Statement of Research Significance
Amnestic mild cognitive impairment (aMCI) is often the transitional stage between healthy aging and dementia, with pathology starting in a brain region called the perirhinal cortex. This region is necessary for feelings of familiarity – that gut sense that something has happened before but lacking any memory for the details. Research Question: However, prior research has been mixed on whether familiarity is intact or impaired in aMCI, because, we argue, recollection – the deliberate recall of past events and their contextual details (e.g., where/when/how something happened before) was also at play in those studies. We, conducted two studies that minimized the role of recollection, with healthy younger and older adults, and older adults with aMCI. Main Findings: Both studies showed impaired familiarity in people with aMCI, compared to the healthy groups. Study Contributions: This research highlights familiarity as an early indicator of aMCI, which could allow for earlier treatment
Familiarity is an integral mnemonic function that notifies us that we have experienced something before (Montaldi et al., Reference Montaldi, Spencer, Roberts and Mayes2006). The feeling of familiarity can be triggered in certain situations. For example, we can feel that a person walking by us looks familiar. Perhaps this person is an employee of a store you visit frequently, an old friend from your childhood soccer team, or a teacher you had in elementary school. Barring certain situations (e.g., Myftaraj et al., Reference Myftaraj, Skrepnek and Anderson2025), familiarity remains intact in healthy aging (Koen & Yonelinas, Reference Koen and Yonelinas2016). In contrast to familiarity, recollection provides the spatiotemporal context to the person we initially felt was familiar (Kelley & Jacoby, Reference Kelley, Jacoby, Tulving and Craik2000; Mandler, Reference Mandler2008). A familiar person one perceives would be, for instance, correctly recollected as an employee of your favorite store.
There are plenty of examples of impaired recollection in aging (see Koen & Yonelinas, Reference Koen and Yonelinas2014, for a review) and amnesia (Allen, Reference Allen2018; Markowitsch & Staniloiu, Reference Markowitsch and Staniloiu2013), and under conditions that reduce attentional resources such as divided attention (Craik, Reference Craik2016; De Brigard, Reference De Brigard2012; Troyer & Craik, Reference Troyer and Craik2000). However, there are relatively few examples of impaired familiarity in clinical cohorts. The goal of this paper is to examine familiarity in amnestic mild cognitive impairment (aMCI), within whom we predicted impaired familiarity, for reasons detailed below.
The single-process theory of recognition memory argues that familiarity has the same memory base as recollection, but is a weaker process (Dobbins et al., Reference Dobbins, Khoe, Yonelinas and Kroll2000; Wixted & Stretch, Reference Wixted and Stretch2004). A strong memory will allow a person to confidently recollect the item, while a weaker memory will only make the item seem familiar. However, the contradictory and dominant viewpoint is the dual-process theory of recognition memory, which argues that familiarity and recollection are independent processes (Jones & Jacoby, Reference Jones and Jacoby2001; Mandler, Reference Mandler2008).
Neuroimaging and case studies have supported this independence of familiarity and recollection. Most typically, recollection is associated with activity in the hippocampus, while familiarity is associated with activity in the perirhinal cortex (PRC) and the entorhinal cortex (ERC) (Davachi et al., Reference Davachi, Mitchell and Wagner2003; Diana et al., Reference Diana, Yonelinas and Ranganath2008; Wolk et al., Reference Wolk, Dunfee, Dickerson, Aizenstein and DeKosky2011; Yonelinas et al., Reference Yonelinas, Otten, Shaw and Rugg2005; Reference Yonelinas, Widaman, Mungas, Reed, Weiner and Chui2007). In individuals with hippocampal damage, recollection is impaired, yet familiarity is spared (Aggleton & Brown, Reference Aggleton and Brown1999; Aggleton et. al, Reference Aggleton, Vann, Denby, Dix, Mayes, Roberts and Yonelinas2005; Bastin et. al, Reference Bastin, Linden, Charnallet, Denby, Montaldi, Roberts and Andrew2004; Brandt et. al, Reference Brandt, Aretouli, Neijstrom, Samek, Manning, Albert and Bandeen-Roche2009; Holdstock et. al, Reference Holdstock, Mayes, Gong, Roberts and Kapur2005). There has also been an opposite case: Patient NB had parts of her left temporal lobe including the PRC and ERC removed to treat intractable epilepsy, leaving the hippocampus intact. NB was able to recollect her experiences, but familiarity was impaired (Bowles et al., Reference Bowles, Crupi, Mirsattari, Pigott, Parrent, Pruessner, Yonelinas and Köhler2007; Reference Bowles, Duke, Rosenbaum, McRae and Köhler2016; Köhler & Martin, Reference Köhler and Martin2020).
Individuals with aMCI experience memory deficits that exceed those occurring with typical healthy aging (Palmer et al., Reference Palmer, Fratiglioni and Winblad2003). AD-associated neurofibrillary tau accumulates first in the transentorhinal (perirhinal) cortex (Braak & Braak, Reference Braak and Braak1991; Taylor & Probst, Reference Taylor and Probst2008). Significant PRC volume loss (Juottonen et al., Reference Juottonen, Laakso, Insausti, Lehtovirta, Pitkänen, Partanen and Soininen1998; Troyer et al., Reference Troyer, Murphy, Anderson, Craik, Moscovitch, Maione and Gao2012), PRC cortical thinning (Krumm et al., Reference Krumm, Kivisaari, Probst, Monsch, Reinhardt, Ulmer, Stippich, Kressig and Taylor2016), and ERC volume loss (Devanand et al., Reference Devanand, Pradhaban, Liu, Khandji, De Santi, Segal, Rusinek, Pelton, Honig, Mayeux, Stern, Tabert and de Leon2007; Du et al., Reference Du, Schuff, Amend, Laakso, Hsu, Jagust and Weiner2001; Juottonen et al., Reference Juottonen, Laakso, Insausti, Lehtovirta, Pitkänen, Partanen and Soininen1998; Pennanen et al., Reference Pennanen, Kivipelto, Tuomainen, Hartikainen, Hänninen, Laakso, Hallikainen, Vanhanen, Nissinen, Helkala, Vainio, Vanninen, Partanen and Soininen2004) have been found in aMCI. Not all cases progress to Alzheimer’s disease (AD; Oltra-Cucarella et al., Reference Oltra-Cucarella, Ferrer-Cascales, Alegret, Gasparini, Díaz-Ortiz, Ríos and Sanchez-SanSegundo2018). Familiarity deficits may be a cognitive marker identifying individuals at higher risk for progression to AD, potentially facilitating early intervention and treatment.
Recollection and familiarity are probed using several methods. The “Remember/Know” paradigm (Tulving, Reference Tulving1985) distinguishes between “remembering” (recollecting details) and “knowing” (a sense of familiarity without details). Jacoby’s (Reference Jacoby1991) process dissociation procedure pits recollection and familiarity in opposition to each other. Receiver operating characteristics (Hanley & McNeil, Reference Hanley and McNeil1982) involve participants rating items on a scale from “certainly old” to “certainly new”. These three methods converge in identifying robust recollection impairments in aMCI, but they have led to conflicting findings about how aMCI affects familiarity. Some studies identify spared familiarity in aMCI (Anderson et al., Reference Anderson, Ebert, Jennings, Grady, Cabeza and Graham2008; Anderson et al., Reference Anderson, Baena, Yang and Köhler2021; Embree et al., Reference Embree, Budson and Ally2012 [for pictures]; Hudon et al., Reference Hudon, Belleville and Gauthier2009; Serra et al., Reference Serra, Bozzali, Cercignani, Perri, Fadda, Caltagirone and Carlesimo2010; Troyer et al., Reference Troyer, Murphy, Anderson, Craik, Moscovitch, Maione and Gao2012), and some find familiarity impairments in aMCI (Ally, Gold et al., Reference Ally, Gold and Budson2009; Embree et al., Reference Embree, Budson and Ally2012 [for words]; Pitarque et al., Reference Pitarque, Meléndez, Sales, Mayordomo, Satorres, Escudero and Algarabel2016; Wolk et al., Reference Wolk, Dunfee, Dickerson, Aizenstein and DeKosky2011; Reference Wolk, Mancuso, Kliot, Arnold and Dickerson2013).
These conflicting findings may stem from flaws in dual process approaches. In the three methods, recollection and familiarity are probed subjectively in relation to each other, rather than independently as they are purported to be. This relates to a second flaw, regarding the mathematical interdependence of recollection and familiarity estimates. Joordens and Hockley (Reference Joordens and Hockley2000) highlighted that when studied items are highly memorable, recollection increases at the expense of familiarity, while less memorable items show the opposite effect. Conversely, Yonelinas et al., (Reference Yonelinas2002) found that high levels of recollection can inflate familiarity.
We argue that familiarity cannot reliably be measured independently when recollection is also at play. We used two other methods to study familiarity, under conditions that minimize the influence of recollection, in individuals with aMCI compared to healthy younger and older adults: A response deadline procedure (Study 1) and a frequency judgment task after incidental learning (Study 2). We predicted that familiarity deficits would be evident under a short response deadline, and during frequency judgments.
Study 1: Response deadline procedure
Familiarity-based responses are often faster than recollection-based responses (Atkinson & Juola, Reference Atkinson and Juola1973, Reference Atkinson and Juola1974; Boldini et al., Reference Boldini, Russo and Avons2004; Hintzman & Caulton, Reference Hintzman and Caulton1997). This aligns with the notion of familiarity as an automatic, fast-acting process, while recollection is a deliberate and controlled process (Jacoby, Reference Jacoby1991). Response deadline procedure (RDP) studies have found that responding under short deadlines relies on familiarity, while responding under longer deadlines allows for recollection to take control (McElree et al., Reference McElree, Dolan and Jacoby1999). In one RDP study, younger and older participants saw words once, twice, or three times, and then heard a second list of words, and were asked to respond “old” only to words that they had heard before (Jacoby, Reference Jacoby1999). When young adults were given a longer deadline, increased repetition of words from the first list resulted in lower false-alarm rates. However, with a short deadline, increased repetition produced higher false alarm rates. For older adults, false alarms to studied words increased with increased repetition in both the short and long deadline. Jacoby argued that familiarity was directing responses in the short deadline; the longer deadline allowed for recollection to direct responses, albeit more effectively in younger than older adults.
Although studies using the RDP have not simultaneously examined recollection and familiarity in aMCI, Besson et al. (Reference Besson, Ceccaldi, Tramoni, Felician, Didic and Barbeau2015) presented participants with a series of object images to study and later tested their recognition using the Speed-and-Accuracy-Boost (SAB) procedure. During the test phase, participants were required to press a button if they recognized the image or withhold their response if they believed the image was new, within a 700 ms response window. Unlike in a traditional yes/no recognition task where individuals with aMCI exhibited deficits in both recollection and familiarity, their performance on the SAB task was comparable to healthy controls, suggesting that fast familiarity-based recognition remains intact in aMCI.
In a later study, Besson et al. (Reference Besson, Simon, Salmon and Bastin2020) used a slightly longer SAB (750 ms) paradigm, where participants studied everyday object images and completed three recognition conditions at test: (1) classical – stimuli was the same as encoding, (2) discriminative – stimuli recognized among same-category exemplars, and (3) entity – stimuli with altered features (e.g., angle, contrast) recognized among same-category distractors. Participants were required to respond “old” if they recognized the object. Individuals with aMCI performed worse than healthy controls across all conditions, suggesting that while fast, automatic familiarity processes may remain relatively intact in aMCI under strict time constraints, extending the response window can expose impairments in later-stage familiarity processes, such as confidence-based decision-making and post-retrieval monitoring.
To address expand on the current findings, we presented healthy younger and older adults and older adults with aMCI with objects in an incidental encoding phase and then showed those objects again in a recognition memory test phase, under long and short response deadlines. We predicted that healthy older adults would perform worse than healthy younger adults in the long response deadline, reflecting impaired recollection, but similarly to healthy younger adults in the short response deadline, signifying spared familiarity in healthy aging (Koen & Yonelinas, Reference Koen and Yonelinas2014). We additionally hypothesized that individuals with aMCI would have significantly lower recognition accuracy than healthy counterparts in the long and short response deadlines, signifying impaired recollection and familiarity, respectively.
Methods
Pilot studies
We conducted two pilot studies. In the first (Pilot A in supplementary material), we used a short deadline of 1 sec, in which we discovered that people missed a considerable number of responses. In our second pilot study, we increased the short deadline to 1.2 sec (Pilot B in supplementary material), involving 35 younger adults, 57 older adults, and 16 older adults with aMCI. Here, missed responses were few, and the aMCI group performed worse than the two healthy groups in the short deadline, supporting the notion of familiarity deficits in aMCI. However, participants were allowed to respond at any time during the long deadline (5 sec), yet 82% of these responses were made within the timeframe of our short deadline (1.2 sec), suggesting that many of their responses may have been driven by familiarity even in the long deadline. This motivated us to require people in the current study to view objects in the long deadline for 3 sec before they could respond. We used Pilot B data to discover a suitable sample size based on group differences in the short deadline (η p 2 = 0.14), which established a sample size of 24 individuals per group, with power of 0.90 and α = 0.05.
Participants
Participants were recruited through the Baycrest Academy for Research and Education. Older adults with or without a designation of aMCI were recruited and administered a battery of neuropsychological tests, until a total sample of 24 individuals with aMCI were identified. Ultimately, the study consisted of 24 younger adults (ages 18–30), 30 older adults (ages 60–85), and 24 older adults with aMCI. These participants were distinct from any pilot participants. Data were excluded from an additional two participants for pressing the same key throughout the entire test, three participants for not providing a response for>20 trials, one participant for not providing any responses, and two for not completing the experiment.
Participants were required to be native English speakers or to have learned English before the age of five. Participants were excluded if they had medical conditions that affected their cognitive functioning (other than aMCI), such as brain injury, dementia, stroke, epilepsy, multiple sclerosis, chemotherapy, heart attacks, and heart disease. Participants with a diagnosis of anxiety, depression, or other psychiatric disorders were also excluded from the study. Individuals who were regularly taking any drugs (recreational or prescription) that could affect cognitive functioning were excluded as well. The study was completed in accordance with Helsinki Declaration and approved by the Baycrest Research Ethics Board (REB #17-16), and all participants provided written informed consent. Participants were given a $30 CAD e-gift card for completing the study.
Neuropsychological testing
Participants completed a neuropsychological assessment on Zoom (n= 76) or in person (n= 2) if they lacked access to a computer. For testing on Zoom, participants were instructed to keep their camera on and have a pen and paper ready for tasks involving drawing/writing, which they were required to display on camera. Participants completed the questions to determine subjective cognitive complaints (Jessen et al., Reference Jessen, Wiese, Bachmann, Eifflaender-Gorfer, Haller, Kölsch and Bickel2010), the Montreal Cognitive Assessment (MoCA; Nasreddine et al., Reference Nasreddine, Phillips, Bédirian, Charbonneau, Whitehead, Collin and Chertkow2005), Shipley Institute of Living Scale: Vocabulary (Shipley, Reference Shipley1946), and Wechsler Abbreviated Scale of Intelligence Matrix Reasoning (Wechsler, Reference Wechsler1999). Older adults also completed the Rey Auditory Verbal Learning Test (Rey, Reference Rey1964), Forward and Backward Digit Span (Wechsler, Reference Wechsler2008), Logical Memory (Wechsler, Reference Wechsler1997), the Boston Naming Test (Kaplan et al., Reference Kaplan, Goodglass and Weintraub1983), FAS (Borkowski et al., Reference Borkowski, Benton and Spreen1967), and Animal Naming (Rosen, Reference Rosen1980). For participants that had impairments indicative of aMCI, an informant completed the Functional Activities Questionnaire (FAQ; Pfeffer et al., Reference Pfeffer, Kurosaki, Harrah, Chance and Filos1982), to rule out dementia. All test batteries were reviewed by a clinical neuropsychologist (NDA). The diagnostic criteria for aMCI was performance at least 1.5 standard deviations below the expected level (based on tests of intelligence) on two or more measures of memory, as well as concern regarding a change in cognition, independence of function in daily life, and no evidence of dementia (Albert et al., Reference Albert, DeKosky, Dickson, Dubois, Feldman, Fox, Gamst, Holtzman, Jagust, Petersen, Snyder, Carrillo, Thies and Phelps2013).
Materials
The 225 images were taken from the Konkle et. al (Reference Konkle, Brady, Alvarez and Oliva2010) database, available at http://cvcl.mit.edu/MM/. Twenty-five of the images were scrambled using matPyrtools in MATLAB (Mathworks, Natick, MA), with source code developed by Portilla and Simoncelli (Reference Portilla and Simoncelli2000). The images were processed to alter their texture, making them unrecognizable as objects, while preserving the original color. This involved 25 iterations of texture synthesis, utilizing three pyramid scales, four orientations, and seven spatial neighbors.
Each test version had equal numbers of tools, vegetation, instruments, sports gear, animals, personal use items, food items, office appliances, kitchen utensils, clothing items, pieces of furniture, and kitchen appliances, in four sets of 50 images. Sets were counterbalanced across studied and unstudied, and short and long response deadline condition assignments. The four stimulus sets did not differ in memorability (Bainbridge, Reference Bainbridge2019), F (3, 96) = .110, p = .954, η p 2 = .00.
Procedure
The task was developed and presented using Psychopy software (version 3.5, Open Science Tools, Ltd; Peirce et al., Reference Peirce, Gray, Simpson, MacAskill, Höchenberger, Sogo, Kastman and Lindeløv2019), and uploaded on Pavlovia, which created a URL for the experiment. The URL was emailed to participants during the Zoom call, and participants were assigned an ID corresponding to their test version.
Upon completion of neuropsychological testing, participants underwent an incidental encoding task, viewing 100 intact and 25 scrambled objects. Participants were asked to determine if an object was intact or scrambled. After a short break, participants were presented with new and old object images. For the first test phase, 50 studied and 50 new images were presented for a longer duration (5s). The longer deadline was presented first to avoid participants adopting quick responses. In the current study, participants had to view each object for three seconds before they could provide a response; once three seconds had passed, the screen background changed from white to gray, indicating that a response was allowed. Participants had two seconds to provide a response. In the second phase, 50 studied and 50 new images (different from the first phase) were presented for a shorter duration (1.2s). For both phases, participants selected whether they had seen the object before by pressing “M”, or whether the object was new by pressing “Z”.
Analyses
Two univariate ANOVAs were conducted to compare education levels across groups and to examine age differences between the healthy older adults and adults with aMCI groups. Univariate ANOVAs were conducted as a function of group for each neuropsychological test, with tests of intellectual functioning including healthy younger adults. Separate 3 × 2 ANOVAs as a function of group and deadline were conducted for number of missed responses and for recognition accuracy (hit rate minus false alarms). Significant effects were probed using Sidak post-hoc analyses.
Results
Participant data
Demographic and neuropsychological test data are shown in Table 1. There was no difference in education between groups, F(2, 75)<1, p = .940, η p 2 = .00, or in age between healthy older adults and adults with aMCI, F(1, 52) = 2.21, p = .143, η p 2 = .04. Healthy younger and older adults performed within normal ranges in neuropsychological functioning. On average, individuals with aMCI performed within normal ranges in the intellectual functioning tasks (SILS Vocabulary, WASI-MR) and the attention and working memory task (Digit Span), but as per diagnostic criteria, performed below average or borderline across measures of memory. On average, individuals with aMCI also had a MoCA score signifying cognitive impairment.
Table 1. Mean (standard deviation) demographic and neuropsychological test data for participants in study 1
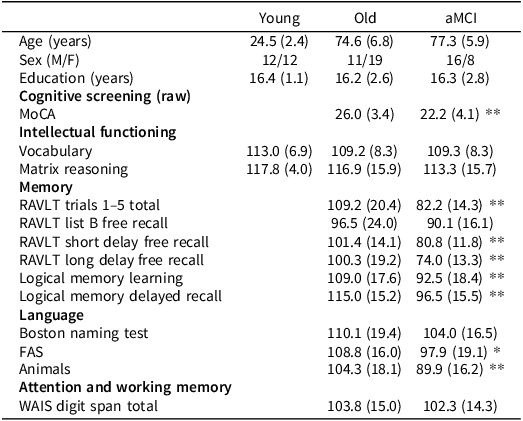
Note: Means are scaled scores unless otherwise stated.
*p < 0.05.
**p < 0.01.
Response deadline task data
Younger adults had fewer missed responses than healthy older adults (p < .001) and older adults with aMCI (p < .001), with no difference between the latter two groups (p = .951), F(2, 75) = 21.03, p < .001, η p 2 = .36. Additionally, participants missed more responses with a shorter deadline than a longer deadline, F(1, 75) = 64.37, p < .001, η p 2 = .46. An interaction between group and deadline, F(2,75) = 15.17, p < .001, η p 2 = .29, revealed that while younger adults missed a comparable number of responses in the long (M = 0.21) and short deadline (M = 0.17), healthy older adults and individuals with aMCI missed more responses in the short (healthy older adults: M = 2.47, aMCI: M = 2.54) than long deadline (healthy older adults: M = 0.33, aMCI: M = 0.50)Footnote 1 .
As evident in Figure 1, older adults with aMCI had significantly lower recognition accuracy than younger adults (p < .001) and older adults (p < .001), with no difference between the latter two groups (p= .617), F(2, 75) = 11.44, p < .001, η p 2 = .23. Additionally, recognition accuracy was higher at the longer than shorter deadline, F(1, 75) = 33.86, p < .001, η p 2 = .31. There was no interaction between group and deadline; F(2, 75) = 1.89, p = .158, η p 2 = .04.
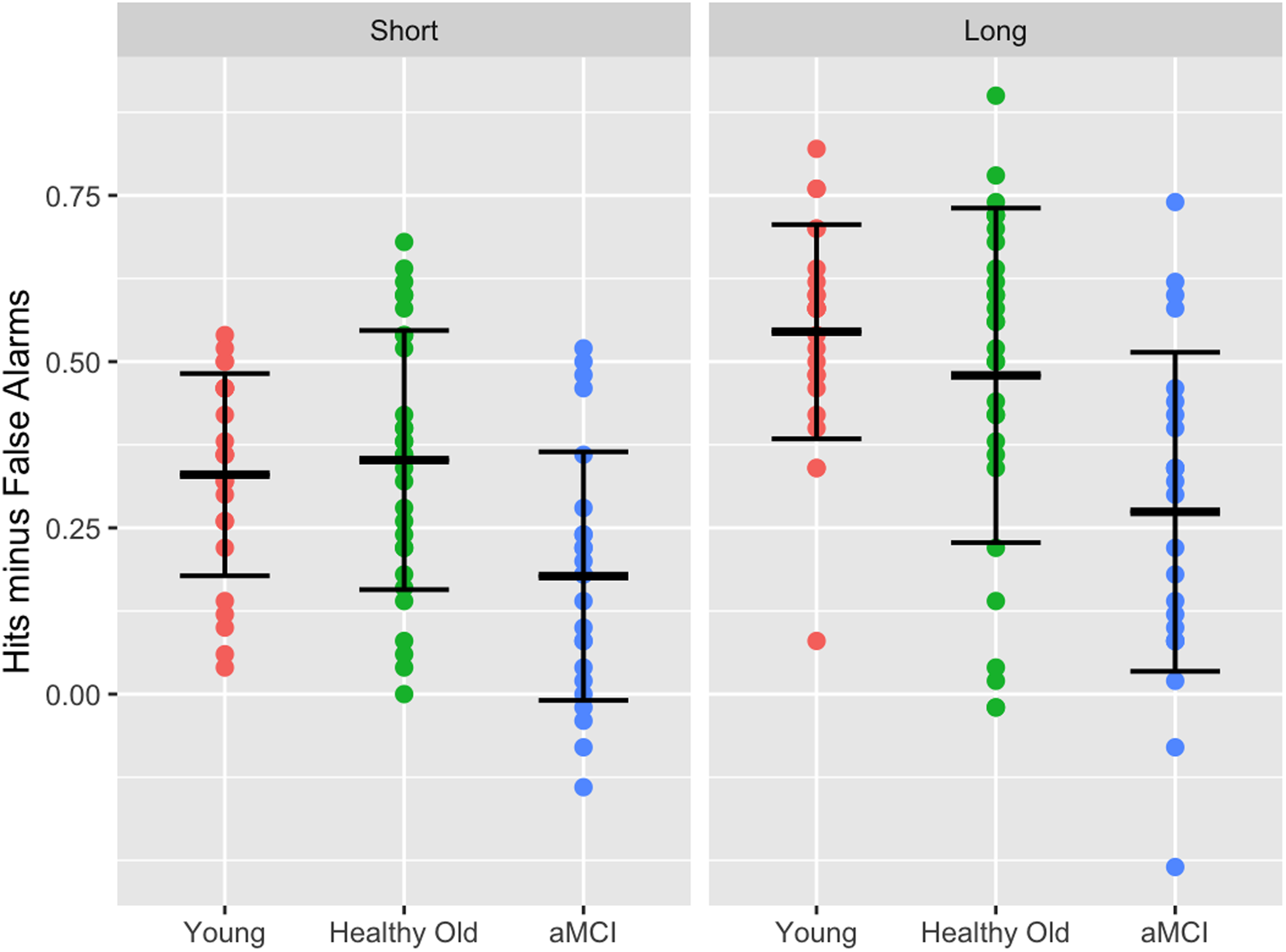
Figure 1. Recognition accuracy (hits – false alarms) in the short and long deadline, per group.
Discussion
In the long deadline, younger and older adults outperformed individuals with aMCI. This aligns with prior evidence of impaired recollection in aMCI from other paradigms (Anderson et al., Reference Anderson, Ebert, Jennings, Grady, Cabeza and Graham2008; Besson et al., Reference Besson, Ceccaldi, Tramoni, Felician, Didic and Barbeau2015; Koen & Yonelinas, Reference Koen and Yonelinas2014), as well as hippocampal atrophy in aMCI (Chen et al., Reference Chen, Zhang and Li2015; Hanseeuw et al., Reference Hanseeuw, Van Leemput, Kavec, Grandin, Seron and Ivanoiu2011; Setti et al., Reference Setti, Hunsberger and Reed2017). The lack of a significant difference in the long deadline between younger and older adults raises questions, but older adults may have used compensatory strategies involving more efficient use of familiarity, even in a recollection-based task (Morcom & Johnson, Reference Morcom and Johnson2015; Yonelinas & Jacoby, Reference Yonelinas and Jacoby1996).
Recognition accuracy was also lower in the aMCI group in the short deadline, indicative of impaired familiarity. Our short deadline was 1200ms, whereas past studies used short deadlines of 600–750 ms (Besson et al., Reference Besson, Ceccaldi, Tramoni, Felician, Didic and Barbeau2015; Reference Besson, Simon, Salmon and Bastin2020; Yonelinas & Jacoby, Reference Yonelinas and Jacoby1994). Although we tested shorter time limits in a pilot study (Pilot A), we found that many participants failed to respond in time, but we cannot rule out the possibility that even shorter deadlines might engage familiarity-based processing that could be intact in aMCI. Besson et al. (Reference Besson, Ceccaldi, Tramoni, Felician, Didic and Barbeau2015) demonstrated that fast familiarity was preserved in aMCI using a go/no-go SAB task with a 700 ms deadline, though it was impaired in a yes/no recognition task. Besson et al., suggested that fast familiarity may rely on perceptual fluency, which is often preserved in aMCI (Perri et al., Reference Perri, Serra, Carlesimo and Caltagirone2007). Thus, some individuals with aMCI might compensate for familiarity deficits by leveraging perceptual fluency. Nonetheless, when Besson et al. (Reference Besson, Simon, Salmon and Bastin2020) extended the time deadline to 750 ms and added additional experimental conditions, familiarity deficits were revealed in aMCI, with most significant declines being in the condition requiring entity-level representations. These findings suggest that extending the response deadline employs later familiarity-related processes, which may be impaired in aMCI. Moreover, the observed deficits in that entity condition support the idea that although perceptual fluency may provide some compensation early on, familiarity deficits become evident when tasks require more abstract, conceptual processing. Future studies should consider implementing different deadlines for younger and older adults, especially in the short deadline condition. Nonetheless, our study confirms that individuals with aMCI exhibit deficits in recollection (Besson et al., Reference Besson, Ceccaldi, Tramoni, Felician, Didic and Barbeau2015; Chen et al., Reference Chen, Zhang and Li2015; Hanseeuw et al., Reference Hanseeuw, Van Leemput, Kavec, Grandin, Seron and Ivanoiu2011; Koen & Yonelinas, Reference Koen and Yonelinas2014; Setti et al., Reference Setti, Hunsberger and Reed2017) and provides further evidence of deficits in familiarity, within the context of RDP.
This discussion highlights one disadvantage of the RDP – the potential contamination of these processes at either response deadline. If recollection can be invoked in the short deadline, or familiarity in the long deadline, then the RDP is not ideal to inform us if familiarity is impaired or spared in aMCI. For this reason, we conducted another study, comparing frequency judgments in healthy younger and older adults, and older adults with aMCI.
Study 2: Frequency judgment task
Frequency judgment tasks typically involve presenting participants with stimuli a variable number of times, without forewarning of a memory task. Participants are later asked to indicate how often they encountered each specific stimulus. Hintzman and Curran (Reference Hintzman and Curran1994) found that individuals first make an automatic familiarity judgment – assessing whether an item is old or new. If the item is judged to be old, a secondary judgment regarding its frequency is then made. Kausler and Puckett (Reference Kausler and Puckett1980) further support the claim that frequency judgments are mediated by automatic processes, showing that neither healthy aging nor intentional learning alter performance (see Zacks & Hasher, Reference Zacks and Hasher2002, for a review).
Bowles et al (Reference Bowles, Duke, Rosenbaum, McRae and Köhler2016) used a frequency judgment task to present control participants and patient NB (who, as previously mentioned, had the left PRC resected) 120 words, each shown 1, 3, 4, 7, or 11 times and then asked how often each word was presented. Patient NB did significantly worse than control participants, represented by the correlation of frequency judgments and the actual frequency of the items. Duke et al. (Reference Duke, Martin, Bowles, McRae and Köhler2017) demonstrated that among various medial temporal lobe regions, activity in the PRC alone tracked frequency in healthy individuals.
Building on this work, Anderson et al. (Reference Anderson, Baena, Yang and Köhler2021) presented healthy older adults and older adults with aMCI 100 words presented 1, 2, 4, 7, or 12 times, as well as 20 pronounceable nonwords each presented twice, for a lexical decision encoding task. During the test phase, participants were asked to rate each word’s relative frequency on a scale of 1 (not frequent) to 5 (highly frequent). Participants with aMCI had significantly lower correlations between their frequency judgments and the actual stimulus frequency compared to healthy participants, indicating reduced familiarity in aMCI.
Sanger and Anderson (Reference Sanger and Anderson2022) sought to replicate and extend these findings, investigating if familiarity deficits in aMCI are more pronounced for objects than words, given the PRC’s crucial role in object recognition (Winters & Bussey, Reference Winters and Bussey2005). Participants viewed four types of stimuli during the encoding phase: intact objects, scrambled objects, intact words, and pronounceable nonwords, with intact objects and words presented 1, 2, 4, or 7 times, and scrambled objects and nonwords presented twice. Participants made intact/not intact decisions. In the test phase, participants were asked to recall how many times each intact item appeared. Like Anderson et al. (Reference Anderson, Baena, Yang and Köhler2021), frequency judgments were less accurate in participants with aMCI, but there was no difference in familiarity deficit for objects versus words. Sanger and Anderson conjectured that this may be because the objects used in their study were highly distinctive. The PRC is particularly involved in resolving highly overlapping, fine-grained features of objects, suggesting that familiarity deficits may become more pronounced when objects with overlapping features are used (Erez et al., Reference Erez, Cusack, Kendall and Barense2016).
In the current study, we assessed frequency judgments for highly overlapping objects (e.g., one coin shown once, another shown twice, another four times, and another seven times). Aligned with the findings of Anderson et al. (Reference Anderson, Baena, Yang and Köhler2021) and Sanger and Anderson (Reference Sanger and Anderson2022), we predicted that familiarity would be comparable in healthy younger and older adults, but that familiarity would be impaired in aMCI, and more so than found by Sanger and Anderson.
Methods
Participants
Young adults, healthy older adults, and older adults with aMCI were recruited from the Baycrest Academy for Research and Education until a total of 24 per group were identified, based on a d w = −0.41 from Koen and Yonelinas (Reference Koen and Yonelinas2014) meta-analysis of familiarity deficits in individuals with aMCI. These recruitment efforts resulted in 24 younger adults (ages 18–30), 40 healthy older adults (ages 60–85) and 24 older adults with aMCI (ages 60–85). These participants were distinct from those who participated in Anderson et al. (Reference Anderson, Baena, Yang and Köhler2021) and Sanger and Anderson (Reference Sanger and Anderson2022). Data were excluded from an additional five participants for pressing the same key throughout the entire test, and two for not completing the experiment.
The same inclusion and exclusion criteria as in Study 1 were applied. The study was completed in accordance with Helsinki Declaration and approved by the Baycrest Research Ethics Board (REB #17-16), and all participants provided written informed consent. Participants were given a $30 CAD e-gift card for completing the study. Thirty-two participants in the current study (4 younger adults, 20 healthy older adults, and 8 older adults with aMCI) also completed Study 1.
Neuropsychological testing
Participants completed the same series of neuropsychological tests as in Study 1. The neuropsychological assessment was also conducted on Zoom (n= 84) or in person (n= 4) if they lacked access to a computer.
Materials
The 80 images for the study were taken from the Konkle et. al (Reference Konkle, Brady, Alvarez and Oliva2010) database. We collected 4 image exemplars within each of 20 object categories. These object categories were divided into four sets containing an equal number of animals, food, clothing, appliances, vegetation, and miscellaneous items (e.g., coin, street sign) represented equally across presentations. Object items were also equated in “memorability” across versions (Bainbridge, Reference Bainbridge2019). A Set X Exemplar ANOVA for object memorability found no main effects of Set, F(1, 72) = .459, p = .500, η p 2 = .01, or Exemplar, F(3, 72) = .074, p = .974, η p 2 = .00, or significant interaction, F(3, 72) = .431, p = .731, η p 2 = .02.
There were three random versions of placements of the repetitions, with the constraint that objects were not repeated within three trials of each other. Additionally, as participants had a break halfway through the study phase, object category sets were also counterbalanced across study half. Lastly, the object exemplars were counterbalanced across four frequency orders. This resulted in 24 versions. As there were a minimum of 24 participants per age group, each participant in their group had a different test version, except for healthy older adults in which some versions were repeated.
Procedure
The computer task for the study was developed and presented using Psychopy software (version 3.5, Open Science Tools, Ltd; Peirce et al., Reference Peirce, Gray, Simpson, MacAskill, Höchenberger, Sogo, Kastman and Lindeløv2019), and uploaded on Pavlovia, which created a URL for the experiment. The URL was emailed to participants during a Zoom call. Participants were assigned an ID corresponding to the test version they were given.
Upon completion of neuropsychological testing, participants underwent an incidental encoding phase of 80 object images. Participants were asked “How much does this image appeal to you?” and had two seconds to respond, ranging from 1 (does not appeal to me) to 5 (appeals to me very much). Object images varied in frequency (once, twice, four, or seven times) within their category for a total of 280 trials. A 30 s break was given to participants halfway through this phase.
In the surprise test phase, participants made frequency judgments indicating how often they viewed an object image. All 80 images were presented in a fixed random orderFootnote 2 , and individuals responded 1, 2, 4, or 7 with no time limit.
Analyses
Two univariate ANOVAs were conducted to compare participants’ education levels across the groups and to examine participants’ age differences between the healthy older adults and adults with aMCI groups. Separate univariate ANOVAs were conducted as a function of group for each neuropsychological test, with tests of intellectual functioning analyzing healthy younger adults as an additional group.
Pearson’s correlations of frequency judgments with actual object frequencies were calculated for each participant and submitted to a univariate ANOVA as a function of group. These analyses were re-run using age as a covariate. Further analyses were conducted to compare the current study with the findings of Sanger and Anderson (Reference Sanger and Anderson2022), in a 2 (study) x 3 (group) ANOVA on familiarity.
Results
Participant data
Demographic and neuropsychological test data are shown in Table 2. There was no difference in education between groups, F(2, 85) = 1.40, p = .252, η p 2 = .03. However, participants with aMCI were significantly older than healthy older adults, F(1, 62) = 7.89, p = .006, η p 2 = .10.
Table 2. Mean (standard deviation) demographic and neuropsychological test data for participants in study 2
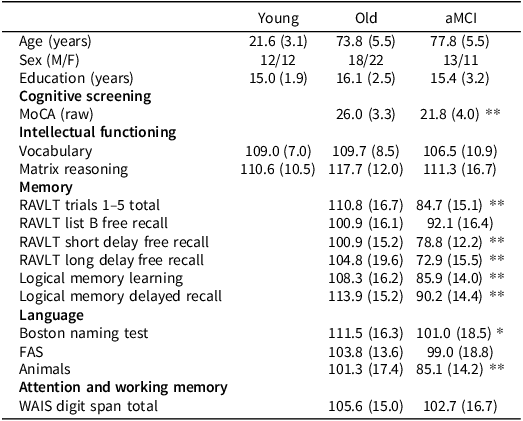
Note: Means are standard scores unless otherwise stated.
*p< 0.05.
**p< 0.01.
Healthy younger and older adults performed within normal ranges in neuropsychological functioning. On average, individuals with aMCI performed within normal ranges in the intellectual functioning tasks (SILS Vocabulary, WASI-MR) and the attention and working memory task (Digit Span) but, as per diagnostic criteria, performed below average or borderline across measures of memory. On average, individuals with aMCI also had a MoCA score signifying cognitive impairment.
Frequency judgment task data
As evident in Figure 2, individuals with aMCI had significantly lower frequency judgment correlations than younger adults (p < .001) and healthy older adults (p < .001), with no significant differences between younger adults and older adults (p = .881), F(2, 85) = 32.05, p < .001, η p 2 = .43. Adding age as a covariate did not alter these results.
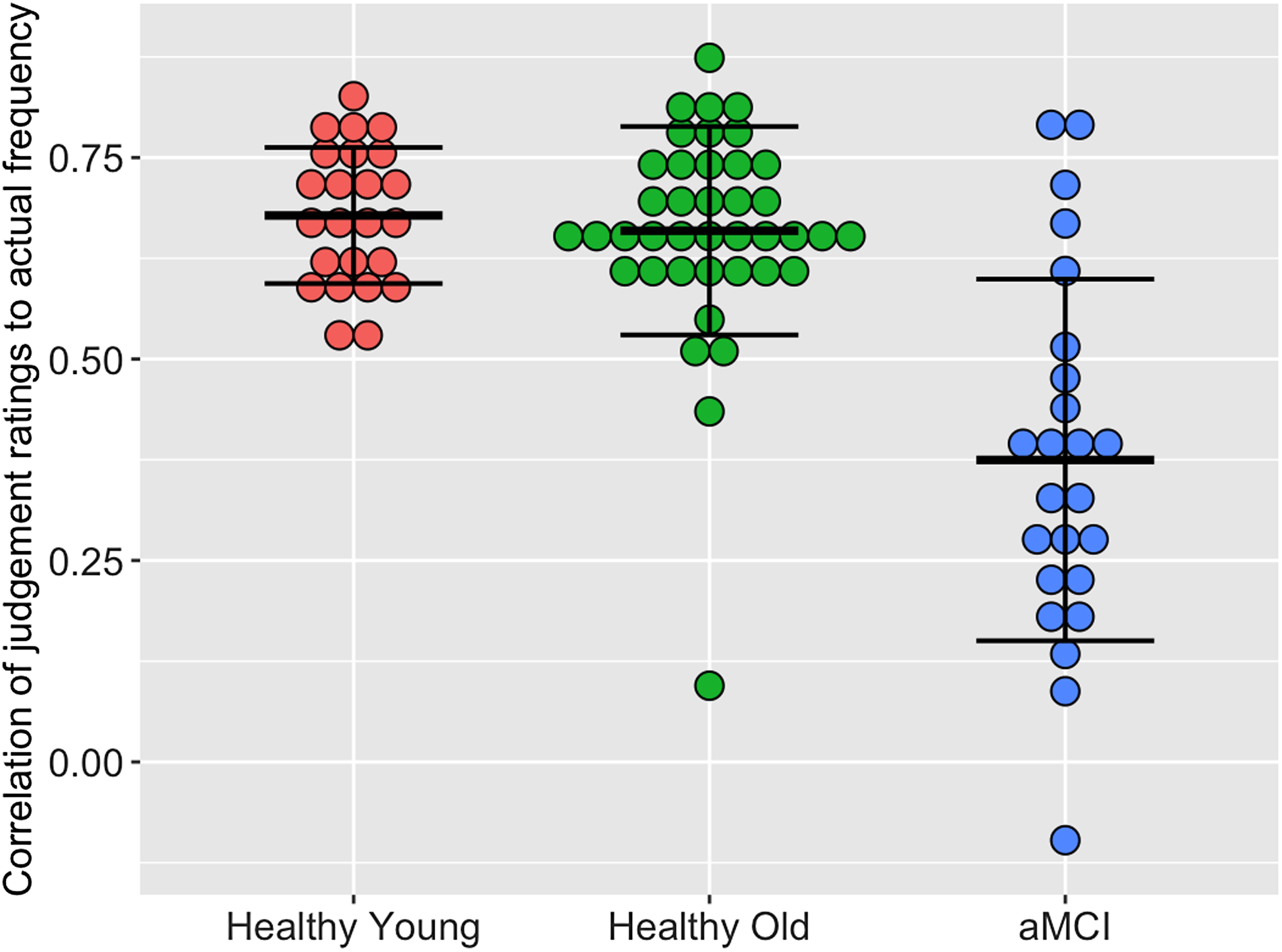
Figure 2. Correlation of judgment ratings to actual frequency. per group.
The ANOVA comparing familiarity estimates for objects in our present study and that of Sanger and Anderson (Reference Sanger and Anderson2022) found that overall, familiarity was comparable across studies, F(1, 186)<1, but was greater in healthy younger and older adults than older adults with aMCI, F(2, 186) = 34.23, p < .001, η p 2 = .27, with no difference between healthy younger and older adults (p = .902). Critically, the Study × Group interaction, F(2, 186) = 3.493, p = .032, η p 2 = .04, resulted from significantly lower familiarity for objects with overlapping features than distinct objects in the aMCI group alone (p = .026) with no study difference in the healthy young (p = .154) or healthy old (p = .934).
Discussion
Younger adults and healthy older adults had similar frequency judgment correlations, but individuals with aMCI were significantly less accurate in their frequency judgments. Our study, together with previous findings from our lab (Anderson et al., Reference Anderson, Baena, Yang and Köhler2021; Sanger & Anderson, Reference Sanger and Anderson2022), provides robust evidence that familiarity is impaired in individuals with aMCI. Furthermore, distinguishing between highly similar objects drives PRC activation (Erez et al., Reference Erez, Cusack, Kendall and Barense2016), which is also associated with familiarity (Bowles et al., Reference Bowles, Crupi, Mirsattari, Pigott, Parrent, Pruessner, Yonelinas and Köhler2007; Davachi et al., Reference Davachi, Mitchell and Wagner2003; Diana et al., Reference Diana, Yonelinas and Ranganath2008; Wolk et al., Reference Wolk, Dunfee, Dickerson, Aizenstein and DeKosky2011; Yonelinas et al., Reference Yonelinas, Otten, Shaw and Rugg2005; Reference Yonelinas, Widaman, Mungas, Reed, Weiner and Chui2007). Indeed, the greater familiarity deficit in aMCI for the highly similar objects used in this study, compared to that of Sanger and Anderson (Reference Sanger and Anderson2022) further highlights the PRC’s role in the decline of familiarity in aMCI.
General discussion
The primary purpose of this work was to evaluate familiarity in aMCI using paradigms that minimize the influence of recollection. We used a response deadline task and a frequency judgment task. Shorter response deadlines can elicit automatic and less resource-intensive familiarity due to the time constraints, while longer deadlines allow for detailed retrieval processes associated with recollection (Jacoby, Reference Jacoby1999; McElree et al., Reference McElree, Dolan and Jacoby1999). Frequency judgments also rely on automatic familiarity processes (Hintzman & Curran, Reference Hintzman and Curran1994; Zacks & Hasher, Reference Zacks and Hasher2002), and discriminating between highly similar items drives PRC activation (Erez et al., Reference Erez, Cusack, Kendall and Barense2016) – the region associated with familiarity (Bowles et al., Reference Bowles, Crupi, Mirsattari, Pigott, Parrent, Pruessner, Yonelinas and Köhler2007; Davachi et al., Reference Davachi, Mitchell and Wagner2003; Diana et al., Reference Diana, Yonelinas and Ranganath2008; Wolk et al., Reference Wolk, Dunfee, Dickerson, Aizenstein and DeKosky2011; Yonelinas et al., Reference Yonelinas, Otten, Shaw and Rugg2005; Reference Yonelinas, Widaman, Mungas, Reed, Weiner and Chui2007). Damage to the PRC also leads to frequency judgment deficits (Bowles et al., Reference Bowles, Crupi, Mirsattari, Pigott, Parrent, Pruessner, Yonelinas and Köhler2007; Reference Bowles, Duke, Rosenbaum, McRae and Köhler2016; Köhler & Martin, Reference Köhler and Martin2020). We hypothesized that in both tasks, familiarity would be preserved in healthy aging (Koen & Yonelinas, Reference Koen and Yonelinas2016). However, as tau begins to accumulate in the PRC (Braak & Braak, Reference Braak and Braak1991), we hypothesized that, on average, familiarity would be impaired in individuals with aMCI (Anderson et al., Reference Anderson, Baena, Yang and Köhler2021; Sanger & Anderson, Reference Sanger and Anderson2022).
Our results verified this hypothesis; individuals with aMCI had poorer performance in the short response deadline and in the frequency judgment task compared to healthy older adults, indicating familiarity impairment. We also established that this impairment is more pronounced when individuals with aMCI distinguish among highly similar, rather than distinct, objects.
Contrary to the literature (Koen & Yonelinas, Reference Koen and Yonelinas2014), recollection impairments were not evident in healthy older adults. This raises questions about the validity of the RDP in distinguishing between familiarity and recollection. Second, due to experimenter error, test images in the frequency judgment task were presented in the same random order for each participant. To examine potential primacy or recency effects, we divided the test trials into eight blocks of ten trials each and compared frequency judgment correlations across blocks. Our analysis revealed no significant performance differences across blocks; F(7, 680) = 1.60, p = .131, η p 2 = .02 and no interaction effect between blocks and group; F(14, 680)<1, p = .613, η p 2 = .02. Lastly, the Baycrest participant pool may not represent the aging population, as this group is typically well-educated, middle to upper class, predominantly Caucasian, and accustomed to participating in research studies. Although participants underwent a battery of neuropsychological tests to assess their cognitive status, and other health conditions that could result in memory impairment were excluded, they were not recruited from a memory clinic. Consequently, while our sample meets the established criteria for aMCI, there remains the possibility that it includes individuals with heterogeneous underlying pathologies, some of which may not reflect the prodromal phase of AD. To enhance the generalizability of these findings, future research should consider recruiting from both community and clinical settings.
Exploring familiarity in subjective cognitive decline could help in understanding the earliest signs of cognitive changes that precede objective cognitive impairment. In addition, future research could examine how these behavioral results map on to electroencephalogram indicators of familiarity, particularly the N400 (Ally & Budson, Reference Ally and Budson2007; Rugg & Curran, Reference Rugg and Curran2007). Individuals with aMCI exhibit smaller amplitudes of N400 response signals during recognition tasks, compared to healthy counterparts (Ally, McKeever et al., Reference Ally, McKeever, Waring and Budson2009; Galli et al., Reference Galli, Ragazzoni and Viggiano2010; Hoppstadter et al., Reference Hoppstädter, King, Frölich, Wessa, Flor and Meyer2013), but to our knowledge, differences in the N400 during frequency judgments in aMCI has not been explored. Finally, an accessible diagnostic tool can be useful to identify familiarity deficits in those at risk of dementia, to monitoring cognitive decline over time, and possibly facilitate intervention.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S1355617725000219.
Acknowledgements
We would like to express our sincere gratitude to Mariam Sidrak, Brahm Sanger, Elizabeth Baker-Sullivan, Hayley Gable, Kevin Tang, Smeet Solanki, and Juliana Springer for their invaluable contributions to this research.
Funding statement
This research was funded by a grant from the Natural Sciences and Engineering Research Council of Canada awarded to NDA (grant number RGPIN-2023-05241).
Competing interests
The authors report no conflicts of interest.






