Recent improvements in the early detection of cancers and post-diagnosis therapy have led to a rapid increase in the number of cancer survivors in the United States, with an estimate of 18 million in January 2022 and projected to reach 26 million by 2040(1–Reference Miller, Nogueira and Devasia3). As living with cancer becomes more common, there is a need to study the lifestyle of cancer survivors, particularly regarding how a healthy lifestyle can enhance survival and improve quality of life. Previous research has shown that obesity, poor nutrition and metabolic disorders are associated with cancer recurrence, worse quality of life and increased mortality(Reference van Zutphen, Kampman and Giovannucci4–Reference Castro-Espin and Agudo6). Although numerous studies have confirmed the association between healthy lifestyles, adhering to dietary recommendations, and improved cancer-related outcomes, there is mixed evidence about dietary behaviours among cancer survivors(Reference Hansen, Nagle and Ibiebele7–Reference van Veen, Mols and Bours13). While some survivors adopt healthier eating habits after diagnosis of cancer, many continue to face challenges aligning their diets with recommended dietary guidelines(Reference Hawkins, Smith and Zhao14–Reference Potter, Collins and Brown23).
Awareness of dietary guidelines plays a crucial role in promoting healthy behaviours among cancer survivors(Reference Di Sebastiano, Murthy and Campbell24,Reference Grundy, Poirier and Khandwala25) . Knowledge about diet-related cancer risks is essential for behaviour change, as seen in frameworks like the Health Belief Model and Social Cognitive Theory(Reference Maiman and Becker26–Reference Ajzen30). These models emphasise that perceptions of risk and cognitive factors such as knowledge and attitudes significantly impact health behaviours. Therefore, further research is warranted to understand these factors better and to develop effective strategies to improve dietary behaviours among cancer survivors. The purpose of this study is to investigate the differences in diet-related awareness of cancer risk and diet-related behaviours among cancer survivors compared with non-cancer individuals using the Health Information National Trends Survey (HINTS).
Materials and methods
Study population
We retrieved data from the HINTS, a cross-sectional, publicly available, nationally representative dataset that provides information on awareness and behaviours of diet-related cancer risk. All survey iterations spanning from year 2003 to 2022, i.e. iteration 1 through iteration 6, were scanned for variables related to awareness of diet-related cancer risk and behaviours. If similar survey items were available in multiple survey iterations, they were harmonised and pooled together using appropriate survey weights to increase the sample size. HINTS uses a two-stage stratified probability sample of non-institutionalised adults. Detailed methodology, including survey weights, has been previously described(Reference Hesse, Moser and Rutten31,Reference Nelson, Kreps and Hesse32) .
HINTS participants were identified as cancer survivors if they answered ‘yes’ to the survey question: ‘Have you ever been diagnosed as having cancer?’. If they answered ‘no’, they were identified as non-cancer individuals for data analysis. The total number of cancer survivors and non-cancer individuals differed in each survey cycle, and we summed up the number of study subjects based on the availability of relevant questions for analyses. This study was deemed exempt by the XXX Institutional Review Board under federal regulation 45.46.101 (b) CFR (www.hhs.gov/ohrp/humansubjects/guidance/45cfr46.html.). All data used in this study can be downloaded from the website of HINTS (https://hints.cancer.gov).
Diet-related cancer risk awareness
Six questions for diet-related cancer risk awareness were extracted from various iterations of HINTS 1, HINTS 5 and HINTS 6, which covered processed meat, red meat, fruit and vegetables, dietary fibre, alcohol consumption and soda or sugar-sweetened drinks For example, awareness of red meat was assessed using the survey items: ‘How much do you think that eating too much red meat can influence whether or not a person will develop cancer?’ and ‘How much do you think that eating too much red meat (for example beef, pork, ham) could increase a person’s chance of developing cancer?’. These questions were harmonised, and response categories were combined as ‘yes’ (a lot), ‘no’ (a little, not at all) and ‘don’t know’.
Diet-related behaviours
Eight questions for diet-related behaviours were extracted from various iterations of HINTS 4, HINTS 5 and HINTS 6. It covers dietary behaviours related to fruit, vegetables, soda drinks, alcohol consumption and energy information. For example, fruit and vegetable intake was measured by the following two survey items: ‘About how many cups of fruit (including 100 % pure fruit juice) do you eat or drink each day?’ and ‘About how many cups of vegetable (including 100 % pure vegetable juice) do you eat or drink each day?’. There were seven response categories ranging from none to ≥4 cups. These responses were dichotomised based on ACS recommendations (consume 1·5–2 cups of fruit and 2–3 cups of vegetables daily)(Reference Rock, Doyle and Demark-Wahnefried33). Previous year’s fruit and vegetable intake intention was evaluated using the following two survey question: ‘At any time in the past year, have you intentionally tried to increase, maintain or haven’t paid attention to the amount of Fruit or 100 % Fruit Juice you eat or drink?’ and ‘At any time in the past year, have you intentionally tried to increase, maintain or haven’t paid attention to the amount of vegetable or 100 % vegetable juice you eat or drink?’ Three response categories were ‘increased’, ‘maintained’ and ‘haven’t paid attention’. Weekly soda consumption was studied using the survey item: ‘Not counting any diet soda or pop, how often do you drink regular soda or pop in a typical week?’. Six response categories were dichotomised as ‘>= 1 d/week’ and ‘< 1 d/week’. Alcohol consumption was assessed using the composite survey item: ‘Average number of alcohol drinks per week’ wherein response options were combined as ‘<= 1–10 drinks’ or ‘> 10 drinks’. Usage of menu energy information was analysed by the survey item: ‘When available, how often do you use menu information on calories in deciding what to order?’. Responses were combined as ‘often’, ‘sometimes’ and ‘rarely’. Survey item, ‘Think about the last time you ordered food in a fast food or sit-down restaurant. Did you notice calorie information listed next to the food on the menu or menu board?’ assessed the behaviour of noticing energy information in a menu using binary response (yes, no).
Statistical analysis
Single-factor analysis was performed using the Pearsons and Rao–Scott chi-square tests to analyse the difference in diet-related cancer risk awareness and behaviours between cancer survivors and non-cancer individuals. Binary and multinomial logistic regression models were used to detect further the difference with adjustment for potential confounders, including age, gender, race/ethnicity, education, marital status and BMI. Age in years was categorised as 18–39, 40–59, 60–69 and 70 or more. Gender was a binary variable as men or women. Race and ethnicity were grouped as Hispanics, non-Hispanic Blacks, non-Hispanic Whites and non-Hispanic others. The last group contains Asians, Native Hawaiians or other Pacific Islanders, American Indians or Alaska Natives and Multiple races. Education was defined as high school graduates or less, some college, college graduates or more. Marital statuses were grouped as married, divorced, single and widowed. BMI was categorised as obese (> 30 kg/m2) or non-obese (≤ 30 kg/m2).
In the binary logistic regression models, the outcome variables were daily fruit and vegetable intake (< 2–3 cups; ≥ 2–3 cups), weekly consumption of soda (< 1 d/week; ≥ 1 d/week), the average number of alcoholic drinks per week (< 1–10 drinks; > 10 drinks) and noticing energy information on the restaurant menu (yes; no), respectively. The multinomial logistic regression models contained outcome variables with more than two categories. For example, the usage of menu energy information contained three categories: often, sometimes and rarely. We performed logistic regression analyses based on basic and fully adjusted models. The basic model was only adjusted for age and gender, whereas the full-adjusted model included all the aforementioned covariates.
Items in the HINTS questionnaire that had missing values, or if responses were inapplicable or selected in error, were all combined to create a separate category labelled as ‘missing’. The percentage of missing was calculated, and the randomness of missing was analysed. If missing data were less than 5 % or missing is completely random, they were removed from the analysis; otherwise, they remained as a separate category.
Multicollinearity was assessed in all models using correlation analysis and the variance inflation factor among independent variables. The results showed that most correlation coefficients were below 0·2, and a few were greater than 0·5 but smaller than 0·7. Moreover, all variance inflation factors were less than 2. Additionally, interactions between cancer status and demographic variables, as well as other independent variables, were analysed. There was no statistically significant interaction between cancer status and demographic variables in most models. Significant interactions were detected between cancer status and race/ethnicity for perception of the influence of dietary fibre on cancer (P = 0·0168) and usage of menu energy information (P = 0·0230). We thus included the interaction items in the model, and the results of cancer status relative to the dependent variables were not significantly affected. Similarly, interaction between gender and race/ethnicity was significant for perception of red meat on cancer (P = 0·0235). However, the result of the perception of red meat on cancer between cancer survivors and non-survivors remained similar when the interaction item was added to the model.
All analyses were weighted to account for complex sampling design and unequal sample size in each survey cycle. This was done using the methodology developed by HINTS, which was published previously(Reference Hesse, Moser and Rutten31,Reference Nelson, Kreps and Hesse32) . Replicated weights were calculated using the Jackknife method, and final sample weights were applied. The statistical significance level was set at P-value < 0·05. Analyses for this study were conducted using the Statistical Analysis System software 9.4(34).
Results
Baseline characteristics
Table 1 summarises the baseline characteristics of adult cancer survivors and non-cancer individuals. The weighted percentage of female cancer survivors was 58·7 % compared with 50·6 % of non-cancer females. About 63 % of cancer survivors were 60 years of age or older, whereas this percentage was approximately 23 % for non-cancer individuals. About 81 % of survivors were non-Hispanic White compared with 64 % among non-survivors. With regards to marital status, about 64 % of survivors were either married or living as married, and about 25 % were separated, divorced, or widowed. About 65 % of cancer survivors had some college education or higher compared with 67 % of non-cancer individuals. Online supplementary material, Supplemental Table 1 contains basic demographic information about the study sample from each of the HINTS survey cycles that have been pooled. Different combinations of the survey cycles have been pooled for each of the variables depending on their harmonisation.
Table 1 Baseline characteristics of adult cancer survivors and non-cancer individuals: Health Information National Trends Survey (HINTS) *
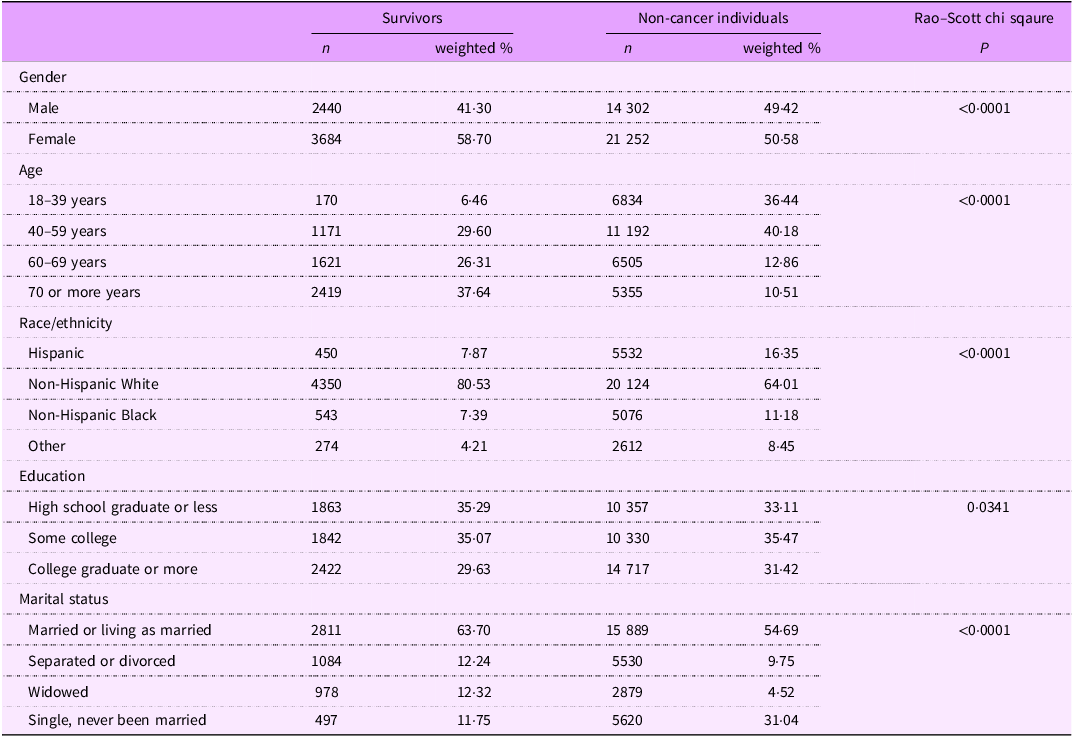
* Pooled and weighted data from HINTS 1 (2003), HINTS 4 cycle 1 (2011), HINTS 4 cycle 2 (2012), HINTS 4 cycle 3 (2013), HINTS 4 cycle 4 (2014), HINTS 5 cycle 1 (2017), HINTS 5 cycle 2 (2018), HINTS 5 cycle 3 (2019), HINTS 5 cycle 4 (2020) and HINTS 6 (2022).
Diet-related cancer risk awareness
Table 2 contains the results of single factor and multinominal logistic regression analysis for the diet-related awareness of cancer risk among cancer survivors and non-cancer individuals. Compared with non-cancer individuals, cancer survivors had slightly lower odds of saying that eating too much processed meat (OR = 0·98; 95 % CL = 0·82, 1·18) or red meat (OR = 0·97; 95 % CL = 0·76, 1·24) is unrelated to cancer risk but the results were not statistically significant. Similarly, cancer survivors might have better awareness regarding fibre (OR = 0·86; 95 % CL = 0·59, 1·24), fruit and vegetable (OR = 0·89; 95 % CL = 0·71, 1·11), alcohol (OR = 0·89; 95 % CL = 0·65, 1·23) and sugar-sweetened beverages (OR = 0·82; 95 % CL = 0·66, 1·02). However, none of the associations were statistically significant, which indicates that diet-related cancer risk awareness in cancer survivors is not different from that of non-cancer individuals.
Table 2 Multinominal logistic regression of diet-related awareness among cancer survivors and non-cancer individuals in HINTS *
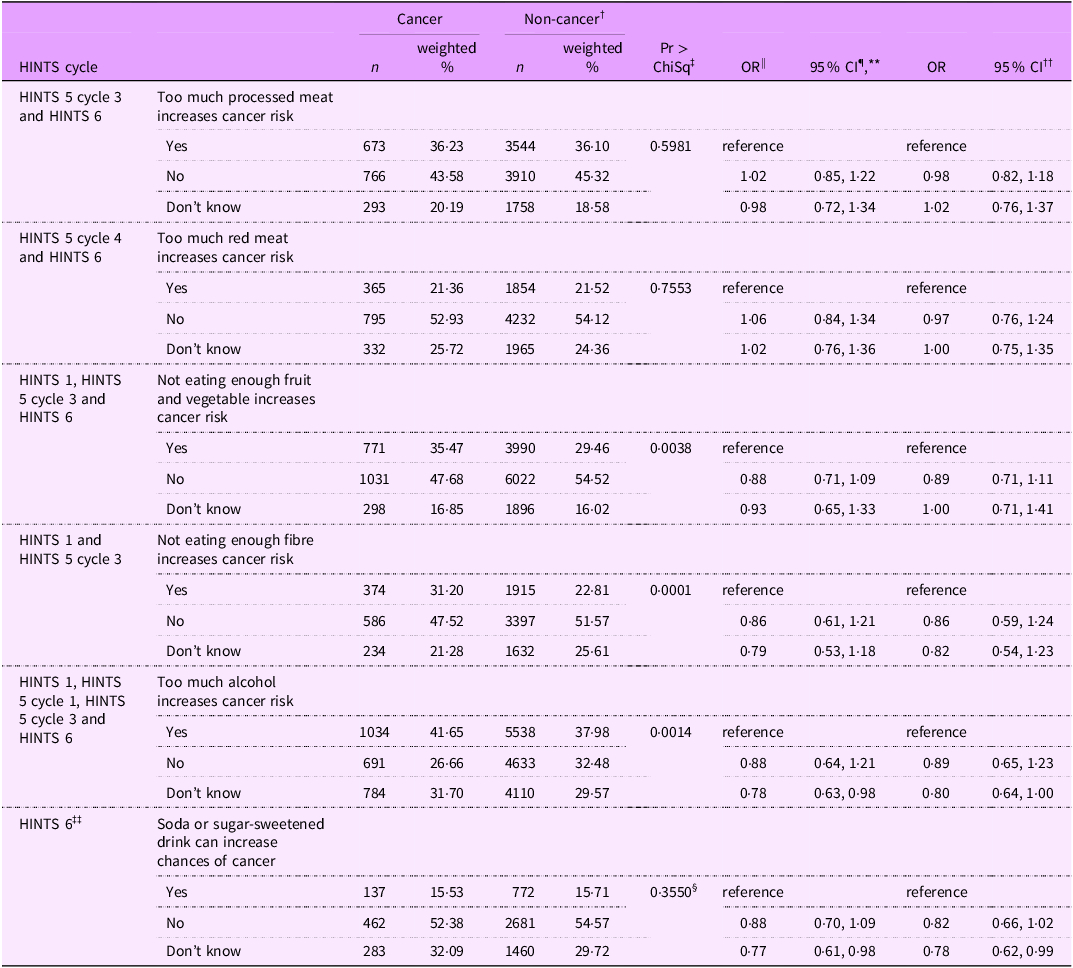
* HINTS = Health Information National Trends Survey.
† Reference.
‡ Rao–Scott Chi-Square test.
§ Pearson Chi-Square test.
** Adjusted for age and gender only.
†† Adjusted for age, gender, education level, BMI status, race/ethnicity and marital status.
‡‡ Unweighted n (%).
Diet-related behaviours
Table 3 includes the results of a single-factor, binary and multinominal logistic regression analysis for diet-related behaviours. Compared with non-cancer individuals, cancer survivors had slightly lower odds (OR = 0·91; 95 % CL = 0·77, 1·06) of not meeting the ACS fruit intake recommendations of consuming >= 2–3 cups of fruit per day. Although this association was not statistically significant, it is important to note that approximately 82 % of cancer survivors and non-cancer individuals failed to meet the ACS recommendations of consuming ≥ 2–3 cups of fruit daily. Similar associations were found among cancer survivors for the consumption of vegetable intake (OR = 0·96; 95 % CL = 0·83, 1·11), and approximately 75 % of both the groups did not meet the ACS recommendations of consuming ≥ 2–3 cups of vegetables per day. Compared with non-cancer individuals, cancer survivors had slightly lower odds of consuming > 10 alcoholic drinks per week on average (OR = 0·86; 95 % CL = 0·65, 1·13). However, approximately 36 % of cancer survivors and approximately 43 % of non-cancer individuals consumed > 10 alcoholic drinks per week on average. There were no significant associations relating to weekly sugar-sweetened beverage (soda) consumption (OR = 0·89; 95 % CL = 0·68, 1·17) as well as noticing (OR = 0·91; 95 % CL = 0·78, 1·06) or the usage (OR = 0·89; 95 % CL = 0·76, 1·03) of menu energy information in the two groups.
Table 3 Multiple logistic regression of diet-related behaviours among cancer survivors and non-cancer individuals in HINTS *
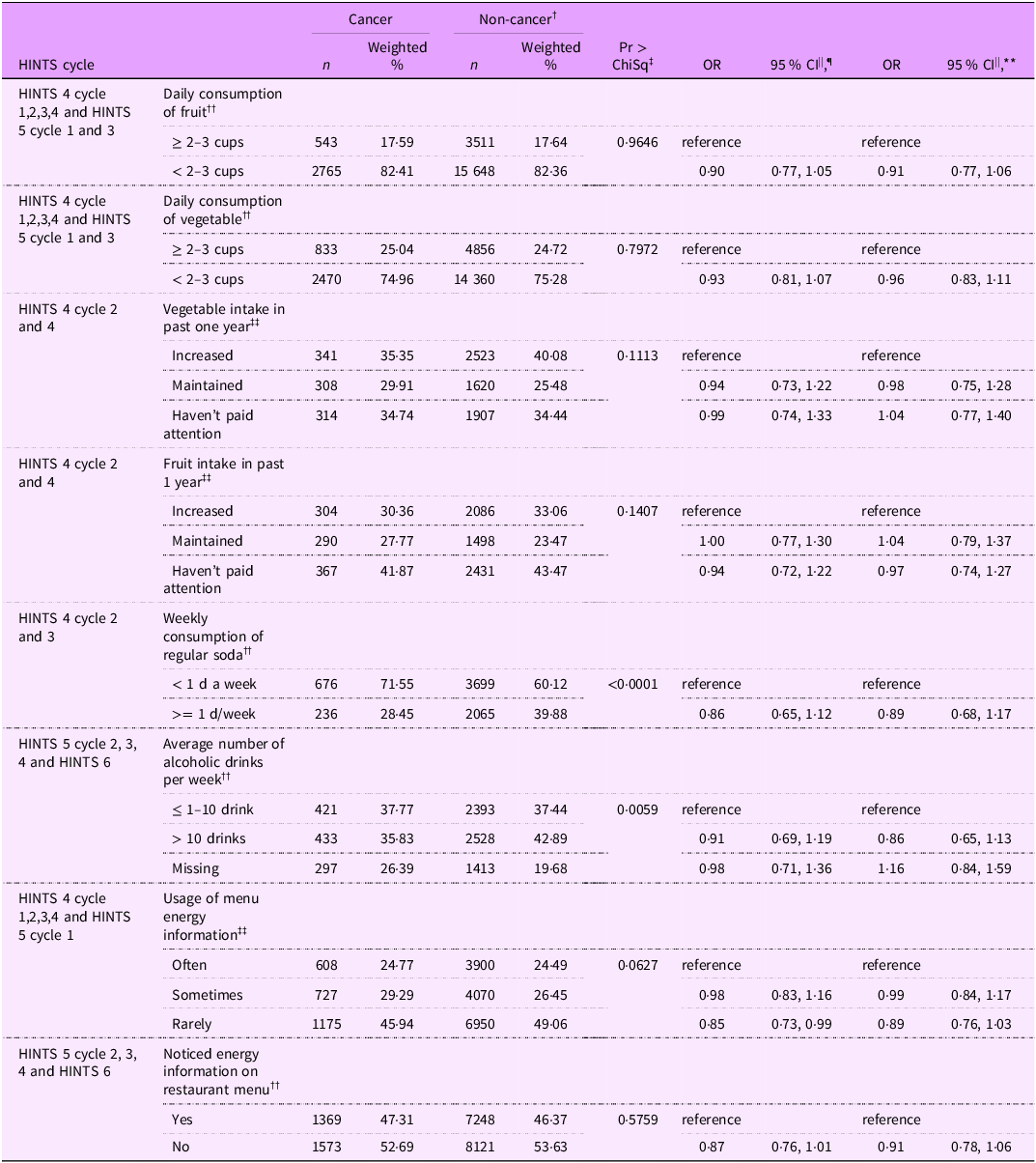
* HINTS = Health Information National Trends Survey.
† Reference.
‡ Rao–Scott Chi-Square test.
¶ Adjusted for age and gender only.
** Adjusted for age, gender, education level, BMI status, race/ethnicity and marital status.
†† Binary logistic regression.
‡‡ Multinominal logistic regression.
Discussion
This study investigated the differences in specific diet-related cancer risk awareness and behaviours between cancer survivors and those without a history of cancer. Our results indicate that there were no significant differences in diet-related cancer risk awareness and behaviours between the two groups. Despite this, the findings warrant further investigation to delve deeper into these dietary behaviours, particularly considering that 82 % and 75 % of cancer survivors failed to meet the ACS recommendations of consuming >= 2–3 cups of fruits and vegetables per day, respectively, which can contribute to increased risk of cancer recurrence, comorbidities and mortality. A cancer diagnosis is often regarded as a ‘teachable moment’ that increases openness to adopting a healthy lifestyle. Previous diet and physical activity interventions leveraging this ‘teachable moment’ have provided promising results(Reference Schulz, Locke and Campbell35–Reference Campbell, Carr and Devellis37). However, the lack of significant differences in dietary cancer risk awareness or behaviours in our study indicated the need for targeted dietary and behavioural interventions in post-cancer diagnosis care. Integrating diet and nutrition education and interventions into routine healthcare practices can help ensure that survivors receive the guidance needed to make beneficial lifestyle adjustments(Reference Bell38).
A study on dietary habits utilising NHANES 1999–2000 data showed consistent results with our findings. No discernible differences were found regarding total fruit and vegetable intake in cancer survivors and non-cancer individuals(Reference Zhang, Liu and John19). Another study based on the 2000 National Health Interview Survey indicated minimal differences in low fruit and vegetable consumption among cancer survivors and non-survivors(Reference Coups and Ostroff39). Similarly, an Australian study by Eakin and colleagues utilising the Australian National Health survey showed no significant differences in fruit and vegetable consumption between groups(Reference Eakin, Youlden and Baade40). Additionally, Joe-Milliron and team, analysing NHANES 2003–2006 data, found no substantial variations in fruit and vegetable intake between female breast cancer survivors and those without a cancer history. The study revealed that over 90 % of both groups fell short of meeting the recommended 5 servings of fruits and vegetables per day, as endorsed by WHO and USDA guidelines(Reference Milliron, Vitolins and Tooze22).
Studies on diet-related awareness of cancer risk in cancer survivors compared with non-cancer individuals were scarce, while many studies examined dietary knowledge or beliefs in cancer survivors. Beeken and colleagues conducted a qualitative study exploring beliefs about diet quality and cancer among adult cancer survivors in the UK through a semi-structured interview. Their results showed that survivors acknowledged the impact of diet on cancer development but exhibited less clarity regarding its role in recurrence(Reference Beeken, Williams and Wardle41). Additionally, their reports revealed a general lack of professional advice on diet. Similarly, other studies also highlighted a prevailing gap in nutritional guidance for cancer survivors(Reference O’Callaghan, Douglas and Keaver42,Reference Keaver, Douglas and O’Callaghan43) . One plausible hypothesis for our findings is that the survivors may not have enough resources or support to leverage the ‘teachable moment’ of cancer diagnosis. Furthermore, cancer survivors, particularly long-term survivors, may overestimate the healthfulness of their current diets(Reference Anderson, Steele and Coyle44,Reference Dowswell, Ryan and Taylor45) . This overestimation can lead to a gap between perceived and actual dietary quality. For instance, findings from a prospective population-based investigation of colorectal cancer survivors found that nutritional information positively influenced their beliefs about the role of nutrition in cancer outcomes(Reference van Veen, Mols and Smeets46). This underscores the need for targeted dietary behaviour change interventions among cancer survivors.
Moreover, the findings from our study might highlight the need for adequate training in nutrition and dietary counselling among healthcare providers, which may offer targeted dietary advice to cancer survivors. Research studies have reported that healthcare professionals often felt unequipped to provide effective dietary guidance due to limited training in this area(Reference Crowley, Ball and Hiddink47,Reference Bassin, Al-Nimr and Allen48) . For example, a study found that fewer than 30 % U.S. medical schools met the recommended 25 h of nutrition education across their curricula. This lack of preparation can be particularly concerning in oncology and primary care. Integrating more robust nutrition training into medical education and ongoing professional development can equip healthcare providers to deliver personalised and culturally sensitive dietary guidance effectively. Additionally, time constraints for providing nutritional advice and gaps in interprofessional collaboration are also noteworthy barriers(Reference DiMaria-Ghalili, Mirtallo and Tobin49–Reference Agwara, Martyn and Macaninch51). Allocating greater resources to nutritional education and service for cancer survivors and enhancing interprofessional collaborations are essential policy considerations. The healthcare providers can play a proactive role in helping patients make meaningful lifestyle changes that support cancer prevention and survivorship.
A limitation of this study is that it uses self-reported data that is prone to social desirability and recall bias. Nevertheless, we anticipate minimal reporting errors for cancer diagnosis, considering that cancer is a life-threatening event. Since data is analysed cross-sectionally, causal inferences cannot be drawn from the results. We cannot evaluate whether dietary awareness and behaviours among cancer survivors changed after cancer diagnosis. The diet-related cancer risk awareness and behaviours in cancer survivors could be influenced by the stage of cancer and the treatments they undergo in each stage, involving intricate pathways. Unfortunately, HINTS did not collect data on these factors, preventing us from assessing how cancer stage and treatment impact the diet-related cancer risk awareness and behaviours of survivors. High percentage of missingness in the outcome variable of alcohol intake may have impacted the relevant results. Despite these limitations, our study population was drawn from a nationally representative sample of the US population collected using a standardised approach by the National Cancer Institute. We harmonised the study variables by pooling several survey iterations using survey weights based on the methodology developed by the HINTS. The multiple logistic regression was adjusted for potential confounders. Furthermore, the test for multicollinearity showed that all variance inflation factors were less than 2 in all models which suggested the effect of multicollinearity on model reliability is minimal. Ultimately, we examined interactions between independent variables and considered them in the final models. The validity of the findings can be strengthened, and potential bias further minimised.
Conclusion
This study represents a pivotal investigation of diet-related cancer risk awareness and behaviours. Utilising the HINTS data, this research brings to light a notable absence of significant differences in diet-related cancer risk awareness and behaviours between cancer survivors and those without a history of cancer. Besides, our study found that approximately 85 % and 75 % of participants do not meet the ACS recommendations for daily fruit and vegetable intake, respectively, emphasises the critical need for targeted and feasible dietary interventions tailored to the distinctive challenges faced by the vulnerable population. Healthcare professionals should integrate culturally sensitive nutritional education and counselling services into routine survivorship care, which may help cancer survivors adopt and maintain healthy dietary behaviours. Policy changes to consider include allocating additional resources and promoting consistent interprofessional collaboration.
Given the cross-sectional nature of this study, the absence of statistically significant differences in dietary awareness or behaviours between cancer survivors and non-cancer survivors does not necessarily imply the absence of an association. Future longitudinal cohort studies assessing dietary awareness and behaviours before and after cancer diagnoses are warranted, as they would provide stronger evidence regarding temporality.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980025100505
Acknowledgements
We thank the participants of HINTS and all persons who contributed to the data collection and management.
Financial support
H.M. received a summer fellowship from the Cancer Epidemiology Education in Special Populations (CEESP) Program, through a grant from the National Cancer Institute (Grant R25 CA112383) and the University of California Presidential Endowment. Dr. Yunxia Lu is her mentor for this work.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Authorship
H.M. contributes to conceptualisation, data retrieval, curation, formal analysis, manuscript writing and editing. Y.L. contributes to conceptualisation, supervision of data retrieval and data analysis, manuscript writing and critical editing.
Ethics of human subject participation
This study was deemed exempt by the University of California Irvine Institutional Review Board under federal regulation 45 46·101 (b) CFR (www.hhs.gov/ohrp/humansubjects/guidance/45cfr46.html.). All data used in this study can be downloaded from the website of HINTS (https://hints.cancer.gov).
Data availability: All data used in this study can be downloaded from the website of HINTS (https://hints.cancer.gov).





