Summations
• Motor functional neurological disorder (FND) poses a substantial clinical burden and has no effective pharmacotherapies. Building upon the encouraging treatment potential of psychedelics in neuropsychiatric disorders, this protocol outlines the first study investigating psilocybin-assisted physiotherapy for refractory motor FND.
• The study will compare a moderate dose of psilocybin that incorporates movement tasks during the acute drug effects versus a conventional, standard-dose approach. Both treatment groups will receive a course of FND-specific physiotherapy pre- and post-dosing, accompanied by psychiatric support. A battery of measures will be assessed at baseline and up to four weeks post-treatment.
• The findings will inform an adequately powered randomised controlled trial in this cohort and provide insights into the feasibility of psychedelic treatment in other functional and neuropsychiatric disorders.
Considerations
• Novel therapies for motor functional neurological disorder (FND) are required because existing treatments are insufficient. Supported by a theoretical basis, psychedelic-assisted physiotherapy in motor FND warrants further investigation.
• Testing psychedelics with physiotherapy will also provide novel insights into the potential for psychedelic-assisted therapy to target broader physiological and sensorimotor mechanisms implicated in other functional and neuropsychiatric disorders.
• Given the nascency of this area, this pilot study will prioritise safety and feasibility, with any observed benefits limited by the study’s open-label design and small sample size. As the field evolves, randomised controlled studies with adequate power and long-term outcomes will contribute to understanding the translational potential of this treatment.
Highlights
• Motor functional neurological disorder is a common illness. Many individuals do not respond to current treatments and experience disabling symptoms in the long term.
• Building on the encouraging treatment potential of psychedelics in neuropsychiatric disorders and their hypothesised mechanism of action, this protocol details a study comparing the feasibility and potential efficacy of two distinct treatment regimens of psilocybin combined with physiotherapy.
• The findings will inform a follow-up, randomised controlled trial in refractory motor functional neurological disorder.
Introduction
Background and rationale
Functional neurological disorder (FND) presents with neurological symptoms that are considered incompatible with other neurological conditions (Hallett et al., Reference Hallett, Aybek, Dworetzky, McWhirter, Staab and Stone2022). Motor FND refers to the presence of functional motor symptoms, such as weakness, tremor, or abnormal gait. It is a common illness, with estimates of incidence of functional motor symptoms at approximately 4–5/100’000 population per year (Binzer et al., Reference Binzer, Andersen and Kullgren1997; Stone et al., Reference Stone, Warlow and Sharpe2010). These are almost certainly underestimates, as many remain undiagnosed for years (Crimlisk et al., Reference Crimlisk, Bhatia, Cope, David, Marsden and Ron2000), and presentations have likely increased since the coronavirus disease 2019 pandemic (Hull et al., Reference Hull, Parnes and Jankovic2021). Many individuals continue to experience disabling symptoms in the long term, as demonstrated by a systematic review which found that 39% of individuals with functional motor symptoms remained the same or worse at follow-up (Gelauff et al., Reference Gelauff, Stone, Edwards and Carson2014).
Available treatment options in motor FND are limited. Pharmacotherapy is not indicated in its direct treatment (Aybek & Perez, Reference Aybek and Perez2022), and trials of psychotherapy remain inconclusive (Hinson et al., Reference Hinson, Weinstein, Bernard, Leurgans and Goetz2006; Kompoliti et al., Reference Kompoliti, Wilson, Stebbins, Bernard and Hinson2014; Dallocchio et al., Reference Dallocchio, Tinazzi, Bombieri, Arnó and Erro2016). Preliminary evidence from pilot studies demonstrated symptom improvement with psychologically informed, FND-specific physiotherapy (Czarnecki et al., Reference Czarnecki, Thompson, Seime, Geda, Duffy and Ahlskog2012; Jordbru et al., Reference Jordbru, Smedstad, Klungsøyr and Martinsen2014; Nielsen et al., Reference Nielsen, Buszewicz, Stevenson, Hunter, Holt, Dudziec, Ricciardi, Marsden, Joyce and Edwards2017b). However, a recently published phase 3 randomised controlled trial (RCT) of FND-specific physiotherapy for motor FND demonstrated mixed findings (Nielsen et al., Reference Nielsen, Stone, Lee, Goldstein, Marston, Hunter, Carson, Holt, Marsden, Le Novere and Nazareth2024). Although a significant difference in the primary outcome measure was not observed, the physical functioning domain of the participant-reported 36-Item Short Form Survey (SF-36) indicated that 59% of the specialist physiotherapy group rated their symptoms as improved compared to 39% of the standard physiotherapy group. This suggests that while a proportion of individuals may benefit from physiotherapy alone, alternative approaches are necessary for those who are refractory to treatment.
While pathophysiological models of motor FND remain an evolving field, several proposed mechanisms have been consolidated into a Bayesian approach based upon the dynamic relationship between sensory input and neural representations of this information (Friston, Reference Friston2010; Edwards et al., Reference Edwards, Adams, Brown, Pareés and Friston2012). This model posits that in motor FND, top-down somatic self-representations, or priors, may become overly precise through factors such as abnormal emotion processing, self-directed attention, and interoceptive dysfunction. In the context of motor control, this generates a disconnect between conscious experience and sensorimotor function, resulting in motor symptoms. Furthermore, these symptoms may become entrenched when interpreted as illness, thus reinforcing aberrant self-representations.
Based on this model, there is emerging interest in the application of classic psychedelics, such as psilocybin, for motor FND (Bryson et al., Reference Bryson, Carter, Norman and Kanaan2017). Psychedelics exert multifaceted effects upon the brain, including synaptogenesis and dendritic arborisation (Ly et al., Reference Ly, Greb, Cameron, Wong, Barragan, Wilson, Burbach, Soltanzadeh Zarandi, Sood, Paddy, Duim, Dennis, McAllister, Ori-McKenney, Gray and Olson2018); altered connectivity across macroscopic cortical networks (Madsen et al., Reference Madsen, Stenbæk, Arvidsson, Armand, Marstrand-Joergensen, Johansen, Linnet, Ozenne, Knudsen and Fisher2021); and both acute changes in perception and conscious state and longer-term changes in beliefs and behaviours (Studerus et al., Reference Studerus, Kometer, Hasler and Vollenweider2011). A synthesis of these effects suggests that psychedelics can promote neuroplasticity and relax overly precise priors while increasing the brain’s sensitivity to sensory input (Carhart-Harris & Friston, Reference Carhart-Harris and Friston2019). Indeed, these changes are hypothesised to underlie their therapeutic effects in several neuropsychiatric disorders, including major depressive disorder (MDD), illness-related anxiety, obsessive compulsive disorder, and substance use disorder (Andersen et al., Reference Andersen, Carhart-Harris, Nutt and Erritzoe2021).
The impact of psychedelics upon brain function suggests a promising role for psychedelic-assisted physiotherapy in motor FND. By relaxing somatic priors, sensorimotor processes may become freed from maladaptive top-down influences and more receptive to sensory input, such as proprioceptive feedback during movement retraining, thus enhancing the therapeutic potential of physiotherapy (Bryson et al., Reference Bryson, Carter, Norman and Kanaan2017). Although an encouraging exploration of psychedelics in FND was underway before the prohibition of these agents, the studies were limited by poor quality, and psychedelic treatment by itself was not universally effective (Butler et al., Reference Butler, Seynaeve, Nicholson, Pick, Kanaan, Lees, Young and Rucker2020). This may suggest a potential role for augmenting psychedelics with modern physiotherapy approaches.
Combining psychedelic treatment with physiotherapy for motor FND raises questions regarding the most appropriate treatment regimen. In previous studies of psychedelics in FND, a ‘psycholytic’ regimen was used in most cases, which involves low-to-moderate doses (typically 3 to 15 mg of psilocybin) combined with psychotherapy during the dosing session (Passie et al., Reference Passie, Guss and Krähenmann2022). An analogous approach could be used in psychedelic-assisted physiotherapy, whereby the participant engages in movement tasks during the drug effects to take advantage of acute changes in brain dynamics and neuroplasticity following psychedelic administration (Carhart-Harris & Friston, Reference Carhart-Harris and Friston2019; Berkovitch et al., Reference Berkovitch, Fauvel, Preller and Gaillard2025; Weiss et al., Reference Weiss, Magnesa, Gambini, Gurrieri, Annuzzi, Elefante, Perugi and Marazziti2025). We recently completed a dose-finding pilot study of movement tasks undertaken during the acute effects of low-to-moderate psilocybin doses in healthy participants – the first study of its kind to assess the impact of psilocybin on motor function (Bhagavan et al., Reference Bhagavan, Kanaan, Carter, Nielsen, Berlowitz, Issak, Braat, Zaloumis, Attard, Oliver, Mayne, McKernon, Roebuck, Rucker, Butler and Bryson2024) – which demonstrated the feasibility of engaging in movement tasks during the acute effects of psilocybin up to 15 mg. However, whether it is also feasible to administer physiotherapy during the acute psychedelic effects in motor FND remains uncertain.
In contrast, modern studies have predominantly adopted a ‘psychedelic’-assisted therapy (PAT) framework involving higher doses (typically 25 mg of psilocybin) and more profound psychoactive effects (Barber and Aaronson, Reference Barber and Aaronson2022; Passie et al., Reference Passie, Guss and Krähenmann2022). In studies of depression and anxiety, psychotherapy is also provided in the weeks following dosing to leverage a persistent ‘window’ of neuroplasticity, the duration of which may correlate with the intensity of the psychedelic experience (Watts and Luoma, Reference Watts and Luoma2020; Lepow et al., Reference Lepow, Morishita and Yehuda2021; Nardou et al., Reference Nardou, Sawyer, Song, Wilkinson, Padovan-Hernandez, De Deus, Wright, Lama, Faltin, Goff and Stein-O’Brien2023). Given the potential practical challenges of administering physiotherapy during acute psychedelic effects, this presents an alternative treatment model in motor FND: a standard ‘psychedelic’ dose alone, followed by physiotherapy in the subsequent weeks. However, whether this sustained window of neuroplasticity fosters movement retraining is also unknown. Ultimately, the optimal psilocybin dose and application of adjunctive physiotherapy for motor FND are unclear and in need of further exploration.
This protocol, therefore, details an individually randomised parallel-group pilot study assessing the tolerability, feasibility, and potential efficacy of psilocybin-assisted physiotherapy in participants with refractory motor FND. This study will compare two distinct psilocybin-assisted treatment paradigms: 1) a moderate ‘psycholytic’ dose (15 mg) that incorporates movement tasks during the acute drug effects, and 2) a standard ‘psychedelic’ dose (25 mg), integrated within a course of FND-specific physiotherapy for both treatment groups.
Objectives
Hypotheses
-
1. Both standard- and moderate-dose psilocybin will be well-tolerated in participants with refractory motor FND.
-
2. It is feasible for participants with refractory motor FND to perform a series of movement tasks during the acute effects of moderate-dose psilocybin.
-
3. Psilocybin-assisted physiotherapy can improve motor symptoms and disability in participants with refractory motor FND compared to the participants’ baseline assessment.
-
4. There is a difference in tolerability and symptom improvement between moderate- and standard-dose psilocybin-assisted physiotherapy in participants with refractory motor FND.
Primary aims
• To assess the tolerability of psilocybin-assisted physiotherapy in participants with refractory motor FND and compare between treatment groups, as measured by vital signs and adverse event (AE) reporting.
• To assess the feasibility of completing movement tasks during the acute effects of moderate-dose psilocybin in participants with refractory motor FND.
• To assess the effect of psilocybin-assisted physiotherapy in participants with refractory motor FND on within- and between-group changes in:
-
○ Clinician-rated changes in motor FND symptoms, using the Simplified Functional Movement Disorder Rating Scale (S-FMDRS).
-
○ Participant-reported changes in motor FND severity and improvement, using the Patient Global Impression of Severity (PGI-S) and Improvement (PGI-I).
-
Secondary aims
• To assess the effect of psilocybin-assisted physiotherapy in participants with refractory motor FND on within- and between-group changes in:
-
○ Motor FND symptom severity and improvement, using the Clinical Global Impression of Severity (CGI-S) and Improvement (CGI-I).
-
○ Motor FND symptom severity, using the S-FMDRS, graded through video assessment by an independent observer.
-
○ Depressive symptoms, using the Patient Health Questionnaire-9 (PHQ-9).
-
○ Anxiety symptoms, using the Generalised Anxiety Disorder-7 (GAD-7).
-
○ Somatic symptoms, using the Patient Health Questionnaire-15 (PHQ-15).
-
○ Health-related quality of life, using the SF-36.
-
• To assess the impact of pre-treatment expectations on outcomes in participants with refractory motor FND and compare between groups, using the Stanford Expectations of Treatments Scale (SETS).
• To assess the impact of psilocybin on altered conscious states and ego-dissolution in participants with refractory motor FND and compare between groups, using the 5-Dimensional Altered States of Consciousness (5D-ASC) and Ego-Dissolution Inventory (EDI).
• To determine the utility of these outcome measures for an adequately powered RCT of psilocybin-assisted physiotherapy in motor FND.
Exploratory aims
• To explore any acute benefits of performing movement tasks during the acute effects of moderate-dose psilocybin in participants with refractory motor FND.
• To explore the impact of psilocybin-assisted physiotherapy in participants with refractory motor FND on within- and between-group changes in:
-
○ Motor function, using the De Morton Mobility Index (DEMMI), Functional Movement Exploration (FME), Action Research Arm Test (ARAT), Box and Block Test (BBT) – original and modified versions – and video footage.
-
○ Sensorimotor function, using the force-matching task.
-
○ Resting-state and task-based measures of functional brain activity, using functional magnetic resonance imaging (fMRI).
-
○ Personality traits, using the Big Five Inventory-2 (BFI-2).
-
○ Experiences of the study treatment, using a study treatment questionnaire.
-
○ Qualitative effects through face-to-face interviews.
-
Trial design
This is a two-arm, individually randomised parallel-group study in 24 participants with refractory motor FND who will be randomly assigned, in a 1:1 ratio, to one of two treatment groups:
• Psilocybin 15 mg with movement tasks during the acute drug effects (moderate-dose arm), or
• Psilocybin 25 mg without movement tasks during dosing (standard-dose arm).
All participants will receive specialist, FND-specific physiotherapy, including two sessions pre- and six sessions post-dosing. Physiotherapy follow-up visits will occur one week and four weeks after completing their treatment. Psychiatric oversight will be provided, including a preparation session before their psilocybin dose, supervision during the dosing session, and follow-up sessions one week and four weeks after completing their physiotherapy treatment. An overview of this design is outlined in Fig. 1. Statisticians and an independent assessor of symptom severity will remain blinded to treatment allocation.

Figure 1. Study Design.
Abbreviation: FND, Functional Neurological Disorder.
Sample size
A sample size of 12 has been chosen because, assuming an 80% chance of a participant successfully completing physiotherapy during acute dosing, this provides over a 90% chance of enrolling at least one participant who is unable to complete the intervention. We consider a completion rate of at least 80% to be tolerable, and so a sample size of 12 is likely sufficient to screen for a poorly tolerated intervention. Participants who withdraw before psilocybin dosing will be replaced.
Methods: participants, interventions, and outcomes
Study setting
All study visits will occur at Austin Health, a public tertiary teaching hospital in Melbourne, Australia. fMRI scans will occur at the Melbourne Brain Centre, a research centre at the hospital. The dosing session will occur within a dedicated room providing a comfortable, monitored, clinical setting with access to temperature control, blankets, headphones, and music. Physiotherapy treatment sessions and follow-up visits will occur within the same room. Psychiatry follow-up visits and remaining scheduled outcome measures will occur within the study psychiatrist’s office.
Eligibility criteria
Study team eligibility
The trial physiotherapists will be registered physiotherapists trained in the Physio4FMD specialist physiotherapy intervention (Nielsen & Holt, Reference Nielsen and Holt2024) and movement tasks administered. The psychiatrists will be registered psychiatrists with experience working with psychedelic medicines.
Recruitment
The treating psychiatrist at Austin Health’s Functional Neurology Clinic will discuss the study with potentially eligible participants. The study team will also distribute a referral flyer to clinicians in Australia working with FND. Interested individuals will then be referred to the study by their treating practitioner and provided with the participant information sheet and consent form (PICF), which contains detailed study information and has been approved by the Austin Health Human Research Ethics Committee (AHHREC).
Consent and screening
Referred participants will be invited to a screening visit with the study psychiatrist, who will undertake informed consent. Information about the study procedures, potential benefits, and risks will be provided in a clear, balanced, and neutral manner. Informed consent will be obtained for the use of de-identified data for both this study and secondary uses, including informing future related projects and safety data to be shared with the Usona Institute.
Those willing to proceed with the study will provide a dated signature on the PICF. The study psychiatrist will then conduct a medical and psychiatric history, physical examination, vital signs, and electrocardiogram to assess eligibility.
Assignment of interventions
Allocation sequence
Participants will be randomised in a 1:1 ratio into one of the two treatment groups until 12 participants in each group have been allocated. The randomisation list will be computer-generated by an independent statistician of the University of Melbourne, using random block sizes, and uploaded as a randomisation module into Research Electronic Data Capture (REDCap) (Harris et al., Reference Harris, Taylor, Thielke, Payne, Gonzalez and Conde2009). After the study psychiatrist confirms eligibility, the study coordinator, who is independent of administering interventions, will action the randomisation module, allocating the treatment group.
Blinding
The statisticians and an independent assessor of symptom severity will be blinded to treatment assignments and will not be permitted access to the randomisation module. Videos for independent assessment of symptom severity will be allocated via a random number generator. If there is an emergency requiring knowledge of a participant’s allocation, the blind may be broken for that individual participant.
Interventions
Intervention description
Following enrolment, all participants will undertake a series of baseline measures, including self-reported outcomes, clinician-rated assessments of motor function and FND symptom severity, treatment expectations, a force-matching task, and an fMRI scan.
Eligible participants will undertake a psilocybin preparation session with the study psychiatrist consistent with existing safety guidelines for psychedelic research (Johnson et al., Reference Johnson, Richards and Griffiths2008). This will involve building rapport and trust, providing education about psilocybin, intention setting, reviewing relaxation strategies, and advice for preparation, dosing, and integration.
Treatment
Participants will then be scheduled for their study treatment. This will comprise two initial physiotherapy sessions, the psilocybin dosing session, and then six physiotherapy sessions within three weeks post-dosing.
Physiotherapy
The specialist physiotherapy is based on the Physio4FMD manual used in previous studies in motor FND (Nielsen & Holt, Reference Nielsen and Holt2024). A workbook will be completed by both the participant and the physiotherapist to help guide the intervention.
The initial two physiotherapy sessions pre-dosing will involve:
• Comprehensive assessment.
• Development of a treatment plan.
• Education on FND following a standardised biopsychosocial model.
The remaining six sessions post-dosing will comprise:
• Video analysis of movement.
• Posture and movement retraining – aiming to redirect attention away from the body and promote positive experiences of symptom-free and automatic, normal movement.
• Advice for managing common co-existing difficulties in FND, such as pain, fatigue, and memory difficulties.
• Codeveloping a self-management plan.
Psilocybin dosing session
The psilocybin dosing session will take place within three days of the initial physiotherapy sessions. Upon arrival, the study psychiatrist will ensure the participant’s continued eligibility and safety by reviewing their medications, measuring vital signs, and conducting a urine drug screen and urine pregnancy test (if applicable). The psychiatrist will then administer the prescribed psilocybin. The participant will be encouraged to relax, with the study psychiatrist available to provide support. Vital signs will be rechecked at 0.5-, 1, 3, and 5 hours post-dose, and AEs monitored throughout. For participants randomised to the moderate-dose arm, the physiotherapist will attend at 1.5 and 4.5 hours post-dose to administer a series of movement tasks and any further physiotherapy as tolerated by the participant to explore any potential treatment benefit of novel experiences of movement during the acute drug effects.
The participant will remain under the supervision of the study psychiatrist or physiotherapist for at least 5 hours post-dose and until the acute drug effects have subsided. The participant will then complete questionnaires regarding the intensity of their experience before being taken home by a support person or taxi. The psychiatrist will telephone the participant the following day to invite any further reflections and monitor safety.
Follow-up
After the final physiotherapy treatment session, the participant will complete the scheduled outcome measures. Follow-up physiotherapy and psychiatry visits will be scheduled for one week and four weeks post-treatment. Follow-up physiotherapy visits will review any areas of difficulty since completing the treatment, the self-management plan, and the goals. Follow-up psychiatry visits will cover psychological integration post-dosing, AEs, mental health, FND symptoms, and any corresponding changes in functioning. The psychiatrist will also conduct a semi-structured, audio-recorded qualitative interview at the one-week follow-up visit. All scheduled outcome measures will be completed at each of these study visits.
Following study exit, participants will return to the care of their usual treating practitioners, and any relevant handovers will be provided by the study psychiatrist and physiotherapist.
Discontinuing or modifying allocated interventions
Participants may withdraw from the study at any point. Investigators can withdraw participants if it is deemed in their best interest, they engage in protocol deviations placing them at risk, exclusion criteria develop, or an AE occurs that affects their safety.
The Principal Investigator has the right to terminate the study at any time and will make the final decision to terminate the study upon completion. If terminated prematurely, investigators will inform participants promptly, and all requirements regarding the storage and secure destruction of study documents and investigational products will be observed.
Concomitant care
Contraindicated medications
To be enrolled in the study, participants must not be taking:
• Opioids within 12 hours of psilocybin dosing.
• Antidepressants within five half-lives of their cessation before psilocybin dosing.
• Potent enzyme inducers or inhibitors.
• Drugs with a narrow therapeutic index within 12 hours of psilocybin dosing.
• Nicotine and caffeine within 2 hours before and 6 hours following psilocybin dosing.
Concomitant care
Medications will be reviewed at screening and before the dosing session. If a contraindicated medication is identified at the screening visit, a discussion between the investigators, participant, and their treating practitioner will take place to determine if it is appropriate to cease the medication before the dosing session. This will include a plan for reviewing and, if required, restarting the medication post-psilocybin dosing.
For contraindicated antidepressant medications, the recommendation will be to wean gradually to mitigate discontinuation effects, cease at least five half-lives before the scheduled dosing session to avoid interactions, and restart (if required) after completing the physiotherapy treatment to avoid initial medication titration adverse effects during the treatment course.
Participants will be required to suspend external physiotherapy for their motor FND throughout study enrolment until their study exit.
Outcomes
Time points for all outcome measures are outlined in Fig. 2, and their details are provided in Table 2.

Figure 2. Study schedule.
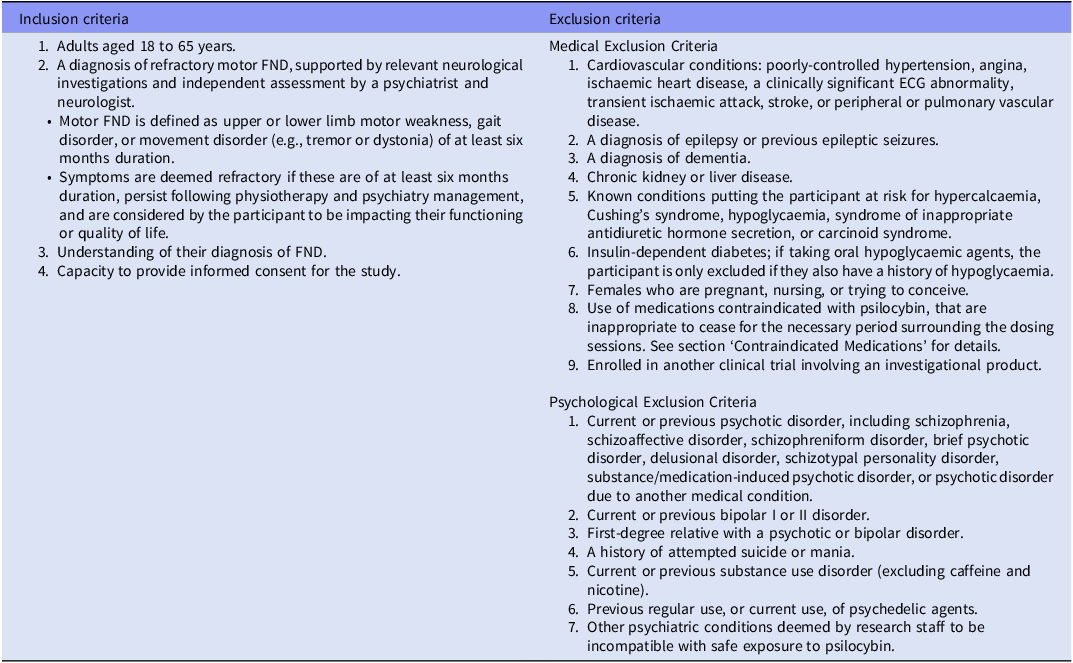
Table 1. Eligibility Criteria

Abbreviations: ECG, Electrocardiogram; FND, Functional neurological disorder.
Table 2. Primary, Secondary, and Exploratory Outcomes

Abbreviations: 5D-ASC, 5-Dimensional Altered States of Consciousness; ARAT, Action Research Arm Test; BBT, Box and Block Test; BFI-2, Big Five Inventory-2; CGI-I, Clinical Global Impressions of Improvement; CGI-S, Clinical Global Impressions of Severity; DEMMI, De Morton Mobility Index; EDI, Ego-Dissolution Inventory; FME, Functional Movement Exploration; fMRI, Functional magnetic resonance imaging; GAD-7, General Anxiety Disorder-7; PGI-I, Patient’s Global Impressions of Improvement; PGI-S, Patient’s Global Impressions of Severity; PHQ-9, Patient Health Questionnaire-9; PHQ-15, Patient Health Questionnaire-15; S-FMDRS, Simplified Functional Movement Disorder Rating Scale; SETS, Stanford Expectations of Treatments Scale; SF-36, 36-Item Short-Form Health Survey.
Primary outcomes
Tolerability will be assessed by checking vital signs regularly during the dosing sessions and recording AEs arising at any time during the study. AEs will be classified based on the type, number, severity, relatedness to the study drug, and whether the event constitutes a serious adverse event (SAE) or significant safety issue (SSI).
The feasibility of participating in physiotherapy during the acute effects of psilocybin 15 mg will be assessed by measuring the successful completion of a series of movement tasks. These will include the S-FMDRS (Nielsen et al., Reference Nielsen, Ricciardi, Meppelink, Holt, Teodoro and Edwards2017a), DEMMI (de Morton et al., Reference de Morton, Davidson and Keating2008), FME, ARAT (Yozbatiran et al., Reference Yozbatiran, Der-Yeghiaian and Cramer2008), and BBT (Mathiowetz et al., Reference Mathiowetz, Volland, Kashman and Weber1985) (original and modified versions) at 1.5 and 4.5 hours post-dosing.
The S-FMDRS assesses seven body regions and two functional movements (gait and speech) commonly affected by motor FND. Severity and duration are each rated from 0 to 3 for each of the nine items (higher scores indicate greater impairment), with items summed to provide a total score between 0 and 54. The scale may be completed by neurologists and physiotherapists, has high inter-rater reliability for the total score, and is sensitive to treatment-related changes. The assessment of the S-FMDRS by the study physiotherapist will comprise the primary outcome for assessing clinician-rated symptom severity. The S-FMDRS will be completed at baseline, 1.5 and 4.5 hours post-dosing for participants receiving psilocybin 15 mg, completion of the physiotherapy treatment, and the one-week and four-week follow-up visits.
The PGI-S and PGI-I are self-rated, single-item scales to capture static and dynamic aspects of disease severity and change, respectively (Food and Drug Administration, 2018). The PGI-S is rated from 1 to 4 (higher scores indicate greater impairment), and the PGI-I is rated from 1 to 7 (lower scores indicate greater improvement, and higher scores indicate worsening). These measures have shown satisfactory feasibility, validity, and sensitivity to treatment-related changes in studies on neuropsychiatric symptoms (Mohebbi et al., Reference Mohebbi, Dodd, Dean and Berk2018; Snyder et al., Reference Snyder, Tao, Svetnik, Lines and Herring2021; Remigio-Baker et al., Reference Remigio-Baker, Hungerford, Bailie, Ivins, Lopez and Ettenhofer2024). The PGI-S will be completed at baseline, and both scales completed on the morning of each day of the physiotherapy treatment and dosing session, following completion of the physiotherapy treatment, and at the one-week and four-week follow-up visits.
Secondary outcomes
An independent assessment of each S-FMDRS measure, as captured using video footage, will be completed by the study neurologist who will be blinded to treatment allocation. This will comprise a secondary outcome to help assess the interrater reliability and utility of this measure for this and subsequent studies. To complement the participant-reported PGI-I and PGI-S scales, the clinician-rated CGI-I and CGI-S (Busner & Targum, Reference Busner and Targum2007) will be administered to objectively assess disease severity and change, respectively, post-treatment.
Depressive symptoms will be measured by the PHQ-9 (Kroenke et al., Reference Kroenke, Spitzer and Williams2001), anxiety symptoms by the GAD-7 (Spitzer et al., Reference Spitzer, Kroenke, Williams and Löwe2006), somatic symptoms by the PHQ-15 (Kroenke et al., Reference Kroenke, Spitzer and Williams2002), and health-related quality of life by the SF-36 (Ware et al., Reference Ware, Snow, Kosinski and Gandek1993). Treatment expectations will be assessed pre-dosing by the SETS (Younger et al., Reference Younger, Gandhi, Hubbard and Mackey2012), and intensity of the acute drug effects assessed post-dosing by the 5D-ASC (de Deus Pontual et al., Reference de Deus Pontual, Senhorini and Corradi-Webster2023) and EDI (Nour et al., Reference Nour, Evans, Nutt and Carhart-Harris2016).
Exploratory outcomes
Video footage and individual scores for each of the DEMMI, FME, ARAT, and BBT (original and modified versions) will be assessed to explore the impact of psilocybin-assisted physiotherapy on several domains of motor function. Resting-state fMRI will be used to investigate alterations in large-scale brain networks and potential treatment mechanisms (Daws et al., Reference Daws, Timmermann, Giribaldi, Sexton, Wall, Erritzoe, Roseman, Nutt and Carhart-Harris2022; Berkovitch et al., Reference Berkovitch, Fauvel, Preller and Gaillard2025). This will be complemented by a Bayesian belief updating fMRI task, which will assess changes in optimism bias and any underlying neuroimaging correlates (Sharot et al., Reference Sharot, Korn and Dolan2011; Korn et al., Reference Korn, Sharot, Walter, Heekeren and Dolan2014; Marks & Baines, Reference Marks and Baines2017). This task has revealed differences between healthy subjects and patients with MDD and is of relevance given the hypothesised role of aberrant predictive processing in motor FND. Given previous findings of reduced sensory attenuation in motor FND, which may arise through related mechanisms, treatment-induced normalisation of sensorimotor performance using a force-matching task will also be assessed (Pareés et al., Reference Pareés, Brown, Nuruki, Adams, Davare, Bhatia, Friston and Edwards2014). In line with previous studies demonstrating changes in personality following psychedelics, such as increased ‘openness’, and the relevance of these changes to therapeutic effects, personality traits will be measured via the BFI-2 (Soto & John, Reference Soto and John2017). Subjective experiences will also be assessed by surveying participant perspectives on the intervention and conducting a qualitative interview following the treatment.
Data collection, management, and analysis
Data collection and assessment
The study physiotherapist will provide the physiotherapy treatment and physiotherapy follow-up visits, administer movement tasks, and assess symptom severity. The study neurologist will conduct independent assessments of the S-FMDRS, as captured using video footage. Qualified radiographers will conduct fMRI scans. The study psychiatrists will conduct the screening visit, preparation session, dosing session, post-dosing phone call, psychiatry follow-up visits, qualitative interview, and remaining scheduled measures at these visits. At all times, the study team member administering the assessment has full responsibility for the accuracy, completeness, and timeliness of all data captured.
Data management
The majority of study data will be collected and managed using REDCap (Harris et al., Reference Harris, Taylor, Thielke, Payne, Gonzalez and Conde2009) – a secure, browser-based application for managing online surveys and databases – hosted at the University of Melbourne. Scanned paper-based Case Report Forms (CRFs), video footage, recordings and transcripts of qualitative interviews, and fMRI images will be uploaded and stored in secure, password-protected servers hosted by the University of Melbourne and available only to permitted trial staff. Results from urine tests will be recorded, and urine samples will be disposed of in biological hazard waste bins.
Confidentiality will be maintained by assigning participants a unique code to record any data collected, and paper-based CRFs will be stored in locked filing cabinets. Videos of physiotherapy task performance will be de-identified using facial blurring, and qualitative interview audio recordings will be transcribed in a de-identified manner.
On study completion, scanned and electronic source documents will be archived on password-protected servers, and paper-based CRFs kept in locked cabinets. All CRFs will be retained for 15 years and then destroyed by secure shredding and deletion from protected servers according to the University of Melbourne records management policy.
Data management will be carried out to a standard of security and confidentiality consistent with Good Clinical Practice (International Council for Harmonisation, 2015). Data will be handled only by the research team and held at the Department of Psychiatry, University of Melbourne, Austin Health.
Adherence and retention
Before their study treatment, participants will receive a detailed appointment handout, which also reiterates pre- and post-dosing requirements and recommendations. Travel costs incurred for attending each study visit will be reimbursed, up to $50 per visit, and food and drink will be provided at the dosing session. No additional financial inducements will be provided. The psychiatry follow-up visits and qualitative interview will be offered either in person or via video call, as per participant preference, to promote retention by reducing the required number of in-person visits. The study team will monitor data in real time to ensure completeness and will document attempts to obtain follow-up data and any protocol deviations.
To assess the fidelity of the physiotherapy treatment (the extent to which the treatment followed the intervention protocol):
-
1. The study physiotherapist will complete a checklist for each participant, based on the template for intervention description and replication (TIDieR) checklist description (Hoffmann et al., Reference Hoffmann, Glasziou, Boutron, Milne, Perera, Moher, Altman, Barbour, Macdonald, Johnston and Lamb2014).
-
2. The content, length, and number of physiotherapy sessions by participant report will be monitored with a structured telephone survey following completion of the physiotherapy treatment.
-
3. A random sample of completed physiotherapy workbooks will be assessed by an independent physiotherapist against predetermined criteria.
Statistical methods
Prior to the study database being locked, a comprehensive statistical analysis plan will be finalised. The analysis will encompass all participants who were randomised, regardless of whether they completed their full study enrolment. All available data from these participants will be included. The reasons for any participant’s early withdrawal from the study will be documented.
An interim analysis using unblinded data will be performed after 50% (n = 12) of the target sample size has completed their study enrolment. This analysis will consist of summary statistics of tolerability and efficacy outcomes by treatment groups. These interim results will support planning for a follow-up RCT in motor FND participants. As such, the goal of the interim analysis is not related to this pilot study, and no sample size adjustment will be made as a result. Blinding will be maintained as described earlier.
Monitoring
The trial management group (TMG) will comprise all authors listed and provide overall supervision of the trial, including protocol development, oversight of trial progress, and publication and dissemination of trial results. A data monitoring committee is not needed for this study because of the limited known risks, short study duration, and Phase 1 objectives centred on feasibility and safety.
Adverse event reporting and harms
Common acute AEs reported following psilocybin administration included headache, nausea, anxiety, dizziness, and blood pressure elevations and were mostly mild-to-moderate in severity and transient (Johnson et al., Reference Johnson, Andrew Sewell and Griffiths2012; Breeksema et al., Reference Breeksema, Kuin, Kamphuis, van den Brink, Vermetten and Schoevers2022; Yerubandi et al., Reference Yerubandi, Thomas, Bhuiya, Harrington, Villa Zapata and Caballero2024). When incorporating long-term outcomes, contemporary clinical studies revealed no reports of death by suicide, persisting psychotic disorder, or hallucinogen persistent perceptual disorder (Hinkle et al., Reference Hinkle, Graziosi, Nayak and Yaden2024). However, concerns for incomplete reporting and identification have been raised, and further research is required into the preferred management of AEs and long-term safety (Hinkle et al., Reference Hinkle, Graziosi, Nayak and Yaden2024; Simonsson et al., Reference Simonsson, Johnson and Hendricks2024). Therefore, safeguards will be implemented throughout this study to comprehensively monitor, report, and respond to safety concerns.
During psilocybin dosing, the study psychiatrist will be available to respond to any psychological distress. If severe, benzodiazepines and antipsychotics will be available for administration. A Medical Emergency Team notification will be initiated if these measures are ineffective, heart rate exceeds 130 beats per minute, systolic blood pressure exceeds 180 mmHg, oxygen saturation falls below 90%, there is a reduced level of consciousness, or symptoms arise that warrant urgent medical assessment. The site physicians will be available via mobile phone throughout enrolment.
All AEs will be monitored by the study physicians until resolution or, if unresolved or chronic, further follow-up is arranged as warranted. All SAEs and SSIs will be reported as per Austin Health’s safety reporting policy.
Dissemination plans
Results will be submitted to peer-reviewed journals for publication and presented at psychiatry, neurology, physiotherapy, or other relevant conferences.
De-identified safety data will be shared with the Usona Institute. Access to the full trial dataset will only be available to investigators and any other relevant regulatory bodies. Study intellectual property arising from this study will be owned by the University of Melbourne.
Discussion
Conceptual frameworks for FND have evolved from exclusively psychological to biopsychosocial models, supported by evidence implicating physiological and sensorimotor processes (Drane et al., Reference Drane, Fani, Hallett, Khalsa, Perez and Roberts2021; Pareés et al., Reference Pareés, Brown, Nuruki, Adams, Davare, Bhatia, Friston and Edwards2014; Ricciardi et al., Reference Ricciardi, Demartini, Crucianelli, Krahé, Edwards and Fotopoulou2016; Perez et al., Reference Perez, Nicholson, Asadi-Pooya, Bègue, Butler, Carson, David, Deeley, Diez, Edwards and Espay2021; Raynor & Baslet, Reference Raynor and Baslet2021; Sojka et al., Reference Sojka, Diez, Bareš and Perez2021). This shift in understanding is reflected in expert consensus recommendations of physiotherapy as part of multidisciplinary treatment of motor FND (Nielsen et al., Reference Nielsen, Stone, Matthews, Brown, Sparkes, Farmer, Masterton, Duncan, Winters, Daniell and Lumsden2015). However, the limited efficacy of existing interventions and the impact of the disorder emphasise the need for new treatments that target other aspects of FND pathophysiology. Research into psychedelics has demonstrated encouraging potential in several neuropsychiatric disorders, and this pilot study was conceptualised based on these advances in the pathophysiological understanding of motor FND and hypothesised treatment mechanisms of psychedelics.
Given the uncertainties regarding the therapeutic mechanisms of psychedelics and the most appropriate treatment model, this study will therefore compare additional active physiotherapy during the acute effects of moderate-dose psilocybin versus a more conventional, standard-dose PAT approach.
This study will enrol participants with treatment-refractory motor FND. This provides an opportunity to evaluate the potential added benefit of psychedelic treatment in those who have already received the current gold-standard physiotherapy approach. The comprehensive screening and eligibility criteria in this trial will help exclude participants at greater risk of harm following psychedelic treatment (MacCallum et al., Reference MacCallum, Lo, Pistawka and Deol2022). The regular safety reporting, psychiatric support, and availability of study physicians throughout the study will enable prompt action to address any concerns arising and provide a greater understanding of the tolerability of this intervention.
The wide range of validated outcome measures spanning motor function, psychiatric and physical symptoms, and quality of life domains will enable assessment of benefits beyond core symptom relief and the potential for this treatment to address shared biopsychosocial factors across these areas (Butler et al., Reference Butler, Shipston-Sharman, Seynaeve, Bao, Pick, Bradley‐Westguard, Ilola, Mildon, Golder, Rucker and Stone2021). Exploratory outcomes examining underlying mechanisms, such as fMRI and the force-matching task, may deepen these insights and identify potential biomarkers and mediators of treatment response. The inclusion of both clinician-rated and self-rated outcomes, including qualitative interviews, will facilitate balanced inquiries into objective and subjective changes. The utility of these measures will also be assessed for their consideration in future, adequately powered studies.
These findings may inform psychedelic-assisted therapy studies in other FND subtypes, such as functional seizures, and related neuropsychiatric disorders with shared pathophysiological mechanisms, such as chronic pain, fibromyalgia, and chronic fatigue syndrome (Castellanos et al., Reference Castellanos, Woolley, Bruno, Zeidan, Halberstadt and Furnish2020; Glynos et al., Reference Glynos, Pierce, Davis, McAfee and Boehnke2023; Wilde, Reference Wilde2023; Butler et al., Reference Butler, Bird, Maggio, Durden, Modlin, Campbell-Coker, Edwards, Pick, Millman L.S., Lowery, Bhagavan, Kanaan, Golder, Mildon, Mehta, Rucker and Nicholson2024). Beyond the rationale for combining psychedelics with physiotherapy for motor FND, there exists a theoretical basis for the use of psychoplastogens in treating other neuropsychiatric disorders associated with motor dysfunction, such as stroke and acquired brain injury (Nardou et al., Reference Nardou, Sawyer, Song, Wilkinson, Padovan-Hernandez, De Deus, Wright, Lama, Faltin, Goff and Stein-O’Brien2023; Allen et al., Reference Allen, Dames, Foldi and Shultz2024; Yang et al., Reference Yang, Wang and Wang2025). This is of considerable interest given the known impact of psychedelics upon perceptual function closely related to motor control, and this will be the first study combining psychedelics with movement retraining in a clinical population, providing valuable insights into the feasibility of this approach in these related conditions.
While not investigated in this pilot study, a future consideration is the role of psychedelic microdosing. This involves consumption of ‘sub-perceptual’ psychedelic doses with purported cognitive and therapeutic benefits (Andersson & Kjellgren, Reference Andersson and Kjellgren2019; Fadiman & Korb, Reference Fadiman and Korb2019; Lea et al., Reference Lea, Amada and Jungaberle2020). However, evidence for micro-dosing is constrained by a lack of controlled studies (Polito & Liknaitzky, Reference Polito and Liknaitzky2022; Murphy et al., Reference Murphy, Muthukumaraswamy and de Wit2024) and the reduced psychoplastogenic effects at these low doses (Jefsen et al., Reference Jefsen, Elfving, Wegener and Müller2021; Barksdale et al., Reference Barksdale, Doss, Fonzo and Nemeroff2024). Nevertheless, it is possible that this treatment paradigm may be worth exploring in the future as the field evolves.
Given this study’s primary objectives focus on tolerability and feasibility, the therapeutic outcomes will be limited by the open-label design, small sample size, and relatively short follow-up. We also acknowledge challenges arising via the temporary cessation of psychotropic medications, including ongoing uncertainty and study into how best to incorporate these pre-existing medications with psychedelic treatment (Goodwin et al., Reference Goodwin, Croal, Feifel, Kelly, Marwood, Mistry, O’Keane, Peck, Simmons, Sisa and Stansfield2023; Tap et al., Reference Tap, Thomas, Páleníček, Stenbæk, Oliveira-Maia, van Dalfsen and Schoevers2025).
This protocol outlines the first study of psychedelic treatment for motor FND in over 50 years. The study design builds upon recent findings of the safety and feasibility of performing physiotherapy following psilocybin administration in healthy participants. The results from this study will inform the design of a planned, adequately powered RCT of psilocybin-assisted physiotherapy in motor FND.
Authors’ contribution
R.K. is the Principal Investigator and the senior researcher. C.B. and A.B. drafted this manuscript. All authors contributed to the development of the protocol and approval of the final manuscript.
Financial support
This work was supported by the Medical Research Future Fund (R.K., O.C., S.B., G.N., D.B., and A.B., grant number MRF2012410); the Wellcome Trust (M.B., grant number 227515/Z/23/Z); and the RANZCP Foundation, the Royal Australian and New Zealand College of Psychiatrists (C.B.).
The Usona Institute provided the study drug. They have not offered or provided payments to the investigators.
Competing interests
R.K. is on the advisory board of Psychae Institute, a non-profit psychedelic research institute. R.K. has received grant funding from the Wellcome Trust, the Medical Research Council (U.K.), the National Health and Medical Research Council (Australia), and the Weary Dunlop Foundation for research on FND. R.K. receives royalties from Guildford Press for a book chapter on FND.
J.R. has undertaken paid advisory boards for Clerkenwell Health (Past), Beckley PsyTech (Past), Delica Therapeutics (Past), and paid articles for Janssen. J.R. has received assistance for attendance at conferences from Compass Pathways (past) and Janssen. J.R. has been awarded grant funding (received and managed by King’s College London) from Compass Pathways, Beckley PsyTech, Multidisciplinary Association for Psychedelic Studies, National Institute for Health Research, Wellcome Trust, Biomedical Research Centre at the South London and Maudsley NHS Foundation Trust.
O.C. has received funding from The Perception Restoration Foundation.
C.B. has received funding from the Graham Burrows Travelling Scholarship, University of Melbourne.
G.N. is a founding member and on the board of directors of the Functional Neurological Disorder Society. He is on the medical advisory boards of the charities FND Hope U.K. and FND Action. He receives research funding from the National Institute for Health and Care Research (U.K.).
Ethical standard
Ethics approval for this protocol has been awarded by the AHHREC (HREC/57390/Austin-2020). Significant protocol amendments will be reviewed by the TMG and submitted to the AHHREC. Active participants affected by amendments will be notified and provided with an updated PICF to review and reconsent if required.
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Trial registration
Trial registered on Australian New Zealand Clinical Trials Registry (ANZCTR).
Registration Number: ACTRN12621000578808
Date Registered: 17 May 2021
URL: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=381745
Protocol version
1 April 2025
Version 6.0
Identifier: 2025_APR_01 PROTOCOL_PsyFND_V6
Trial sponsor
Austin Health, Austin Hospital, 145 Studley Rd, Heidelberg VIC 3084.
Role of sponsors and funders
The study sponsors and funders are not involved in study design; collection, management, and analysis of data; writing of the report; and the decision to submit the report for publication.