Introduction
Psychotic disorders are among the leading causes of disability (Navarro-Mateu et al., Reference Navarro-Mateu, Alonso, Lim, Saha, Aguilar-Gaxiola and Al-Hamzawi2017) and a public health concern worldwide (Anderson, Reference Anderson2019). Besides genetic predisposition, severe forms of childhood adversity (CA), such as childhood abuse or neglect, parental discord or loss, peer bullying, or household poverty, have consistently been shown to strongly increase the risk for mental disorders, including psychotic disorders (Arango et al., Reference Arango, Dragioti, Solmi, Cortese, Domschke, Murray and Fusar-Poli2021; Morgan & Gayer-Anderson, Reference Morgan and Gayer-Anderson2016). CA is the most robust and potentially modifiable risk factor for schizophrenia spectrum disorders (Dragioti et al., Reference Dragioti, Radua, Solmi, Arango, Oliver, Cortese and Fusar-Poli2022) and can also influence the clinical manifestations and course of the illness (Rosenfield, Jiang, & Pauselli, Reference Rosenfield, Jiang and Pauselli2022; Sideli et al., Reference Sideli, Murray, Schimmenti, Corso, La Barbera, Trotta and Fisher2020; Turner et al., Reference Turner, Harvey, Hayes, Castle, Galletly, Sweeney and Spittal2019), including social and functional outcomes (Christy et al., Reference Christy, Cavero, Navajeeva, Murray-O'Shea, Rodriguez, Aas and Alameda2023; Fares-Otero et al., Reference Fares-Otero, Alameda, Pfaltz, Martinez-Aran, Schäfer and Vieta2023a). General population-based studies indicate that approximately half of all children will experience at least one form of adversity by the time they reach adulthood (McLaughlin, Weissman, & Bitrán, Reference McLaughlin, Weissman and Bitrán2019), and approximately half of youth with first-episode psychosis (FEP) report at least one type of CA (Vila-Badia et al., Reference Vila-Badia, Del Cacho, Butjosa, Serra Arumí, Esteban Santjusto, Abella and Usall2022).
At a neurobiological level, CA has been found to trigger a cascade of neurobiological processes that may impact brain structure and function (Begemann et al., Reference Begemann, Schutte, van Dellen, Abramovic, Boks, van Haren and Sommer2023; Lim, Howells, Radua, & Rubia, Reference Lim, Howells, Radua and Rubia2020; Quinlan et al., Reference Quinlan, Barker, Luo, Banaschewski, Bokde, Bromberg and Schumann2020). CA can lead to lasting structural changes in a number of brain regions that include cortical regions relevant for processing trauma information, as well as key areas implicated in emotion processing, such as the anterior cingulate cortex and hippocampus, as well as in threat processing and higher-order cognitive functions, such as the amygdala (Calem, Bromis, McGuire, Morgan, & Kempton, Reference Calem, Bromis, McGuire, Morgan and Kempton2017; Gold et al., Reference Gold, Sheridan, Peverill, Busso, Lambert, Alves and McLaughlin2016; Teicher, Samson, Anderson, & Ohashi, Reference Teicher, Samson, Anderson and Ohashi2016) and the orbitofrontal cortex (Bounoua, Miglin, Spielberg, Johnson, & Sadeh, Reference Bounoua, Miglin, Spielberg, Johnson and Sadeh2022). Such structural changes may be adaptive in the face of expected future adversity and seem to extend to most individuals exposed to adversity, regardless of psychopathology. Interestingly, there seems to be an overlap between brain structures and regions affected in people exposed to CA and those reported to be altered in psychotic disorders (Hoy et al., Reference Hoy, Barrett, Shannon, Campbell, Watson, Rushe and Mulholland2012), including reductions in hippocampal volume (Adriano, Caltagirone, & Spalletta, Reference Adriano, Caltagirone and Spalletta2012) and fronto-temporal, insular and occipital abnormalities with a similar pattern in both FEP (Vieira et al., Reference Vieira, Gong, Scarpazza, Lui, Huang, Crespo-Facorro and Mechelli2021) and those exposed to CA (Calem et al., Reference Calem, Bromis, McGuire, Morgan and Kempton2017; Pollok et al., Reference Pollok, Kaiser, Kraaijenvanger, Monninger, Brandeis, Banaschewski and Holz2022).
The vulnerability-stress and traumagenic neurodevelopmental models of psychosis posit that CAs may impact the structural development of the brain over time, increasing the vulnerability of exposed individuals to FEP (Lardinois, Lataster, Mengelers, Van Os, & Myin-Germeys, Reference Lardinois, Lataster, Mengelers, Van Os and Myin-Germeys2011; Read, Fosse, Moskowitz, & Perry, Reference Read, Fosse, Moskowitz and Perry2014). However, to date, neuroimaging studies of CA in individuals with FEP have focused mostly on volume changes in some brain structures, e.g., the hippocampus and/or amygdala (Aas et al., Reference Aas, Navari, Gibbs, Mondelli, Fisher, Morgan and Dazzan2012; du Plessis et al., Reference du Plessis, Scheffler, Luckhoff, Asmal, Kilian, Phahladira and Emsley2020; Hoy et al., Reference Hoy, Barrett, Shannon, Campbell, Watson, Rushe and Mulholland2012). Although this work has been important for understanding how CA shapes key stress-related brain mechanisms in people with FEP, this approach has prevented comprehensive research on whole-brain regions. Cortical thickness, which reflects the number of neurons within cortical columns (Narr et al., Reference Narr, Bilder, Toga, Woods, Rex, Szeszko and Thompson2005; Rakic, Reference Rakic1988), appears to be highly susceptible to environmental factors and could constitute a more specific and biologically meaningful metric of neurodevelopmental processes than brain volume (Panizzon et al., Reference Panizzon, Fennema-Notestine, Eyler, Jernigan, Prom-Wormley, Neale and Kremen2009).
A number of studies have indicated the effects of CAs on cortical thickness (Cassiers et al., Reference Cassiers, Sabbe, Schmaal, Veltman, Penninx and Van Den Eede2018). For instance, one study demonstrated reduced visual cortex thickness in adults who witnessed domestic violence in childhood (Tomoda, Polcari, Anderson, & Teicher, Reference Tomoda, Polcari, Anderson and Teicher2012). A meta-analysis revealed that neglect and abuse were associated with reduced thickness in the superior temporal sulcus, supramarginal gyrus, parietal lobe, middle temporal lobe, and the praecuneus (Tozzi et al., Reference Tozzi, Garczarek, Janowitz, Stein, Wittfeld, Dobrowolny and Frodl2020). Additionally, in individuals with FEP, several studies have shown cortical alterations in fronto-temporal networks (Schultz et al., Reference Schultz, Koch, Wagner, Roebel, Schachtzabel, Gaser and Schlösser2010) and the prefrontal cortex (Wiegand et al., Reference Wiegand, Warfield, Levitt, Hirayasu, Salisbury, Heckers and Shenton2004). These cortical alterations have been mostly observed in those with non-affective (v. affective) FEP (Zhao et al., Reference Zhao, Zhang, Shah, Li, Sweeney, Li and Gong2022).
While several studies have previously reported data on reduced cortical thickness in people with FEP (Crespo-Facorro et al., Reference Crespo-Facorro, Roiz-Santiáñez, Pérez-Iglesias, Rodriguez-Sanchez, Mata, Tordesillas-Gutierrez and Vázquez-Barquero2011; Pigoni et al., Reference Pigoni, Dwyer, Squarcina, Borgwardt, Crespo-Facorro and Dazzan2021; Pina-Camacho et al., Reference Pina-Camacho, Martinez, Diaz-Caneja, Mezquida, Cuesta and Moreno2022; Wiegand et al., Reference Wiegand, Warfield, Levitt, Hirayasu, Salisbury, Heckers and Shenton2004), as well as in those exposed to CAs (McCrory, De Brito, & Viding, Reference McCrory, De Brito and Viding2011; Teicher et al., Reference Teicher, Samson, Anderson and Ohashi2016; Yang et al., Reference Yang, Jin, Duan, Yu, Ping, Shen and Zhou2023), no previous study has explored potential similarities between patterns of CA and FEP on cortical thickness alterations or assessed the link between the effects of exposure to different types of CAs and FEP on cortical thickness across brain regions. Disentangling the effects of FEP from those of CAs is an important step in better understanding psychotic disorders and could have important consequences for prevention, diagnosis, and treatment. Furthermore, previous evidence indicates differential effects of specific types of CA (physical, emotional, sexual abuse, neglect) (Cancel et al., Reference Cancel, Comte, Truillet, Boukezzi, Rousseau, Zendjidjian and Fakra2015; Stevelink et al., Reference Stevelink, Abramovic, Verkooijen, Begemann, Sommer, Boks and Vinkers2018) and dimensional approaches (threat v. deprivation) on brain architecture (LoPilato et al., Reference LoPilato, Goines, Addington, Bearden, Cadenhead, Cannon and Walker2019; Thomas et al., Reference Thomas, Rakesh, Whittle, Sheridan, Upthegrove and Cropley2023), which could help disentangle the neurobiological correlates of CA in psychosis; however, further studies are needed in individuals with FEP.
Consequently, we explored the differential and shared patterns of cortical thickness alterations associated with exposure to CA and FEP and we investigated the interactive effects of different types/dimensions of CA and FEP on cortical thickness across brain regions. On the basis of collective evidence from previous studies (Cascino et al., Reference Cascino, Canna, Russo, Monaco, Esposito, Di Salle and Monteleone2023; Kim et al., Reference Kim, Lee, Kang, Kang, Kim, Tae and Han2023; Luo et al., Reference Luo, Chen, Li, Lin, Yu, Lin and Peng2023; Rapado-Castro et al., Reference Rapado-Castro, Whittle, Pantelis, Thompson, Nelson, Ganella and Bartholomeusz2020), we hypothesized that FEP and CA would be associated with reduced cortical thickness and that interactive effects across brain regions would be dependent on CA types and dimensions.
Methods
Participants and study design
This study includes participants from the ‘Genes and Environment in Schizophrenia’ (AGES-CM) project (https://web.agescm.es/), an ongoing multicentric study carried out at the seven largest university hospitals in the Community of Madrid, Spain, that recruits patients seeking help for recent-onset psychosis. The study protocol, sampling characteristics and methods are described in detail elsewhere (Izquierdo et al., Reference Izquierdo, Cabello, Leal, Mellor-Marsá, Ayora, Bravo-Ortiz and Malpica2021). Briefly, the study included sociodemographic, clinical and functional examinations, CA questionnaires, and brain magnetic resonance (MRI) scanning.
The study obtained ethical approval from the medical research ethics committee of the coordinating center Hospital General Universitario Gregorio Marañón (identification number 355/12), and was subsequently reviewed and approved by the research ethics committee of each participating center. The study was conducted in accordance with the Declaration of Helsinki and its later amendments. Prior to study inclusion, participants and/or their legal guardians provided written informed consent.
The inclusion criteria for individuals with FEP were as follows: (a) aged between 7 and 40 years at the time of the first evaluation; (b) experienced FEP (Breitborde, Srihari, & Woods, Reference Breitborde, Srihari and Woods2009) with a total lifetime duration of positive psychotic symptoms shorter than 24 months; and (c) provided written informed consent or parental consent for the minors. The exclusion criteria were (a) meeting diagnostic criteria for another current Axis-I mental disorder (except for substance use disorder); (b) the presence of intellectual disability, defined as an intelligence quotient (IQ) < 70 with impaired functioning; (c) a history of neurodevelopmental and/or neurological disorders (e.g. epilepsy) or head injury with loss of consciousness; and (d) were pregnant at the time of signing the consent form. The inclusion criteria for HCs were as follows: (a) aged between 7 and 40 years at the time of the first evaluation and (b) provided written informed consent or parental consent for the minors. The exclusion criteria were (a) met the diagnostic criteria for current Axis-I mental disorders; (b) had a history of intellectual disability or a history of neurodevelopmental and/or neurological disorders or head injury with loss of consciousness; (c) had a family history of a psychotic disorder in a first- or second-degree relative; (d) were pregnant at the time of signing the consent form; and (e) refused to perform MRI or blood tests to ensure that neuroimaging data and laboratory samples were obtained.
Additional exclusion criteria, for both participants with FEP and HCs, to undergo MRI assessment were contraindications such as metal implants or claustrophobia.
Sociodemographic data and clinical assessment
Relevant sociodemographic data, including age, sex, ethnicity, educational level, marital status, and work status were collected. Parental socioeconomic status (SES) was determined using Hollingshead's Two-Factor Index of Social Position (Hollingshead & Redlich, Reference Hollingshead and Redlich2007), a common system that is based upon parental occupation and educational levels (i.e. years of education and highest educational degree of the parent with the highest level).
Clinical assessment was conducted by trained psychiatrists or psychologists who confirmed the diagnosis of FEP, including non-affective (i.e. schizophrenia spectrum and other psychoses) v. affective FEP (i.e. bipolar disorder type I or major depressive disorder with psychotic symptoms), using the Semistructured Diagnostic Interview for DSM-IV-TR Axis I Disorders (SCID-I) or the Spanish version of the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime version (K-SADS-PL) (Ulloa et al., Reference Ulloa, Ortiz, Higuera, Nogales, Fresán, Apiquian and de la Peña2006), as appropriate. Further clinical assessment details and the reliability of the instruments used are presented in S1 in the Supplement.
Childhood adversity
Self-reported exposure to different types of CA was determined using an adapted version of the Childhood Experience of Care and Abuse Questionnaire (CECA-Q) (Bifulco, Bernazzani, Moran, & Jacobs, Reference Bifulco, Bernazzani, Moran and Jacobs2005). The CECA-Q is designed to assess CA that occurs before the age of 17 years, including emotional or physical abuse by the main parental figures (usually but not necessarily the biological mother or father), sexual abuse by any adult or an individual at least five years older than the recipient, neglect, i.e. physical neglect or failure to give needed care or attention, and emotional neglect, including neglectful failure to supply emotional needs (Golden, Samuels, & Southall, Reference Golden, Samuels and Southall2003), household poverty, parental discord (including frequent disagreements and insults between parents), separation from a parent that involved living apart from the parent for at least 6 months, death of a parent or parental loss, and/or being expelled or suspended from school and/or high school. Each CA (occurring < 17 years) was scored dichotomously as being present or absent for the participant. The items were dichotomously scored as 0 = no (‘did not apply to me at all’) or 1 = yes (‘applied to me’) based on a previous report (Fisher et al., Reference Fisher, Morgan, Dazzan, Craig, Morgan, Hutchinson and Fearon2009).
A history of bullying by peers, including emotional or verbal and/or physical victimization, before 17 years of age was assessed using an adapted short version of the Retrospective Bullying Questionnaire (RBQ) (Guloksuz et al., Reference Guloksuz, Pries, Delespaul, Kenis, Luykx, Lin and van Os2019; Schäfer et al., Reference Schäfer, Korn, Smith, Hunter, Mora-Merchán, Singer and Van der Meulen2004), which measures the self-reported severity of bullying exposure as follows: 0 = ‘none’; 1 = ‘some (no physical injuries)’; 2 = ‘moderate (minor injuries or transient emotional reactions)’; 3 = ‘marked (severe and frequent physical or emotional harm)’. For the purposes of this study, exposure to childhood bullying was dichotomized using ≥ 1 as the cut-off point (0 = ‘absent’ and ≥1 = ‘present’).
Based on the dimensional model of early adversity (McLaughlin, Sheridan, & Lambert, Reference McLaughlin, Sheridan and Lambert2014) and prior evidence synthesis (Thomas et al., Reference Thomas, Rakesh, Whittle, Sheridan, Upthegrove and Cropley2023), we operationalized deprivation as experiences of emotional and/or physical neglect (or failure to meet the child's basic needs, including food and clothing), household poverty, and separation from parents (i.e. when the child's attachment to his or her caregiver is significantly broken due to no or poor quality care being given to the child) > 6 months. This period is long enough for the child-caregiver bond to be seriously damaged. We operationalized threat as emotional, physical or sexual abuse; bullying (i.e. emotional and/or physical peer victimization); and parental discord (including witnessing parental victimization) (Peverill et al., Reference Peverill, Rosen, Lurie, Sambrook, Sheridan and McLaughlin2023; Sumner, Colich, Uddin, Armstrong, & McLaughlin, Reference Sumner, Colich, Uddin, Armstrong and McLaughlin2019).
MRI acquisition, image data processing, and cortical thickness calculation
Structural whole-brain T1-weighted images were acquired using a 3T General Electric Signa HDxt scanner with a 3D FSPGR (coronal slices parallel to the anterior commissure-posterior line without a gap; repetition time, 1900 ms; echo time, 2.6 ms; field of view, 220 mm; matrix size, 256 × 256; slice thickness, 1 mm; voxel volume, 0.86 × 0.86 × 1 mm2; flip angle, 16°; number of excitations, 1). The quality of the scans was visually assessed prior to image processing, and no scans were deemed of insufficient quality. FreeSurfer (v6.0, http://surfer.nmr.mgh.harvard.edu/) was used to estimate cortical thickness (Fischl et al., Reference Fischl, Salat, Busa, Albert, Dieterich, Haselgrove and Dale2002) for 68 cortical regions of interest (ROIs, 34 in the left hemisphere and 34 in the right hemisphere) of the ‘Desikan-Killiany’ cortical atlas (Desikan et al., Reference Desikan, Ségonne, Fischl, Quinn, Dickerson, Blacker and Killiany2006). All the segmentation methods were found to be accurate after visual inspection.
Statistical analyses
Frequency analysis was performed to evaluate the characteristics of the sample. To test for differences in categorical variables, chi-square tests (χ2) were performed between groups. To test for differences in continuous variables, Student's t test was used.
Pearson correlations were performed between the brain maps of CA effects and the brain map of FEP effects (in participants not exposed to CA). General linear models (GLMs) were performed to assess the interactive effects between different CAs and FEP on cortical thickness. The Family-Wise Error Rate (FWER) Holm method (Holm, Reference Holm1979) was used to correct p values (pcorr) for multiple comparisons across multiple cortical regions.
Age and sex were considered covariates given that these factors have been associated with neural structure developmental trajectories between childhood and early adulthood (Cheng et al., Reference Cheng, Mills, Miranda Dominguez, Zeithamova, Perrone, Sturgeon and Mackiewicz Seghete2021; Kim et al., Reference Kim, Lee, Kang, Kang, Kim, Tae and Han2023; Luo et al., Reference Luo, Chen, Li, Lin, Yu, Lin and Peng2023).
Power and sample size calculation for analyzing the different interaction effects was performed using the R package InteractionPoweR (Baranger et al., Reference Baranger, Finsaas, Goldstein, Vize, Lynam and Olino2023). With 214 individuals, the power to detect a weak to moderate interaction correlation (0.35) with p < 0.05/1292 (the number of interactions to test: 19 CAs × 68 cortical regions) was 80.7%.
For all analyses, p values less than 0.05 and 95% confidence intervals (CIs) were used to indicate statistical significance. Statistical analyses were conducted using R version 4.1.2 (R Core Team, 2021), and brain figures were created using the ggseg package (Mowinckel & Vidal-Piñeiro, Reference Mowinckel and Vidal-Piñeiro2020).
Results
Participant characteristics
Of 357 individuals recruited in the AGES-CM 2-CM project, 214 who completed MRI scans and CA measurements at baseline were included; 116 individuals with FEP (mean age ± s.d. = 23.8 ± 6.9 years, 34% female, 80.2% non-affective FEP), and 98 HCs (mean age ± s.d. = 25.1 ± 5.3 years, 43% female). No significant differences were found between groups with respect to age, sex, or educational level. Further sociodemographic and clinical characteristics of the study population are presented in Table 1.
Table 1. Sociodemographic and clinical characteristics of the sample (N = 214)
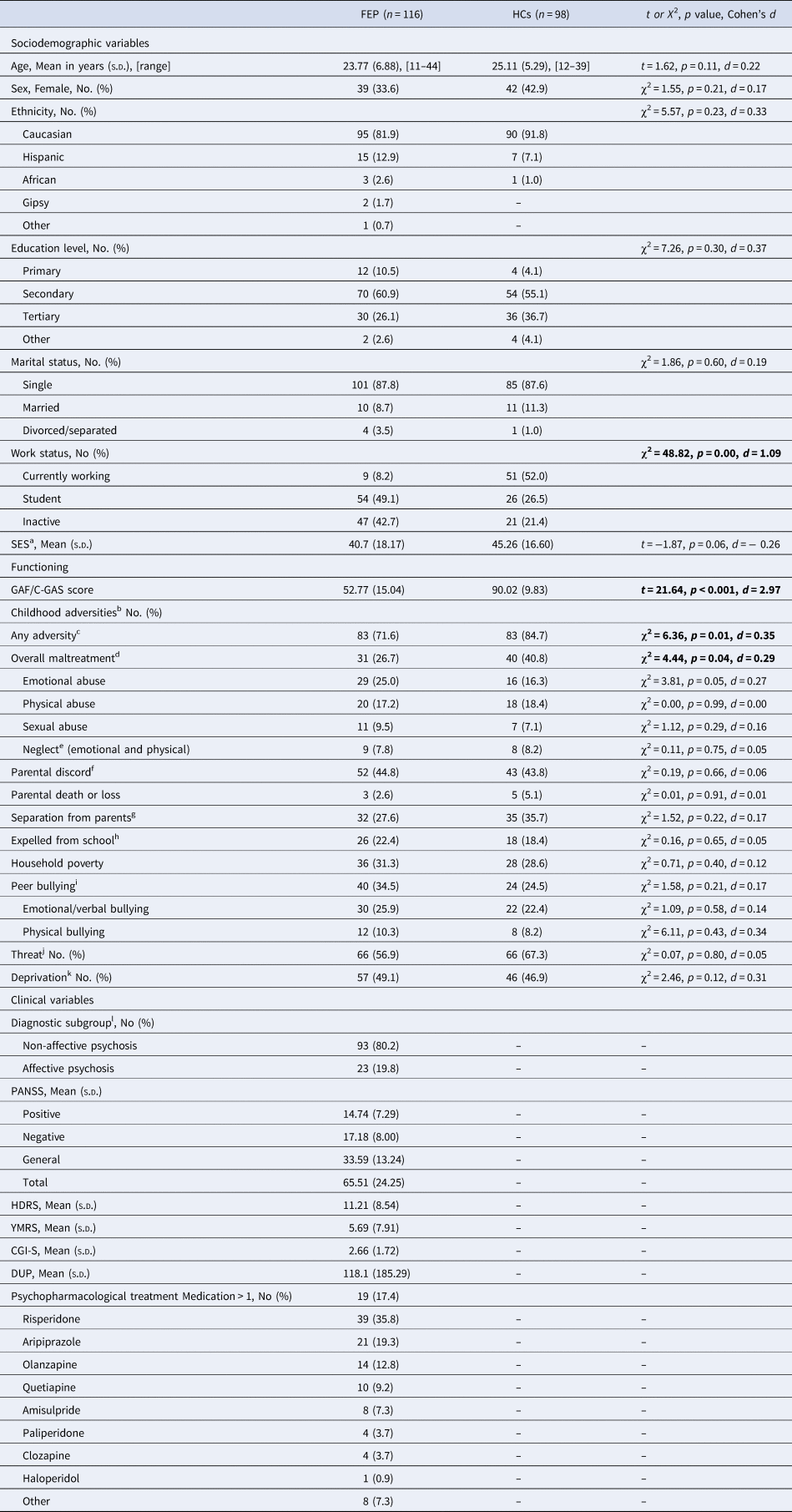
C-GAS, Child Global Assessment Scale; CGI-S, Illness severity; DUP, Duration of Untreated Psychosis (in days); GAF, Global Assessment of Functioning; HC, Healthy control group; HDRS, Hamilton Depression Rating Scale; NA, Not available; YMRS, Young Mania Rating Scale; PANNS, Positive and Negative Syndrome Scale; s.d., Standard deviation.
Note: In all cells, % refers to percentages (within columns) of participants for whom information was available. For qualitative variables, Chi-square (χ2) test was used. For quantitative variables, Student t test was used, significant at p < 0.05; Cohen's d was used for standardized effect sizes (0.20 = small, 0.50 = medium, and 0.80 = high) (Cohen, Reference Cohen1988).
a SES: Socioeconomic Status defined with the Hollingshead's Index.
b Frequencies reflect experience of at least one behavior associated with each childhood adversity (CA) subtype occurring before the age of 17 years.
c With at least one CA.
d Overall maltreatment: Frequencies reflect experience of at least one behavior associated with each abuse or neglect subtype occurring before the age of 17 years.
e Neglect: the failure to meet child's basic needs (emotional and/or physical).
f Parental discord: domestic violence including witnessing the abuse or violence of their parents.
g Separation from parents: a parent-child separation that involved living apart from the parent > 6 months.
h Expelled from school and/or high school.
i Peer bullying: Frequencies reflect experience of at least one behavior associated with each bullying subtype (i.e. emotional/verbal, or physical).
j Threat: experiences of harm or threat of harm (i.e. emotional, physical, sexual abuse, bullying, parental discord).
k Deprivation: the absence of expected inputs from the environment or absence of stimulation that occurs in the context of caregiver interactions (i.e. experiences of neglect, household poverty, separation from parents > 6 months).
l Non-affective FEP: schizophrenia spectrum disorders and other psychoses; Affective FEP: bipolar disorder type I or major depressive disorder with psychotic symptoms.
Exposure to CA types in the sample
Frequencies of exposure to each CA type in participants with FEP are shown in Table 1, and in those with non-affective v. affective FEP are shown in Table S2 in the Supplement. The percentages of individuals who reported at least one CA (72% FEP v. 85% HCs, p = 0.01) and overall maltreatment (i.e. physical/emotional/sexual abuse and/or physical/emotional neglect) (27% FEP v. 41% HCs, p = 0.04) were greater in HCs than in individuals with FEP.
Correlations between brain maps of CA effects and FEP effects
The spatial distribution of cortical thickness abnormalities was similar (r = 0.82) in individuals with CA and in those with FEP (v. HCs). When different types of CA were considered, cortical thickness alterations in individuals with FEP were similar to those found in individuals with exposure to separation from parents (>6 months), bullying, parental discord, household poverty, and sexual abuse (r = 0.50 to 0.25), see online Supplementary Table S3 and for diagnoses (non-affective v. affective psychosis) see Table S4 in the Supplement.
Interaction between CA and FEP effects on cortical thickness
In terms of CA and FEP, exposure to any adversity (p = 0.008), threat (p = 0.042), or overall maltreatment (p = 0.045) was associated with experiencing FEP. This significant association between CA and experiencing FEP was observed in non-affective psychosis (see Table S5.1 in the Supplement).
In terms of FEP and cortical thickness, experiencing FEP was associated with cortical thinning in both the left and right hemispheres across the occipital, temporal, parietal, and frontal regions (p < 0.001 to p = 0.039) (see Fig. 1). This significant association between FEP and cortical thickness was observed in non-affective psychosis (see Tables S5.2 and S5.3 in the Supplement).
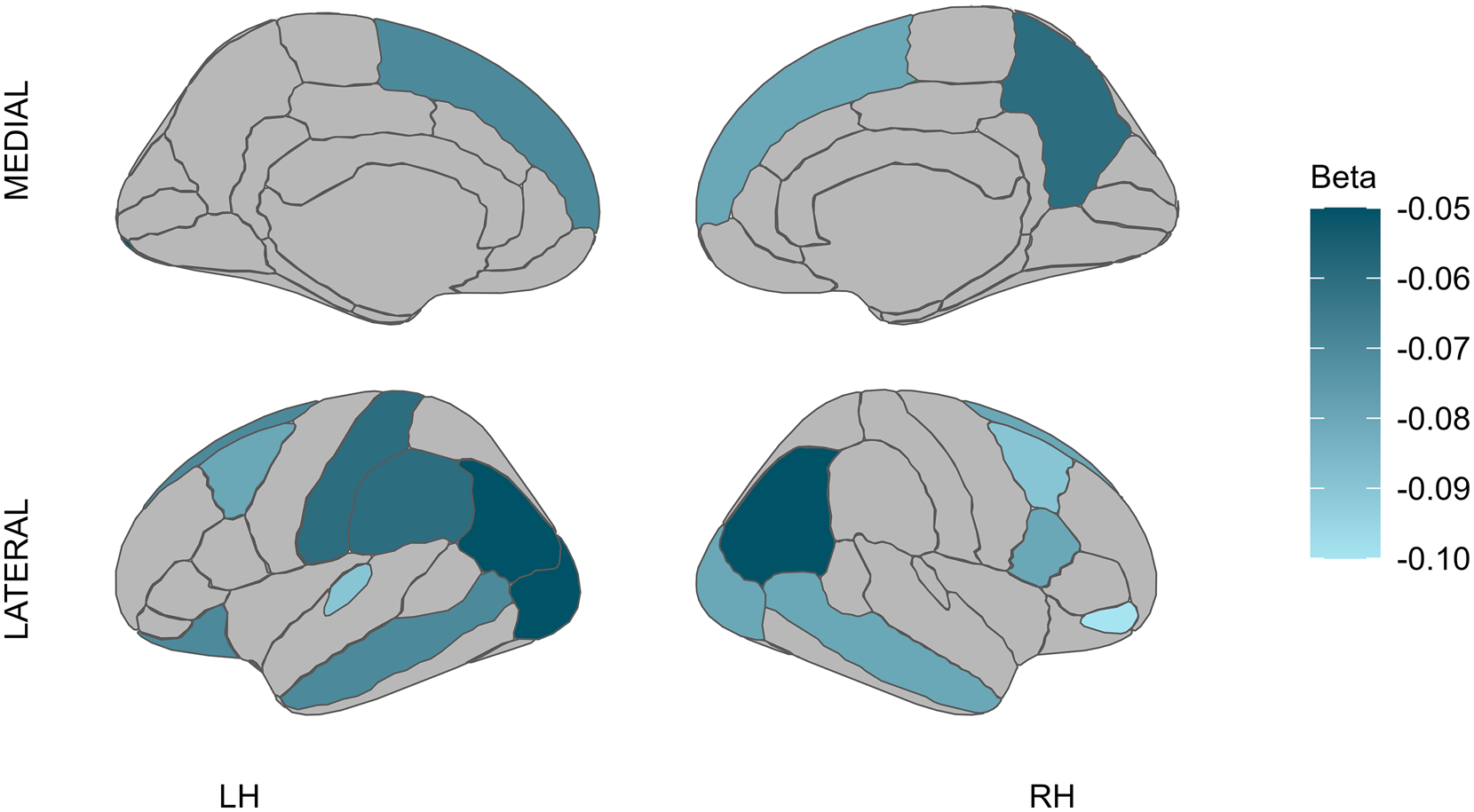
Figure 1. Effects of FEP on cortical thickness.
Note: LH, left hemisphere; RH, right hemisphere; lighter blue represents cortical thinning.
In HCs, exposure to emotional abuse (β = −0.09, [−0.14 to −0.04], pcorr = 0.026) was associated with cortical thinning in the left hemisphere paracentral region, whereas sexual abuse (β = 0.14, [0.06–0.22], pcorr = 0.040) was associated with cortical thickening in the left hemisphere medial orbitofrontal region (see Fig. 2).
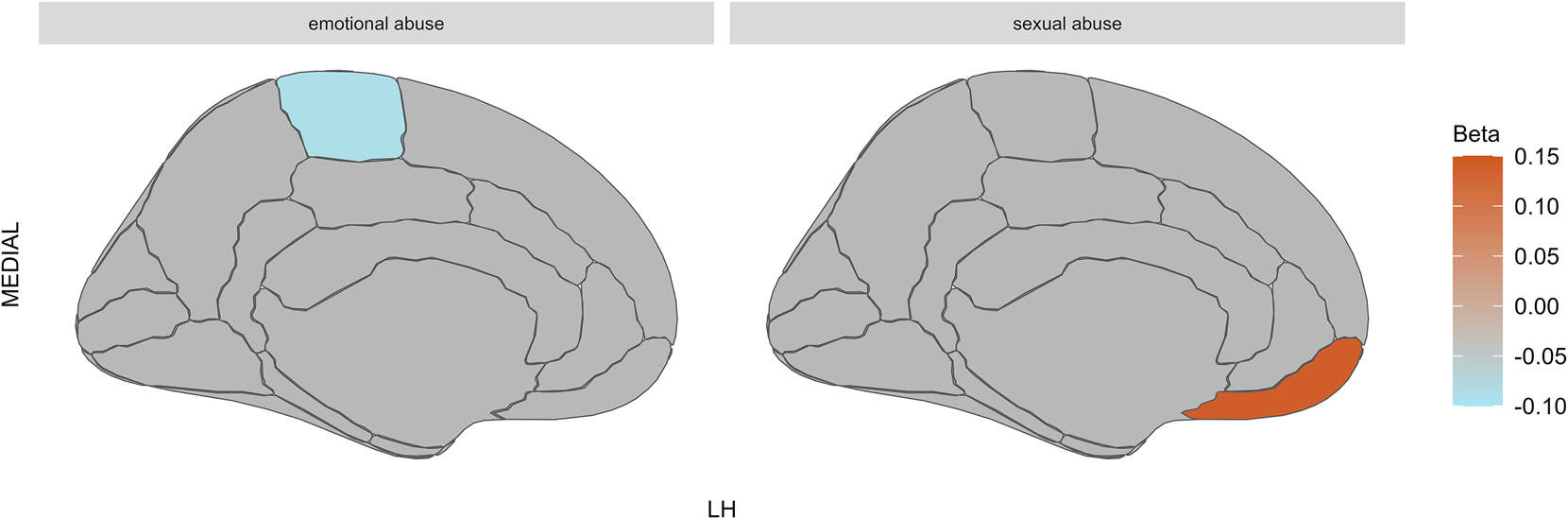
Figure 2. Effects of CA on cortical thickness in HCs.
Note: HCs, healthy controls; LH, left hemisphere; MEDIAL, medial view of the brain; lighter blue represents cortical thinning; stronger orange represents cortical thickening. The figure on the left shows that exposure to emotional abuse was associated with cortical thinning in the left hemisphere paracentral region; the figure on the right shows that sexual abuse was associated with cortical thickening in the left hemisphere medial orbitofrontal region in HCs.
In the overall FEP sample, exposure to neglect was associated with cortical thinning in the right medial orbitofrontal region (β = −0.24, [−0.37 to −0.12], pcorr = 0.016). This significant interaction effect between FEP and neglect was observed for both non-affective FEP (β = −0.22, [−0.35 to −0.10], uncorrected p = 0.001) and affective FEP (β = −0.31, [−0.51 to −0.10], uncorrected p = 0.004). We also found that exposure to overall maltreatment (β = −0.13, [−0.20 to −0.06], pcorr = 0.043) was associated with cortical thinning in the right medial orbitofrontal region. This significant interaction effect between FEP and overall maltreatment was observed for both non-affective FEP (β = −0.12, [−0.19 to −0.04], uncorrected p = 0.002) and affective FEP (β = −0.17, [−0.29 to −0.04], uncorrected p = 0.011) (see Figs 3 and 4).
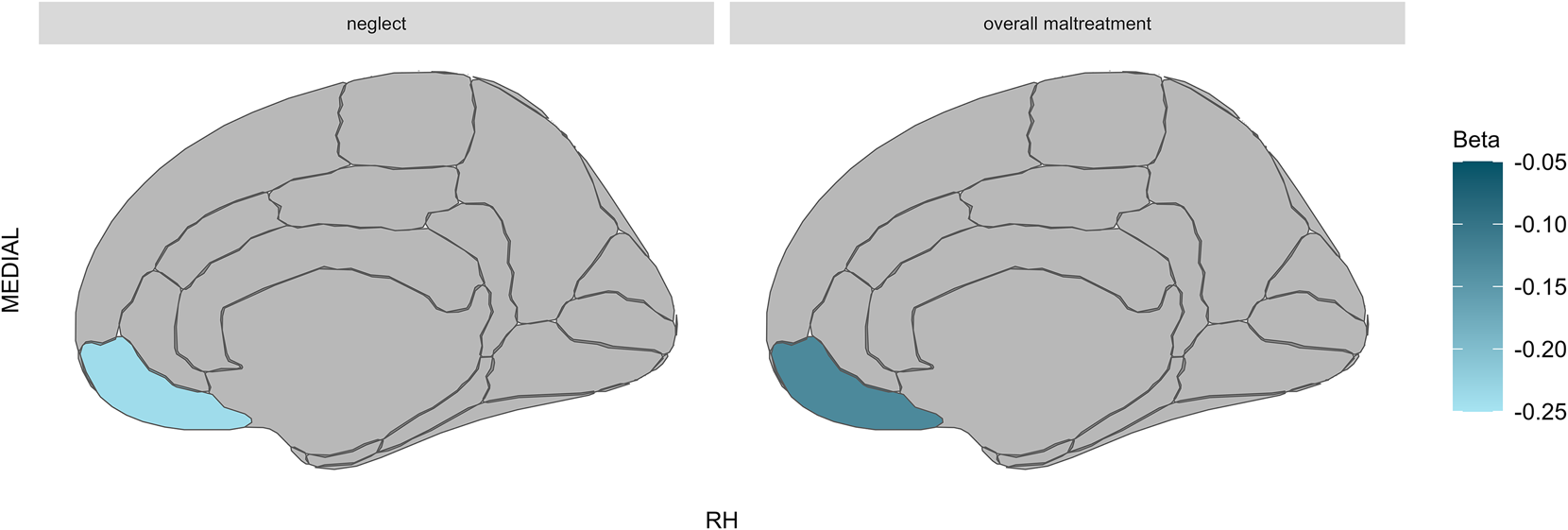
Figure 3. Interaction effects of FEP and CA on cortical thickness.
Note: MEDIAL, Medial view of the brain; RH, Right hemisphere; Lighter blue represents cortical thinning. Brain figures show significant interaction effects of FEP and both exposure to neglect (left) and overall maltreatment (right) on cortical thinning in the right medial orbitofrontal region.
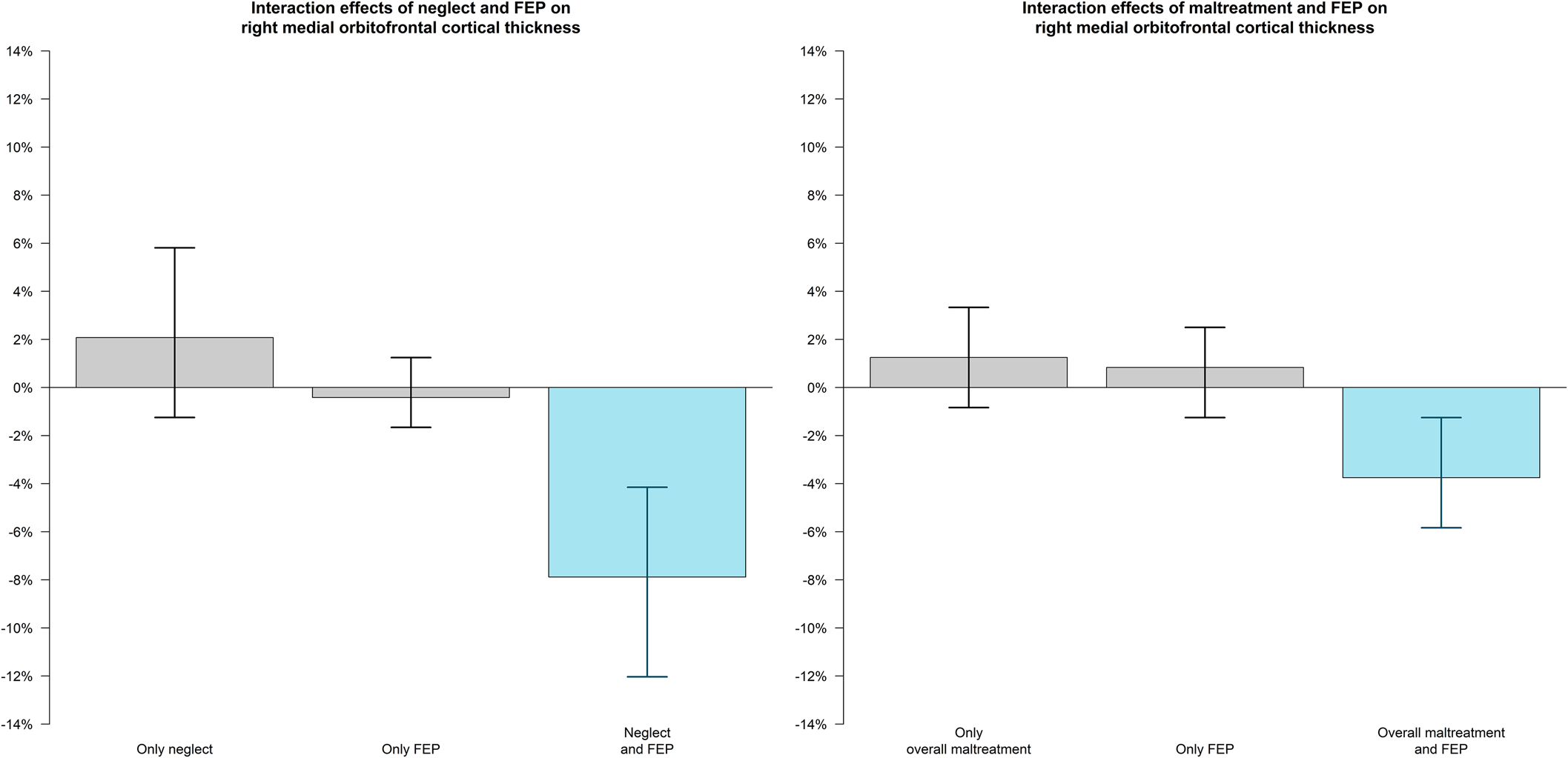
Figure 4. Interaction effects of neglect/overall maltreatment and FEP on right medial orbitofrontal cortical thickness.
Note: Experiencing FEP together with neglect (A) or overall maltreatment (B) was associated with cortical thinning in the right medial orbitofrontal region.
In those with affective psychosis, a significant interaction effect was observed between experiencing FEP and exposure to emotional bullying on cortical thickening in the right hemisphere posterior cingulate cortex (β = 0.34, [0.19–0.49], pcorr = 0.002); (see Fig. S1 in the Supplement). In those with non-affective psychosis, no interaction effects were observed between FEP and exposure to CA on cortical thickness measures.
Discussion
Here, we aimed to identify differential and shared patterns of cortical thickness alterations associated with exposure to CAs and FEP and to explore the interactive effects of different types of CA and FEP on cortical thickness across brain regions. This approach allowed us to disentangle the effects of FEP from those of CA on cortical alterations.
First, when exploring deviant cortical thickness patterns associated with CA and FEP across brain regions, we found that cortical thickness alterations observed in people with FEP were similar to those observed in people exposed to CA. This finding is in line with prior neuroimaging studies on brain volume (Aas et al., Reference Aas, Navari, Gibbs, Mondelli, Fisher, Morgan and Dazzan2012; du Plessis et al., Reference du Plessis, Scheffler, Luckhoff, Asmal, Kilian, Phahladira and Emsley2020; Hoy et al., Reference Hoy, Barrett, Shannon, Campbell, Watson, Rushe and Mulholland2012). Importantly, when different types/dimensions of CA were considered, cortical alterations in people with FEP were similar to those associated predominantly with socio-environmental exposures reflecting enduring social adversity and isolation or interpersonal hostility (e.g. socio-economic deprivation, family disadvantage, negative peer relationships) relative to other types of CA, such as parental death or having been expelled from school. Further studies are needed to explore the moderating factors and underlying pathways through which this association occurs, e.g., attachment, mood symptoms, or cognitive ability (Cortes Hidalgo et al., Reference Cortes Hidalgo, Hammerton, Heron, Bolhuis, Madley-Dowd, Tiemeier and Jones2024; Fares-Otero et al., Reference Fares-Otero, Alameda, Pfaltz, Martinez-Aran, Schäfer and Vieta2023a; Newbury et al., Reference Newbury, Arseneault, Moffitt, Odgers, Howe, Bakolis and Fisher2023).
Second, we found that exposure to certain types/dimensions of CA (i.e. maltreatment, threat) was associated with an increased likelihood of experiencing FEP and that experiencing FEP was related to reduced cortical thickness in several cortical regions of both hemispheres, which is in line with findings of previous studies (Gong, Lui, & Sweeney, Reference Gong, Lui and Sweeney2016; Scanlon et al., Reference Scanlon, Anderson-Schmidt, Kilmartin, McInerney, Kenney, McFarland and McDonald2014; Wen et al., Reference Wen, Zhao, Gong, Zhu, Li, Pan and Biswal2021). Consistent with previous research (Trauelsen et al., Reference Trauelsen, Bendall, Jansen, Nielsen, Pedersen, Trier and Simonsen2015; Zhao et al., Reference Zhao, Zhang, Shah, Li, Sweeney, Li and Gong2022), cortical thickness deficits were found in non-affective psychosis only. However, future studies in larger samples with affective psychosis are warranted.
Intriguingly, in our sample of healthy subjects, we found that emotional abuse was associated with cortical thinning in the left hemisphere paracentral region, whereas sexual abuse was associated with cortical thickening in the medial orbitofrontal region. Notably, no effects of sexual abuse on cortical thickness have been shown in trauma-exposed adolescents (Rinne-Albers et al., Reference Rinne-Albers, Boateng, van der Werff, Lamers-Winkelman, Rombouts, Vermeiren and van der Wee2020), although prospective research is needed to further evaluate the differential biological and pathological consequences of sexual abuse.
In addition to the independent effects of FEP and CAs on cortical thickness in certain brain regions, we confirmed the interactive effects of CAs and FEP on cortical thickness, with differential effects of some CAs on specific brain regions, which is in line with previous research (LoPilato et al., Reference LoPilato, Goines, Addington, Bearden, Cadenhead, Cannon and Walker2019; Rapado-Castro et al., Reference Rapado-Castro, Whittle, Pantelis, Thompson, Nelson, Ganella and Bartholomeusz2020). This finding also supports converging evidence on common underlying mechanisms and unique pathways of different types of CA that emerge from the immediate surroundings of an individual (Vaidya, Marquand, Nees, Siehl, & Schumann, Reference Vaidya, Marquand, Nees, Siehl and Schumann2024). It is crucial to further investigate the cumulative/interactive effects resulting from exposure to multiple adverse events, both simultaneously and successively, i.e., by mirroring the complex and interconnected nature of real-life situations.
As a main finding, exposure to neglect and overall maltreatment were significantly associated with reduced cortical thickness in the right medial orbitofrontal cortex in FEP. Our findings may also support emerging preliminary data suggesting that patients with psychotic disorders and a history of maltreatment may constitute a distinct neurobiological subgroup (Kaufman & Torbey, Reference Kaufman and Torbey2019). Notably, in our study, cortical alterations in FEP were specifically related to interpersonal (family and peers) dysfunction. Furthermore, comparable results in both non-affective and affective FEP suggest potential common neurobiological pathways for CA across the psychosis spectrum. Understanding these neurobiological mechanisms could provide new intervention targets for individuals with FEP and CA.
The medial orbitofrontal cortex is a subregion of the ventromedial prefrontal cortex that regulates sensitivity to outcome value and is involved in evaluation, goal decision-making, and maintenance of a choice over successive decisions (Molinaro & Collins, Reference Molinaro and Collins2023). An essential component of goal-directed decision-making is the ability to maintain flexible responses based on the value of a given reward or ‘reinforcer’ (Noonan, Kolling, Walton, & Rushworth, Reference Noonan, Kolling, Walton and Rushworth2012), and the medial orbitofrontal cortex is uniquely positioned to regulate this process (Gourley, Zimmermann, Allen, & Taylor, Reference Gourley, Zimmermann, Allen and Taylor2016). Hypoactivation of brain areas of the reward system has been found in people with both CA (Armbruster-Genç et al., Reference Armbruster-Genç, Valton, Neil, Vuong, Freeman, Packer and McCrory2022; Boecker et al., Reference Boecker, Holz, Buchmann, Blomeyer, Plichta, Wolf and Laucht2014; Dillon et al., Reference Dillon, Holmes, Birk, Brooks, Lyons-Ruth and Pizzagalli2009; Oltean, Șoflău, Miu, & Szentágotai-Tătar, Reference Oltean, Șoflău, Miu and Szentágotai-Tătar2023) and FEP (Fett et al., Reference Fett, Mouchlianitis, Gromann, Vanes, Shergill and Krabbendam2019).
In fact, goal-oriented reward decision-making dysfunction is a key feature of psychotic psychopathology and has been identified as a variable that increases individuals' vulnerability to negative symptoms (Cooper et al., Reference Cooper, Barch, Reddy, Horan, Green and Treadway2019; Reddy et al., Reference Reddy, Horan, Barch, Buchanan, Gold, Marder and Green2018). The localization of cortical thickness deficits in the medial orbitofrontal cortex in the presence of FEP is consistent with previous findings that suggest an association between medial orbitofrontal cortical thinning and negative symptom severity in patients with schizophrenia (Walton et al., Reference Walton, Hibar, van Erp, Potkin, Roiz-Santiañez, Crespo-Facorro and Ehrlich2018). Taken together, our findings further support the involvement of reward mechanisms in the pathophysiology of FEP and suggest that the dysregulation of reward mechanisms may be, at least in part, linked to CA. These effects of CA on behavior and reward-related cortical areas could underlie deficits in autonomy and social life through alterations in motivation and effortful decision making observed in people with psychotic disorders (Fares-Otero et al., Reference Fares-Otero, Alameda, Pfaltz, Martinez-Aran, Schäfer and Vieta2023a) and affective disorders with psychotic features (Fares-Otero et al., Reference Fares-Otero, De Prisco, Oliva, Radua, Halligan, Vieta and Martinez-Aran2023b).
Interestingly, we found that exposure to emotional bullying (verbal victimization) was associated with increased cortical thickness in the posterior cingulate cortex in those with affective FEP. Specifically, the posterior cingulate cortex has been demonstrated to be involved in both narrative comprehension and autobiographical memories, such as those concerning friends and family, and in emotional memory imagery. In addition, the posterior cingulate cortex forms a central node in the default mode network of the brain (Leech, Braga, & Sharp, Reference Leech, Braga and Sharp2012), and it has been linked to psychotic symptoms such as hallucinations, delusions, disorganized thinking, and a lack of emotional intelligence (Maddock, Garrett, & Buonocore, Reference Maddock, Garrett and Buonocore2001). Further research on the effects of bullying on cortical thickness in larger samples with affective psychosis using functional neuroimaging may provide new avenues for studying connectivity, mapping brain networks, and decoding cognitive and emotional processes.
Finally, while we observed cortical thickness alterations in the left hemisphere associated with FEP, we found significant interaction effects between FEP and CA in the right hemisphere only. Although there is some evidence supporting associations of reduced left middle temporal cortical thickness with altered left hemisphere language network organization in individuals with schizophrenia (Schijven et al., Reference Schijven, Postema, Fukunaga, Matsumoto, Miura, de Zwarte and Francks2023), to our knowledge, no previous study has reported laterality effects of CA and FEP on cortical thickness. Our results may provide new clues on laterality in those with FEP and CA exposure. Future studies should assess these associations including clinical and cognitive measures.
Implications and future directions
Our study adds to previous evidence supporting the independent and joint effects of exposure to CAs and FEP on specific brain circuits. This could improve our understanding of the mechanisms of environmental risk factors and how they interact with other variables to guide preventive and therapeutic strategies.
Further studies should include other psychophysiological and/or neuroimaging measures (e.g. functional magnetic resonance) and cognitive/emotion processing assessments, to test and understand the extent of CA effects on cortical alterations and brain function, and their associations with (social) goal-oriented reward and decision-making, through virtual reality and/or interactive and (close to) real-life assessments (Bell et al., Reference Bell, Pot-Kolder, Rizzo, Rus-Calafell, Cardi, Cella and Valmaggia2024; Fares-Otero, Halligan, Vieta, & Heilbronner, Reference Fares-Otero, Halligan, Vieta and Heilbronner2024b) in FEP.
Although early childhood neglect is associated with alterations in adult brain structure despite subsequent environmental enrichment (Mackes et al., Reference Mackes, Golm, Sarkar, Kumsta, Rutter, Fairchild and Sonuga-Barke2020), whether cortical thickness increases in response to interventions enhancing cognitive reserve (Fares-Otero et al., Reference Fares-Otero, Borràs, Solé, Torrent, Garriga, Serra-Navarro and Verdolini2024a; Sánchez-Torres et al., Reference Sánchez-Torres, Amoretti, Enguita-Germán, Mezquida, Moreno-Izco, Panadero-Gómez and González-Blanco2023) or whether resilience (Fares-Otero et al., Reference Fares-Otero, O, Spies, Womersley, Gonzalez, Ayas and Seedat2023c) protects against FEP needs to be established. Understanding specific changes in brain circuits and compensatory mechanisms may help in the design of interventions that augment such beneficial strategies. Future studies should also attempt to map differentially sensitive periods (Fares-Otero & Schalinski, Reference Fares-Otero and Schalinski2024) of cortical thickness effects across brain regions resulting from adversity exposure and assess the effects of these exposures on neurodevelopment and vulnerability to psychotic disorders. Additionally, granular data on CA severity and duration, the number of CA exposures and the number of repeated exposures to a single CA type (e.g. sexual abuse) should be collected (Fares-Otero & Seedat, Reference Fares-Otero and Seedat2024).
Strengths and limitations
This study has several methodological strengths, such as the use of a relatively large and well-characterized sample of people meeting the diagnostic criteria for FEP. We included a sample of HCs to disentangle the effect of FEP from that of CAs. We explored the associations between the effects of many types of CAs and FEP on cortical thickness in a wide variety of brain regions. We assessed parental discord and peer bullying (making distinctions between emotional v. physical types), which have been relatively understudied thus far but are often experienced by children and adolescents (Finkelhor, Reference Finkelhor2018) – even more so in children with neurodevelopmental disorders and at risk for mental disorders (Abregú-Crespo et al., Reference Abregú-Crespo, Garriz-Luis, Ayora, Martín-Martínez, Cavone, Carrasco and Díaz-Caneja2024). We also used a dimensional model of adversity to help disentangle effects unique to certain adversity types to identify both shared and distinct mechanisms through which different adversities affect cortical thickness across brain regions.
Several limitations should be considered when our findings are interpreted. First, because data on CA and neuroimaging were collected at the same point in time, causal relationships between study constructs cannot be addressed. Further large-scale and longitudinal studies are needed. Second, CAs were retrospectively reported by the participants through retrospective assessments. The reliability of retrospective reports on CA is often questioned because they are prone to a certain recollection bias (Hardt & Rutter, Reference Hardt and Rutter2004). However, empirical studies have shown that retrospective self-reports on the presence of CA by individuals with psychotic disorders are sufficiently reliable and provide strong support for their validity and reliability (Fisher et al., Reference Fisher, Craig, Fearon, Morgan, Dazzan, Lappin and Morgan2011). Surprisingly, we found that exposure to any CA and overall maltreatment was more prevalent in HCs than in those with FEP in our sample. However, previous studies have reported similar prevalence rates of CA in both the general population (McLaughlin et al., Reference McLaughlin, Weissman and Bitrán2019) and FEP (Vila-Badia et al., Reference Vila-Badia, Del Cacho, Butjosa, Serra Arumí, Esteban Santjusto, Abella and Usall2022). Furthermore, our exclusion criteria did not exclude the possibility that some participants could have presented a lifetime but not a current psychiatric diagnosis. This could have been the case for mood and anxiety disorders as well as disorders with childhood onset that are not prominent later in life (e.g. Attention Deficit Hyperactivity Disorder). Third, different traumatic and stressful experiences throughout life can contribute to brain structure changes, which may vary based on the individual's experience (Ansell, Rando, Tuit, Guarnaccia, & Sinha, Reference Ansell, Rando, Tuit, Guarnaccia and Sinha2012). Future research on the cumulative and interactive effects of CA and traumatic life events may contribute to explaining brain structure in people with FEP. Finally, the use of a sample composed of individuals with FEP limits the generalizability of our results to other stages of the illness. Fourth, notably, the present study sample was drawn from public hospitals in Madrid. The degree to which the current findings may be generalized more broadly to children, youth, and adults from demographically and culturally dissimilar contexts is unknown.
Conclusions
The results of this study suggest that the cortical thickness alterations observed in people with FEP are similar to those observed in people exposed to socio-environmental adversity. Our findings also reveal the interactive effects of FEP and exposure to neglect and overall maltreatment on reduced cortical thickness in the right medial orbitofrontal cortex. These findings suggest that neural markers of the CA in regions involved in decision-making and reward mechanisms potentially underlie the association between CA and psychosis, thus revealing shared vulnerability pathways and mechanisms that could become targets for preventive and therapeutic efforts. Future longitudinal neuroimaging studies aimed at addressing the biological mechanisms underlying the interactive effects of CA and psychosis risk on brain development are warranted.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291724002393.
Data availability statement
N. E. F.-O. and J. R. have full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analyses. The data are not publicly available due to privacy restrictions. The R code is available from the authors upon reasonable request.
Acknowledgements
We thank the participants of the AGES-CM research project for their contributions and the AGES-CM group for their dedication to the collection and stewardship of the data used in this study.
Author contributions
Term, Conceptualization, Methodology: N. E. F.-O., J. R. Data collection and data curation: N. E. F.-O., H. M., M. R.-C., V. C., B. G.-B., P. A.-C., A. I., D. M.-H., P. M. C., I. L., M. D., A. O.-T., I. M.-G., A. M.-S., C. L. L., N. M., and AGES-CM group. Writing – original draft: N. E. F.-O., C. M. D.-C., J. R. Writing – reviewing & editing: All the authors. Formal analysis, Software, Validation, Interpretation of the data: N. E. F.-O., C. M. D.-C., J. R. Visualization: N. E. F.-O., E. Vila, J. R. Investigation: N. E. F.-O., J. R., and AGES-CM group. Resources and funding acquisition: R. R.-J., M. D. M., M.-F. B.-O., A. I., E. B.-G., E. V., J. L. A.-M., N. M., C. A., and C. M. D.-C. All the authors approved the final version of the submitted manuscript.
AGES-CM group
Miriam Ayora1, Raquel Álvarez-García2, Ana Sánchez-Cámara1, Santiago Ovejero-García3, Daniel Hernández Huerta4, Iosune Torío-Palmero3, Daniel Lourido4, Ángeles Sánchez-Cabezudo5, Beatriz Serván Rendón-Luna6, Aggie Núñez-Doyle5, Maria Dolores Saiz-Gonzalez6, Karina McDowell7, Katya B. March8, M. Paz Vidal-Villegas8, Juan Carlos Leza7
1Department of Child and Adolescent Psychiatry, Institute of Psychiatry and Mental Health, Hospital General Universitario Gregorio Marañón, IiSGM, CIBERSAM, ISCIII, School of Medicine, UCM, Madrid, Spain.
2Hospital Rey Juan Carlos, Madrid, Spain.
3Department of Psychiatry, University Hospital Fundación Jiménez Díaz, Madrid. Universidad Autónoma de Madrid, Spain.
4Department of Psychiatry, Hospital Universitario Ramón y Cajal, Madrid, Spain.
5Department of Psychiatry, Instituto de Investigación Sanitaria Hospital 12 de Octubre (imas12), Madrid, Spain.
6Health Research Institute, Hospital Clínico San Carlos (IdISSC), Madrid, Spain.
7Department of Pharmacology y Toxicology, School of Medicine, UCM, Instituto de Investigación Hospital 12 de Octubre (imas12), Instituto Universitario de Investigación en Neuroquímica (IUIN), CIBERSAM, ISCIII, Madrid, Spain.
8Department of Psychiatry, Clinical Psychology, and Mental Health, Instituto de Investigación Hospital Universitario La Paz (IdiPaz), Hospital Universitario La Paz, Madrid, Spain.
The work by NEF-O was made possible by the support of the European Union's Horizon 2020 Research and Innovation Program (PSY-PGx, EU.3.1.3. Treating and managing disease, Grant agreement No. 945151), and DAAD (Deutscher Akademischer Austauschdienst) (ID-57681229 – Ref. No. 91629413). PAC has received grant support from Programa Intramural de Impulso a la I + D + i 2023 (Instituto de Investigación Sanitaria Gregorio Marañón). MR-C has received funding from the Instituto de Salud Carlos III, ISCIII, (PI15/00723, PI18/00753, PI21/00701, PI24/01298), and the Spanish Ministry of Science, Innovation and Universities (RYC-2017-23144; CNS2023-144038) and was supported by a NARSAD independent investigator grant (No. 24628) from the Brain & Behavior Research Foundation. AIzq was supported by Grant JDC2022-048291-I funded by MCIN/AEI/10.13039/501100011033 and by the ‘European Union NextGenerationEU/PRTR’. RR-J received funding from the Instituto de Salud Carlos III (PI19/00766; Fondo de Investigaciones Sanitarias/FEDER) and the Madrid Regional Government (S2017/BMD-3740; P2022/BMD-7216). AIba acknowledges the support of CIBER-Consorcio Centro de Investigación Biomédica en Red- (CB/07/09/0025), Instituto de Salud Carlos III, and Ministerio de Ciencia e Innovación; the Madrid Regional Government (S2022/BMD-7216 – AGES 3-CM) and European Union Structural Funds; and grants PI19/01295 and PI22/01183, which were integrated into the Plan Nacional de I + D + I and co-financed by the ISCIII-Subdirección General de Evaluación and the Fondo Europeo de Desarrollo Regional (FEDER). EV acknowledges the support of CIBER-Consorcio Centro de Investigación Biomédica en Red- (CB07/09/0004), Instituto de Salud Carlos III, the Spanish Ministry of Science and Innovation and grants PI18/00805 and PI21/00787, which were integrated into the Plan Nacional de I + D + I and co-financed by the ISCIII-Subdirección General de Evaluación and the Fondo Europeo de Desarrollo Regional (FEDER); the Instituto de Salud Carlos III; the Secretaria d'Universitats i Recerca del Departament d'Economia i Coneixement (2021 SGR 01358), the CERCA Programme, and the Departament de Salut de la Generalitat de Catalunya for the PERIS grant SLT006/17/00357; and the support of the European Union Horizon 2020 Research and Innovation Program (EU.3.1.1. Understanding health, wellbeing and disease: Grant No 754907 and EU.3.1.3. Treating and managing disease: Grant No 945151). CA has received funding from the Spanish Ministry of Science and Innovation, Instituto de Salud Carlos III (ISCIII), co-financed by the European Union; ERDF Funds from the European Commission, ‘A way of making Europe’, financed by the European Union – NextGenerationEU (PMP21/00051), PI19/01024. CIBERSAM, Madrid Regional Government (B2017/BMD-3740 AGES-CM-2), European Union Structural Funds, European Union Seventh Framework Program, European Union H2020 Program under the Innovative Medicines Initiative 2 Joint Undertaking: Project PRISM-2 (Grant agreement No. 101034377), Project AIMS-2-TRIALS (Grant agreement No. 777394), Horizon Europe: Project Youth-GEMs (Grant agreement No. 101057182), the National Institute of Mental Health of the National Institutes of Health under Award Number 1U01MH124639-01 (Project ProNET) and Award Number 5P50MH115846-03 (project FEP-CAUSAL), Fundación Familia Alonso, and Fundación Alicia Koplowitz. CMD-C has received funding from Instituto de Salud Carlos III, the Spanish Ministry of Science and Innovation (JR19/00024, PI17/00481, PI20/00721, PI23/00625) and the European Union under grant number 101057182 (project Youth-GEMs). JR is thankful for the support from Instituto de Salud Carlos III, the European Regional Development Fund (FEDER) (CPII19/00009) and the Secretaria d'Universitats i Recerca del Departament d'Economia i Coneixement (2021 SGR 1128).
Funding statement
This study was supported by the Madrid Regional Government (R&D activities in Biomedicine, grant number S2022/BMD-7216 – AGES 3-CM). The funder had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. This article has been funded for open access by University of Barcelona.
Competing interests
N. V. has received financial support for CME activities and travel funds from the following entities (unrelated to the present work): Angelini, Janssen, Lundbeck, Otsuka. A. Iba has received research support from or served as speaker or advisor for Janssen-Cilag, Lundbeck, Otsuka Pharmaceutical SA and Alter. R. R.-J. has been a consultant for, spoken in activities of, or received grants from Instituto de Salud Carlos III, Fondo de Investigación Sanitaria (FIS), Centro de Investigación Biomédica en Red de Salud Mental (CIBERSAM), Madrid Regional Government (S2010/BMD-2422 AGES; S2017/BMD-3740; P2022/BMD-7216), JanssenCilag, Lundbeck, Otsuka, Pfizer, Ferrer, Juste, Takeda, Exeltis, Casen-Recordati, Angelini, Rovi. E. V. has received grants and served as a consultant, advisor or CME speaker for the following entities: AB-Biotics, AbbVie, Adamed, Angelini, BeckleyPsych, Biogen, Biohaven, Boehringer-Ingelheim, Celon Pharma, Compass, Dainippon Sumitomo Pharma, Ethypharm, Ferrer, Gedeon Richter, GH Research, Glaxo-Smith Kline, HMNC, Idorsia, Janssen, Lundbeck, Luye Pharma, Medincell, Merck, Newron, Novartis, Orion Corporation, Organon, Otsuka, Roche, Rovi, Sage, Sanofi-Aventis, Sunovion, Takeda, Teva, and Viatris, outside of the submitted work. C. A. has been a consultant to or has received honoraria or grants from Abbot, Acadia, Angelini, Biogen, Boehringer, Gedeon Richter, Janssen Cilag, Lundbeck, Medscape, Menarini, Minerva, Otsuka, Pfizer, Roche, Sage, Servier, Shire, Schering Plough, Sumitomo Dainippon Pharma, Sunovion, Takeda and Teva. C. M. D.-C. has received honoraria from Angelini and Viatris and support in attending conferences from Janssen and Angelini. J. R. has received CME honoraria from Inspira Networks for a machine learning course promoted by Adamed, outside the submitted work. The remaining authors have no conflicts of interest to declare.







