elliðaárdalur has various landscapes, with a mixture of forest and grasslands habitats, and the Elliðaár River flows in the middle of the area. Elliðaárdalur is home to 25 bird species and at least 315 vascular plant species, of which 179 (57%) are native plants (Anonymous 2016a; Pálsson Reference Pálsson2004). Our study area in Elliðaárdalur included Geirsnef (a popular dog park) and its surroundings, as well as Háubakkar (a protected area) (Anonymous 2018). Elliðaárdalur is a very large and heterogenous area, and therefore only Geirsnef can be described as habitat type 5, an urban park that is mowed frequently and has 10% to 20% tree cover of downy birch (Betula pubescens Ehrh.) and willow (Salix spp.) (Lososová et al. Reference Lososová, Chytrý, Tichý, Danihelka, Fajmon, Hájek, Kintrová, Kühn, Láníková, Otýpková and Řehořek2012a). In addition, Elliðaárdalur is surrounded by both habitat type 4, a residential area built after the 1960s, with shrubs, trees, and scattered lawns, and habitat type 3, consisting of older family houses with gardens (Lososová et al. Reference Lososová, Chytrý, Tichý, Danihelka, Fajmon, Hájek, Kintrová, Kühn, Láníková, Otýpková and Řehořek2012a).
The Ægisíða study site is a flat area between a protected shoreline and a residential street in the northwest of Reykjavík (Figure 3). Ægisíða is a grassland habitat with some large monocultures of Norwegian angelica (Angelica archangelica L.) and A. sylvestris near a residential house. Previously, this area was farmland that became urbanized, and a 1954 aerial photo shows more houses and industrial buildings than are currently present (Borgarvefsjá 1954). Ægisíða is near to the Reykjavík airport and is popular for cycling, dog walking, and jogging. Overall, this area represents habitat type 3, with a series of older family houses with gardens lining the street across from the shoreline (Lososová et al. Reference Lososová, Chytrý, Tichý, Danihelka, Fajmon, Hájek, Kintrová, Kühn, Láníková, Otýpková and Řehořek2012a).
Data Collection and Statistical Analyses
AllTrailsPro (2017) and ArcGIS mobile applications were used to survey the study areas between May 31 and October 25, 2017 (Luoma Reference Luoma2019). GPS locations of A. sylvestris and M. odorata were recorded in the four study sites (Luoma Reference Luoma2019). ArcGIS was used to draw polygonal shapes of the distribution of A. sylvestris and M. odorata and their potential overlap. The SHAPE_Area polygon feature was used to calculate the distribution area of both plants. Plant distribution was classified as either scattered plants or dense plant stands/patches of A. sylvestris and M. odorata (Luoma Reference Luoma2019). The classification was either (1) ≥3 plants m−2 for a dense plant stand/patch or (2) a plant patch >0.5 m2 but with ≤2 scattered plants. Typically, a scattered distribution for M. odorata was an individual plant in ≤1 m2. ArcMap 10.4.1. was used to create the distribution maps of A. sylvestris and M. odorata. We used WGS 1984 with a prime meridian set for the Greenwich geographic coordinate system to gather the data, which was projected as WGS 1984 with a metric linear unit. Given that A. sylvestris and M. odorata look similar to each other (Figures 1 and 2) and to other plants such as bishop’s goutweed (Aegopodium podagraria L.), the surveys were conducted on foot rather than using a drone. Guides by Kristinsson (Reference Kristinsson2013), Kristinsson et al. (Reference Kristinsson, Hlíðberg and Þórhallsdóttir2018), and Flora of Iceland (2024) were used for plant identification. The data were normally distributed, and there was homogeneity of variance, so we used ANOVA and a Bonferroni correction for the post hoc test (α = 0.05) to compare the distribution and patch size of both plants among the four study sites, running the analyses on SPSS® 24 software (Luoma Reference Luoma2019).
Results and Discussion
Anthriscus sylvestris and Myrrhis odorata Distribution Patterns
Overall, 158 ha (all four study areas) were surveyed, of which A. sylvestris and M. odorata covered 11% (Luoma Reference Luoma2019). Anthriscus sylvestris was more prevalent, encompassing 10% of the total area surveyed and having the greatest cover per area surveyed in Vatnsmýri (23%) (Table 1). Myrrhis odorata coverage was low (≤0.5%) with the exception of Laugarnes (>8%), where it was more common than A. sylvestris. We found no significant difference in plant cover among the study areas for A. sylvestris (F(3, 16) = 0.119, P = 0.947) (Luoma Reference Luoma2019). Elliðaárdalur had the largest total area with A. sylvestris, encompassing 13 ha (Table 1).
Table 1. Distribution of Anthriscus sylvestris (As) and Myrrhis odorata (Mo) in open areas of Reykjavík in 2017. a

a The total area (ha = hectares) where A. sylvestris or M. odorata (Mo) were present is shown, as well as the total area surveyed for each site. In addition, the cover (%) of Anthriscus sylvestris and Myrrhis odorata for each area is presented.
We found 98 patches of M. odorata, ranging from <1 m2 to a dense stand of 3,875 m2 (Luoma Reference Luoma2019; Figure 4). The mean M. odorata patch was 158 m2 (± 58 SE), but because the number of patches per study site varied from 1 to 83, we were unable to compare their size statically among the study areas. A total of 396 A. sylvestris patches (Figure 5) were present in the study sites, including 34 scattered plant patches. Anthriscus sylvestris patch sizes varied greatly, ranging from <1 m2 to >1.2 ha, and were on average 392 m2 (± 55 SE) (Luoma Reference Luoma2019; Table 2). We found significant differences between the sites regarding the mean patch size of A. sylvestris (F(3, 392) = 5.584, P = 0.001), with Vatnsmýri (P = 0.041) and Elliðaárdalur ( P = 0.001) having significantly larger patches than Laugarnes (Luoma Reference Luoma2019; Table 2). We also found that patch size varied significantly within Elliðaárdalur (F(5, 239) = 11.948, P = 0.000). For example, the A. sylvestris patches in the Háubakkar protected area were significantly smaller (P < 0.001; patch size = 106 m2 ± 57) than patches to the east of Geirsnef (patch size = 2,425 m2 ± 780) (Luoma Reference Luoma2019). Similarly, below Geirsnef, there were significantly smaller A. sylvestris patches (241 m2 ± 44) than those in the east (P < 0.001) and northeast (P = 0.017; patch size = 1,428 m2 ± 644) of Geirsnef (Luoma Reference Luoma2019). Moreover, Geirsnef patches were significantly smaller (P < 0.001; patch size = 574 m2 ± 168) than patches east of Geirsnef.

Figure 4. Size and frequency of Myrrhis odorata patches in open areas of Reykjavík in 2017.

Figure 5. Size and frequency of Anthriscus sylvestris patches in open areas of Reykjavík in 2017.
Table 2. Average patch size (m2) of Anthriscus sylvestris among open areas of Reykjavík in 2017
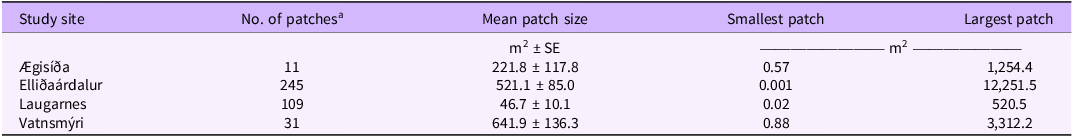
a No. of patches includes both dense plant stands (≥3 plants m−2) and scattered plant patches (>0.5 m2 but with ≤2 plants).
Study Site Distribution
In Laugarnes, there was minimal overlap between A. sylvestris and M. odorata. Anthriscus sylvestris was a mosaic of monoculture stands, scattered plants, and smaller patches around the site (Figure 6) (Luoma Reference Luoma2019). In addition, three very large M. odorata stands were present, two of them near a former building site in the western part. A few large M. odorata stands were found next to patches of A. sylvestris near residential buildings. The majority of the patches (64%) of A. sylvestris and M. odorata in Laugarnes were <10 m2 (Figure 6).

Figure 6. Distribution of Anthriscus sylvestris and Myrrhis odorata in Laugarnes, summer 2017. The distribution of M. odorata in Laugarnes includes both individual plants and dense patches.
Vatnsmýri had a very large M. odorata stand mixed with A. sylvestris next to the heavily trafficked Hringbraut Street along the northeast pathway (Luoma Reference Luoma2019; Figure 7). The majority of A. sylvestris stands (77%) were large (>100 m2) and dense (Figure 4), scattered along the pathways and water (Figure 7).

Figure 7. Distribution of Anthriscus sylvestris and Myrrhis odorata in Vatnsmýri, summer 2017.
In Elliðaárdalur, large, scattered, and dense patches of A. sylvestris were present in and around Geirsnef by the Elliðaár River (Figure 8). Myrrhis odorata was absent in Geirsnef (Figure 8), but was found by the walking paths in the inner parts of Elliðaárdalur as dense stands (Figure 8) without overlapping with A. sylvestris (Luoma Reference Luoma2019). Furthermore, these inner areas in Elliðaárdalur had predominantly dense stands of A. sylvestris along the river and pathways. Most stands (75%) of A. sylvestris and M. odorata in Elliðaárdalur were ≥10 m2 (Figures 4 and 5).

Figure 8. Distribution of Anthriscus sylvestris and Myrrhis odorata in Elliðaárdalur, summer 2017.
Anthriscus sylvestris and M. odorata occurred in scattered and dense stands in the south and northwest areas of Ægisíða (Figure 9), and there was no overlap between the species (Luoma Reference Luoma2019). Anthriscus sylvestris was more common in Ægisíða than M. odorata, and most patches (71%) of both plants were >10 m2 (Figures 4 and 5).

Figure 9. Distribution of Anthriscus sylvestris and Myrrhis odorata in Ægisíða, summer 2017.
Current Status and Recommended Management Actions
The distribution of A. sylvestris was more widespread than expected, totaling 10% of the area surveyed in Reykjavík (Luoma Reference Luoma2019). In contrast, M. odorata distribution is more localized, with the plant found in fewer areas as remnants of abandoned gardens or farmland. Although there is very little overlap between the two species, they both thrive in urban settings, particularly near waterways, buildings, pathways, and roads. Similar distribution patterns have also been recorded in northern and northwestern Iceland, the United Kingdom, the Netherlands, and North America (Bjarnadóttir Reference Bjarnadóttir2014; Darbyshire et al. Reference Darbyshire, Hoeg and Haverkort1999; GBIF 2023; Jónsson and Þórðarson Reference Jónsson and Þórðarson2018; National Museums Northern Ireland 2023; Nature Conservancy 2010; van Mierlo and van Groenendael Reference van Mierlo and van Groenendael1991).
Overall, large monoculture stands of A. sylvestris are enabling the spread of the species in Reykjavík. However, Ægisíða is an exception, where A. sylvestris does not seem to be expanding in large, scattered stands, but rather in smaller patches of one to three plants. Frequent mowing in Ægisíða (four times per summer) may be preventing seed dispersal and the formation of larger stands from individual plants, as the larger stands of A. sylvestris and M. odorata are beyond the grass-cutting range (Luoma Reference Luoma2019). A combination of control actions is needed to manage A. sylvestris (ISC 2019). We advise digging out individual seedlings and their taproots before they bloom in areas where the grass is mowed or where small patches of A. sylvestris are present (Nova Scotia Department of Agriculture 2003). If feasible, Canadian resource managers recommend herbicide use (e.g., glyphosate) to prevent seeding in large stands of A. sylvestris. If herbicides are not an option, then mowing frequently every 2 wk before plants bloom or at peak flowering and thereafter is recommended (ISC 2019; Nova Scotia Department of Agriculture 2003). Moreover, the most effective control strategy for A. sylvestris is a combination of mowing before flowering, application of herbicides, tillage, and native grass seeding (Luoma Reference Luoma2019; Miller and D’Auria Reference Miller and D’Auria2011). Based on management experiments in north and northwest Iceland, researchers suggest applying glyphosate (e.g., Clinic at a 1:120 concentration) at least three to four times for two consecutive years to eradicate A. sylvestris (Bjarnadóttir Reference Bjarnadóttir2014; Jónsson and Þórðarson Reference Jónsson and Þórðarson2018). This treatment seems best for eradicating smaller, isolated stands of A. sylvestris but not large, well-established communities (Bjarnadóttir Reference Bjarnadóttir2014). Bjarnadóttir (Reference Bjarnadóttir2014) and Jónsson and Þórðarson Reference Jónsson and Þórðarson2018) also mention grazing (e.g., sheep) as another potential tool warranting research. In urban nature reserves such as Vatnsmýri, where access is partly restricted during the summer, a strategy that combines hand pulling seedlings and controlled grazing (e.g., sheep, goats, or pigs) can be tested for A. sylvestris. In addition, for open access areas, frequent mowing four or more times before plants bloom and pulling out individual plants with roots seems to be effective to control the spread of both A. sylvestris and M. odorata (ISC 2019). Furthermore, monitoring the plant community in urban nature reserves would help detect changes in species composition and the effects on biodiversity. Urban nature reserves such as Vatnsmýri have high conservation value (Garðarsson et al. Reference Garðarsson, Þorvaldsdóttir, Guðmundsdóttir, Jónsson, Haradsdóttir, Hrafnsdóttir and Sigurðsson2016), making them a priority to manage invasive and problematic alien plants to prevent the loss of biodiversity (Darbyshire et al. Reference Darbyshire, Hoeg and Haverkort1999; Hansson and Persson Reference Hansson and Persson1994).
Another important management strategy is to identify urban hotspots of invasive and problematic alien plants. In Reykjavík, Laugarnes seems to be a hub for invasive or problematic alien plants, where A. sylvestris and M. odorata grow together with L. nootkatensis and H. mantegazzianum. The presence of H. mantegazzianum is a great concern, because it is a serious health hazard, and it shades native plants with its tall stems (≥2 m) and large leaves. In sunny weather, when H. mantegazzianum sap is exposed to ultraviolet radiation, it can cause serious skin burns (Nielsen et al. Reference Nielsen, Ravn, Nentwig and Wade2005). The lowest native plant cover of all the study sites was in Laugarnes, making the presence of A. sylvestris worrisome. Several studies in central Europe have shown that areas with A. sylvestris are vulnerable to further alien plant invasions, particularly plants that thrive in nutrient-rich soils (Godefroid and Koedam Reference Godefroid and Koedam2003; Hansson and Persson Reference Hansson and Persson1994; Pyšek et al. Reference Pyšek, Chytrý, Jarošík, Perrings, Mooney and Williamson2009). Areas like Laugarnes require a management strategy that targets multiple invasive plants. In the past, Reykjavík has implemented eradication efforts for H. mantegazzianum in parts of Laugarnes that included mowing, manual cutting, and herbicide treatment. However, an assessment of the effectiveness of these efforts is needed, as well as testing of additional control actions to manage other problematic alien plants. Furthermore, we recommend monitoring the distribution and soil nitrogen levels of green spaces with L. nootkatensis such as Elliðaárdalur, as they may be invaded by A. sylvestris (Magnússon Reference Magnússon2011). In the future, the abundance and distribution of A. sylvestris is likely to increase with climate change, emphasizing the need to manage this plant now. Wasowicz et al. (Reference Wasowicz, Przedpelska-Wasowicz and Kristinsson2013) predicted that A. sylvestris’s climatic niche in Iceland will increase greatly, enabling it and other alien plants like M. odorata to colonize new habitats. Our study shows that foot surveys using AllTrailsPro and ArcGIS Collector applications to record the distribution of alien plants is effective. These mapping actions may potentially be complemented by remote sensing, a method that also requires ground truthing to train and validate the data collection. Currently, researchers at the University of Iceland are exploring drones and satellite imaging as additional tools to distinguish and map A. sylvestris and M. odorata in Reykjavík.
Future Research Priorities
Currently, M. odorata is less prevalent in green spaces of Reykjavík compared with A. sylvestris. The largest total area with M. odorata is 1 ha (Laugarnes), likely planted several decades ago. Most of the M. odorata’s distribution in Reykjavík is scattered plants and smaller patches (<100 m2). This distribution may be due to M. odorata not having spread to all available habitat or to differences in habitat suitability; however, further research is needed to test these hypotheses. For example, studies looking at differences in soil moisture and nutrients, as well as levels of disturbance, among others, will help assess the influence of environmental and anthropogenic factors in alien plant distribution. Although M. odorata is not as common as A. sylvestris, local eradication and control actions should be implemented to prevent it from spreading and potentially causing negative impacts. The ongoing and increasing alien distribution of M. odorata is not limited to Iceland, as it has also been spreading in the United Kingdom and more recently in New Zealand (Braithwaite Reference Braithwaite2020; GBIF 2023; iNaturalist-NZ 2024; Seebens et al. Reference Seebens, Blackburn, Dyer, Genovesi, Hulme, Jeschke, Pagad, Pyšek, Winter, Arianoutsou, Bacher, Blasius, Brundu, Capinha and Celesti-Grapow2017). Like A. sylvestris, M. odorata is abundant in roadsides close to residential buildings and is suspected to have escaped from gardens (Gederaas et al. Reference Gederaas, Moen, Skjelseth and Larsen2012; Luoma Reference Luoma2019; National Museums Northern Ireland 2023; Stroh et al. Reference Stroh, Humphrey, Burkmar, Pescott, Roy and Walker2020; USDA 2024b). Pyšek et al. (Reference Pyšek, Danihelka, Sádlo, Chrtek, Chytrý, Jarošík, Kaplan, Krahulec, Moravcová, Pergl, Štajerová and Tichý2012) classify M. odorata as a plant escaping cultivation and forming stable populations in the wild. Nonetheless, M. odorata is not categorized as an invasive plant in Iceland or Europe; however, it is classified as having a very high invasion potential in Norway (Gederaas et al. Reference Gederaas, Moen, Skjelseth and Larsen2012; Solstad et al. Reference Solstad, Hegre, Alm, Fløistad, Pedersen, Schei, Vandvik, Vollering, Westergaard and Skarpaas2023). We recommend long-term monitoring and plant community surveys to assess whether M. odorata is becoming an invasive plant in Iceland. Regarding proactive management to test and track, areas with single plants and small patches of M. odorata can be dug out with relatively low effort in early summer. In contrast, large stands may require multiple mowing treatments before plants bloom, but the frequency of mowing needs to be evaluated locally. In addition, public campaigns focusing on the culinary uses of M. odorata would help increase awareness about the plant and foster control efforts in private gardens and green spaces in the future (Hussain et al. Reference Hussain, Poveda, Pezzuto, Soejarto and Kinghorn1990; Lim Reference Lim2016). We have, for example, organized M. odorata and A. sylvestris harvesting events in various green spaces in Reykjavík and neighboring towns since 2021, which have become popular with the public. Moreover, we have created a social media platform, Borgarnáttúra-Urban Biodiversity Iceland, to further promote awareness and collaboration with multiple stakeholders.
Monitoring both invasion pathways and alien plant distributions, which are expected to accelerate with climate change in the Arctic, will facilitate effective strategies that promote urban biodiversity (Wasowicz et al. Reference Wasowicz, Przedpelska-Wasowicz and Kristinsson2013, Reference Wasowicz, Sennikov, Westergaard, Spellman, Carlson, Gillespie, Saarela, Seefeldt, Bennett, Bay, Ickert-Bond and Väre2019). A single alien plant can have detrimental effects when it increases its distribution and becomes problematic by outcompeting native plants (Vilà et al. Reference Vilà, Espinar, Hejda, Hulme, Jarošík, Maron, Pergl, Schaffner, Sun and Pyšek2011). Several central European cities have seen such pattern, showing that some alien plants promote biotic homogenization, thus causing major problems in highly urbanized areas (Lososová et al. Reference Lososová, Chytrý, Tichý, Danihelka, Fajmon, Hájek, Kintrová, Láníková, Otýpková and Řehořek2012b). This is a concern for Reykjavík and other subarctic cities, even though this process can take time. Urban areas can provide suitable habitats for alien plants, as disturbed urban habitats often enable these plants to form large populations (Cadotte et al. Reference Cadotte, Yasui, Livingstone and MacIvor2017). Cadotte et al. (Reference Cadotte, Yasui, Livingstone and MacIvor2017) also emphasize that to prevent invasive plants from spreading beyond city limits, it is important to monitor and control them in urban areas.
As a case study, our research establishes a baseline for problematic alien plants in heterogenous green spaces of a relatively young and remote subarctic city. Anthriscus sylvestris and M. odorata are spreading in Reykjavík’s green spaces, but with different distribution patterns. These species show minimal overlap in distribution; A. sylvestris is more widespread, while the invasive potential of M. odorata still needs to be assessed. It is crucial to understand the ecology and abundance of alien plants in a city to achieve sustainable urban management and planning of green spaces. For example, large and dense patches of A. sylvestris alter landscape aesthetics and may negatively impact native plant habitats for birds, insects, and invertebrates, possibly changing ecosystem functions (Førde and Magnussen Reference Førde and Magnussen2015; Willow Reference Willow2017). Our study suggests that various management strategies will be needed given the different patterns of distribution of A. sylvestris and M. odorata in Reykjavík. Ultimately, an adaptive management strategy that is proactive and long term, involving monitoring, public participation, and various management actions, will increase the success in managing problematic alien plants and fostering biodiversity in subarctic cities like Reykjavík (Zalba and Ziller Reference Zalba and Ziller2007).
Acknowledgments
We greatly appreciate the input and valuable comments of Borgþór Magnússon from the Icelandic Natural History Institute.
Funding statement
This work was supported in part by the Náttúruverndarsjóður Pálma Jónssonar (2017).
Competing interests
The authors declare no conflicts of interest.













