Psychotic disorders affect approximately 1% of the global population and profoundly impact health and quality of life. 1,Reference Jongsma, Turner, Kirkbride and Jones2 Prognoses following a first episode of psychosis (FEP) vary, but many people experience recurrent relapses, long-term disability, social exclusion and high levels of mental health service use. Reference Fusar-Poli, McGorry and Kane3 People with psychosis are also at increased risk of poor physical health, with mortality rates several times higher than those of the general population. Reference Walker, McGee and Druss4,Reference Hayes, Marston, Walters, King and Osborn5 Evidence indicates that intensive treatment during the early stages of psychosis can mitigate long-term impacts. Reference Birchwood, Todd and Jackson6–Reference Penttila, Jaaskelainen, Hirvonen, Isohanni and Miettunen8 Consequently, Early Intervention in Psychosis (EIP) services have been established as a key element of mental healthcare, both in the UK Reference Joseph and Birchwood9 and internationally. Reference Kotlicka-Antczak, Podgorski, Oliver, Maric, Valmaggia and Fusar-Poli10 EIP services provide a comprehensive package of evidence-based treatments emphasising prompt access, multidisciplinary care and recovery-oriented approaches. EIP involvement has been shown to improve outcomes compared with treatment as usual, Reference Fusar-Poli, McGorry and Kane3,Reference Correll, Galling, Pawar, Krivko, Bonetto and Ruggeri11 in a cost-effective manner. Reference Aceituno, Vera, Prina and McCrone12 However, despite calls for research into the ‘active ingredients’ of EIP care, it remains unclear which specific components drive these improved outcomes. Reference Singh13 One recent component meta-analysis suggested that care coordination and psychological interventions are key, but findings were limited by reliance on symptom-based outcomes and an inability to isolate the impact of specific components. Reference Williams, Ostinelli, Agorinya, Minichino, De Crescenzo and Maughan14 Exact service models differ internationally, Reference Csillag, Nordentoft, Mizuno, McDaid, Arango and Smith15 and in the UK the National Clinical Audit of Psychosis (NCAP) identified significant variation in care delivery among services 16 in spite of implementation guidelines. 17 The impact of this variation on outcomes remains unknown.
Aims
This retrospective cohort study of 14 874 individuals aimed to identify associations between EIP care components and real-world outcomes including relapse, compulsory hospitalisation and mortality, using linked data from NCAP and routine health records. By pinpointing components linked to positive outcomes, we aimed to provide actionable insights to enhance EIP delivery, inform policy and resource allocation across mental health services and, ultimately, to better support individuals experiencing psychosis.
Method
This study follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for observational studies. Reference von Elm, Altman, Egger, Pocock, Gotzsche and Vandenbroucke18 A full checklist and deviations from the published protocol Reference Williams, Penington, Gupta, Tsiachristas, French and Lennox19 are provided in the Appendix. This study was informed by patients and carers and their priorities for research.
Study design
We conducted a retrospective cohort study using data from NCAP and routine health data-sets, over a 3-year period. NCAP is a quality improvement programme led by the Royal College of Psychiatrists that provides high-quality data on psychosis care. 16 Since 2017, NCAP has recorded patient-level data from every EIP service in England.
Our cohort comprised 14 874 individuals treated by all EIP services in England during the period 2019–2020. During each NCAP round, a random sample of 100 patients were selected from each service. Where a service’s total case-load comprised <100 eligible patients, all were included.
Inclusion criteria were:
-
(a) Diagnosed ‘first episode’ of any ‘non-organic’ psychotic disorder. To facilitate inclusivity and real-world generalisability of results, no specific diagnostic framework such as ICD or DSM was specified.
-
(b) Under EIP care for >6 months at NCAP audit date (April 2019 or April 2020).
-
(c) Aged 14–65 years – reflecting EIP access standards. 17
Patients were excluded from NCAP (and therefore this study) if they had a diagnosis of psychosis due to an ‘organic cause’.
NCAP exposure data (care components delivered by EIP services) were linked to routine health outcome data for a 3-year follow-up period ending January 2023. Linked data-sets were the Mental Health Services Data Set (MHSDS), Hospital Episode Statistics (HES), the Emergency Care Data Set (ECDS) and the ONS Civil Registration Death data-set. Data were pseudonymised and stored within the Office for National Statistics Secure Research Service (SRS).
Exposure variables
Our exposures are specific components of care specified by the National Institute for Health and Care Excellence (NICE) as necessary constituents of EIP: 20 receipt of an antipsychotic; receipt of ‘cognitive–behavioural therapy for psychosis’ (CBTp); receipt of a family intervention; receipt of vocational support; receipt of a carer-focused intervention; offer and initiation of clozapine where appropriate (patients were eligible if they had ‘treatment-resistant’ symptoms, i.e. inadequate response to two previous antipsychotics); receipt of NICE-approved EIP physical health interventions (smoking/alcohol/psychoactive substance cessation, weight reduction); and average care coordinator case-load size per service and waiting time (whether waiting time standard was met prior to initiation of EIP treatment).
Outcome variables
Our primary outcome was time to ‘relapse’, as indicated by in-patient admission or referral to a ‘crisis resolution and home treatment team’ (CRHTT). Secondary outcomes were time to in-patient admission or CRHTT referral alone, duration (bed days) of in-patient stay over the follow-up period, time to detention under the Mental Health Act, number of acute general hospital admissions and emergency department attendances and all-cause mortality.
Statistical analyses
Analyses were performed using R version 4.3.3 for Windows (R Core Team, R Foundation for Statistical Computing, Vienna, Austria; see https://www.r-project.org/). Initially we generated descriptive statistics for all exposure variables, outcome measures and covariates, and used unadjusted tests to explore pairwise associations.
Next we conducted multivariable analyses using Cox regression for time-to-event outcomes and zero-inflated negative binomial regression for count outcomes. For time-to-event outcomes, the time of exposure was taken as date of the relevant NCAP audit period for each individual (1 April 2019 or 1 April 2020). Multilevel models were used to account for clustering effects within EIP services, and were adjusted for covariates identified via a directed acyclic graph (DAG), including age, sex, ethnicity, employment status and prior admissions (within the data extract window, i.e. post April 2016; see ‘Detailed Statistical Methods’ and Fig. A1 in the Appendix for further details). Interactions (between all demographics variables and exposures) were checked for each outcome and included in model exploration if influential.
Final models were selected using a ‘goodness-of-fit’ approach, aiming to establish models that captured as much information in the data with as few parameters as possible (see Appendix for further details of statistical methods). We assessed model assumptions using appropriate statistical tests.
Given the large number of comparisons, we adopted a significance threshold of P ≤ 0.001 to reduce spurious findings. This threshold was chosen as a heuristic to focus on robust associations rather than applying formal corrections, which may be overly conservative in observational analyses. Results with P-values between 0.001 and 0.05 were considered suggestive but not definitive. This threshold of P ≤ 0.001 is broadly consistent with the significance levels used when controlling type 1 error rate at 5% in large data-sets. Reference Colquhoun21 Missing data for service-level variables were imputed using multiple imputation, with sensitivity analyses to test robustness (see the Appendix).
Results
Data were obtained for 14 874 participants; mean age was 33.5 years (s.d. 11.5) and 9225 were male (62.0%). Demographic characteristics and a summary of exposure and outcome variables are given in Table 1, with more detailed breakdowns (specific ethnicity categories, distribution of comorbidities) available in Figs A2–4 and Tables A1–3 in the Appendix.
Table 1 Cohort demographics
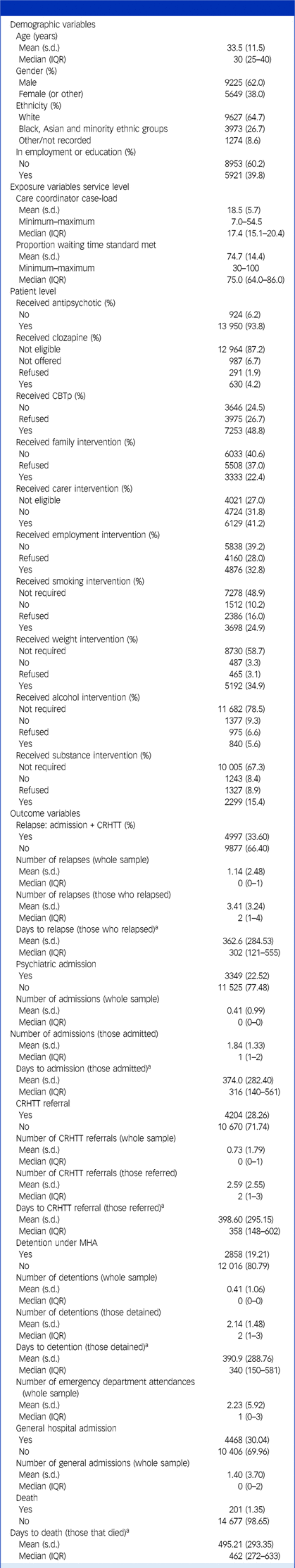
Summarised demographics, exposures and outcomes for the cohort (N = 14 874). Note that for age, interquartile range (IQR) is reported rather than the full range, to avoid potentially identifiable data as per Office for National Statistics Secure Research Service requirements. For gender, the ‘Other’ category (containing a very small number of participants) has been combined with ‘Female’, again to avoid potentially identifiable data.
CBTp, cognitive–behavioural therapy for psychosis; CRHTT, crisis resolution and home treatment team.
a. For time-to-event outcomes, the time of exposure was taken as date of the relevant National Clinical Audit of Psychosis audit period for each individual (1 April 2019 or 1 April 2020).
Primary outcome
Associations among exposure variables, covariates and adjusted hazard rates of relapse (i.e. the risk of a psychiatric admission or referral to a CRHTT, occurring at any given time point during the follow-up period) are reported in Table 2. Hazard rate ratios indicate the likelihood that a participant with the given exposure experienced the outcome at any given time point, relative to the ‘reference’ category for each variable. A hazard ratio >1 indicates an increased likelihood of outcome, while <1 indicates a decreased likelihood.
Table 2 Associations between exposures and relapse (primary outcome)

Unadjusted and adjusted hazard ratio with 95% CIs for the primary outcome (relapse, defined as in-patient admission or crisis resolution and home treatment team (CRHTT) referral). The full model includes all exposure variables and covariates, while the final model is based on a refined selection of variables informed by statistical and theoretical considerations. Hazard ratios represent the relative likelihood of relapse occurring at any given time for individuals in one category of a variable compared with the reference (Ref.) category, holding all other variables constant: hazard ratio >1 indicates an increased likelihood of relapse, while <1 indicates a decreased likelihood. Results are adjusted for clustering within services. Results in bold indicate P-values ≤0.001, which were considered strong evidence; results in italics indicate P-values between 0.001 and 0.05, which were considered suggestive, but not definitive, evidence.
BAME, Black and minority ethnic; EIP, Early Intervention in Psychosis; NA, not applicable; Ref., reference.
a For the continuous variables ‘age’, ‘care coordinator case-load’ and ‘proportion meeting waiting time standard’, stated hazard ratios indicate the change in hazard with a one-unit increase in the exposure. For example, each additional person on a care coordinator’s case-load increased the hazard of relapse by 2% (hazard ratio 1.02, 95% CI 1.01–1.02, P < 0.001).
b Interaction effects indicate the change in hazard ratio for the second variable for each unit of change in the first. For example, for individuals taking antipsychotic medication, each additional year of age decreases the hazard rate by 2% compared with those not taking it. This suggests that the increased hazard rate of relapse associated with antipsychotic medication reduces with age.
We found strong evidence that smaller care coordinator case-loads and the use of clozapine were associated with reduced relapse rates. Hazard rates were increased by 2% for each additional person on the case-load of an individual’s care coordinator (hazard rate 1.02, 95% CI 1.01–1.02, P < 0.001).
For clozapine, we found substantially increased hazard rates (compared with those who were not eligible to receive clozapine) for those who were eligible to receive it and refused it (hazard ratio 1.38, 95% CI 1.17–1.63, P < 0.001), or who were not offered it (hazard ratio 1.50, 95% CI 1.36–1.67, P < 0.001). However, those who were eligible for clozapine and received it showed no significant difference compared with ineligible individuals (hazard ratio 0.97, 95% CI 0.85–1.10, P = 0.614).
Effects on these variables on probability of relapse over the follow-up period are illustrated by cumulative incidence plots in Figs. 1 and 2.
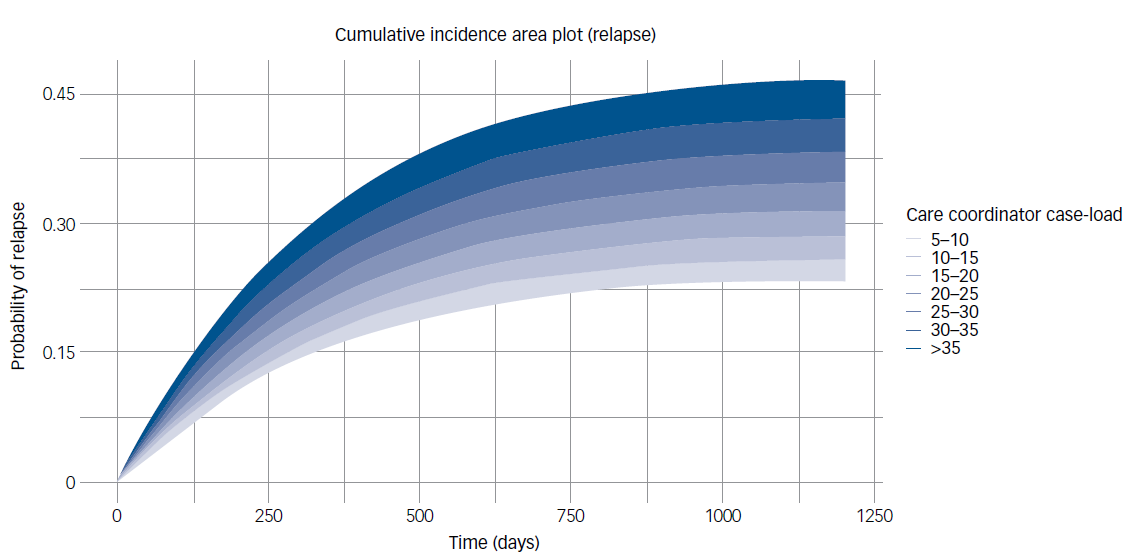
Fig. 1 Probability of relapse by average care coordinator case-load. This plot illustrates the cumulative probability of relapse over time (in days) when care coordinator case-load is varied for an otherwise ‘typical’ Early Intervention in Psychosis (EIP) patient. This typical patient is defined as having mean age and the most prevalent characteristics across other variables (e.g. male, White, unemployed, in receipt of cognitive–behavioural therapy for psychosis). Survival times were probabilistically simulated using parameterised hazard rates for each group, from a Cox proportional hazards model (with relapse probabilities increasing only at discrete event times), reflecting the plausible time-to-relapse patterns from the real population.
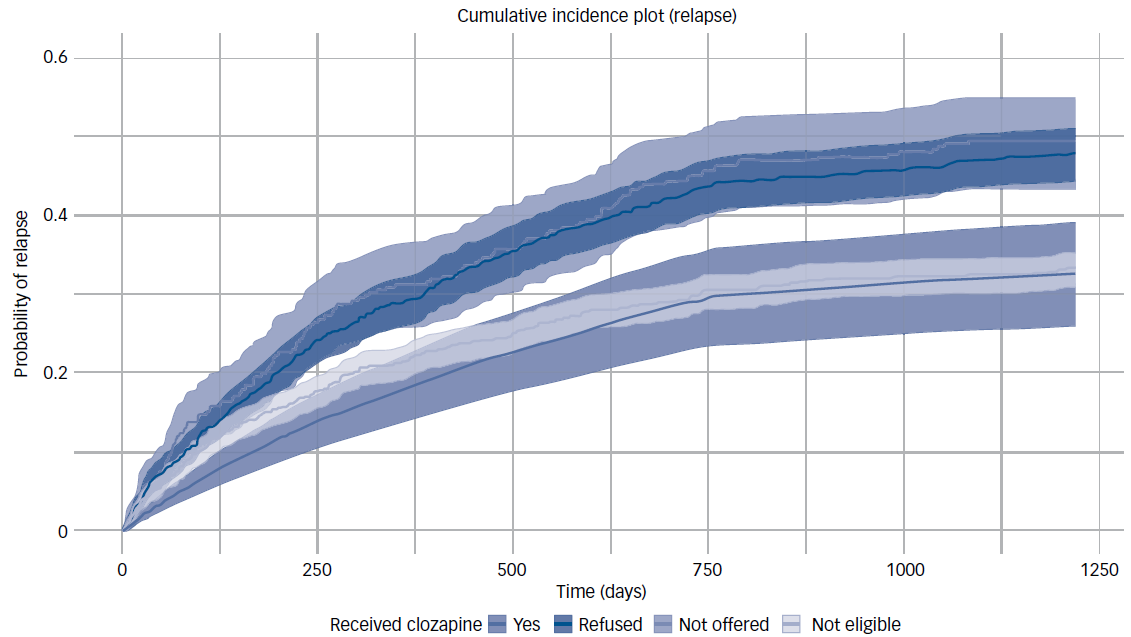
Fig. 2 Probability of relapse by eligibility/receipt of clozapine. This plot illustrates the cumulative probability of relapse over time (in days) based on clozapine eligibility and receipt for an otherwise ‘typical’ Early Intervention in Psychosis (EIP) patient. This typical patient is defined as having mean age and the most prevalent characteristics across other variables (e.g. male, White, unemployed, in receipt of cognitive–behavioural therapy for psychosis). Survival times were probabilistically simulated using parameterised hazard rates for each group from a Cox proportional hazards model (with relapse probabilities increasing only at discrete event times), reflecting the plausible time-to-relapse patterns from the real population.
Hazard rates were also higher for those requiring substance use interventions, regardless of whether these were refused (hazard ratio 1.45, 95% CI 1.32–1.59, P < 0.001) or received (hazard ratio 1.52, 95% CI 1.42–1.63, P < 0.001). No other components of care were associated with significant differences in rates of relapse.
Individuals from Black, Asian and minority ethnic (BAME) groups had higher relapse rates than White individuals (hazard ratio 1.18, 95% CI 1.11–1.26, P < 0.001), and relapse rate decreased by 2% per additional year of age (hazard ratio 0.98, 95% CI 0.98–0.98, P < 0.001). Individuals who had already had an inpatient admission prior to the NCAP census period had substantially higher hazard rates of subsequent relapse compared with those who had not (hazard ratio 2.25, 95% CI 2.11–2.40, P < 0.001).
Secondary outcomes
Findings for time-to-event secondary outcomes (psychiatric admission, CRHTT referral, Mental Health Act detention, mortality) and count outcomes (hospital admissions, emergency department attendances, admission duration) are detailed in Tables A4–10 in the Appendix.
For the outcomes ‘time to psychiatric hospital admission’, ‘time to CRHTT referral’ and ‘time to detention under Mental Health Act’, findings broadly mirrored those for relapse. This was unsurprising given the high degree of correlation between these outcomes (those with increased likelihood of a relapse overall also had increased likelihood of an in-patient admission or a CRHTT referral by definition, but also tended to have increased likelihood of detention under the Mental Health Act). Poorer outcomes were seen for higher care coordinator case-loads, those who were eligible for clozapine and did not receive it (compared with those who were ineligible) and for those who required interventions for substance use.
However, there were some specific differences. For CRHTT referral specifically there was suggestive evidence that those who were eligible for clozapine and received it might have lower (rather than equivalent) hazard ratios than ineligible individuals (hazard ratio 0.85, 95% CI 0.73–0.99, P = 0.033). In addition, those who received carer interventions had higher rates of referral than those with no identified carer involved (hazard ratio 1.16, 95% CI 1.07–1.25, P < 0.001).
For psychiatric admission specifically, there was very weak evidence that those who received CBTp had reduced hazard rates compared with those who were not offered it (hazard ratio 0.91, 95% CI 0.83–1.00, P = 0.057).
For rates of detention under the Mental Health Act, clozapine recipients had lower (rather than equivalent) hazard rates than those ineligible for clozapine (hazard ratio 0.82, 95% CI 0.68–0.98, P < 0.001).
Detention rates were also reduced for those that received CBTp (hazard ratio 0.85, 95% CI 0.77–0.94, P = 0.001), but were higher for BAME (hazard ratio 1.47, 95% CI 1.36–1.59, P < 0.001) and other non-White ethnic groups (hazard ratio 1.27, 95% CI 1.11–1.44, P < 0.001) than for White individuals.
For mortality, hazard rates were substantially higher for individuals requiring interventions for alcohol use who had either refused them (hazard ratio 2.59, 95% CI 1.46-4.58, P< 0.001) or did not receive them for some other reason (hazard ratio 1.80, 95% CI 1.06–3.39, P < 0.001) compared with those who did not require them. However, mortality rates were not increased for those who required an intervention and received it (hazard ratio 1.14, 95% CI 0.66–1.97, P = 0.638). A similar pattern was seen for weight loss interventions, with increased mortality for those refusing them (hazard ratio 1.85, 95% CI 1.05–3.24, P < 0.001) or not receiving them for other reasons (hazard ratio 2.36, 95% CI 1.32–4.22, P < 0.001), but not for those who received them. Mortality rates were increased for those who received interventions for substance use compared with those who did not require them (hazard ratio 2.50, 95% CI 1.74-3.59, P < 0.001), and increased by 7% for each 1-year increase in age (hazard ratio 1.07, 95% CI 1.05–1.08, P < 0.001).
For the following count outcomes, incidence rate ratios indicate the relative frequency with which a participant with the given exposure experienced the outcome, relative to the reference category for each variable. An incidence rate ratio >1 indicates increased frequency of outcome, while <1 indicates decreased frequency.
For the outcome ‘admission duration (bed days)’, we found suggestive evidence that individuals who were eligible for clozapine but did not receive it spent longer admitted over the follow-up period than those who were not eligible – those who were not offered it (incidence rate ratio 1.47, 95% CI 1.07–2.08, P = 0.024) or weaker evidence for those that refused it (incidence rate ratio 1.67, 95 CI 1.00–3.08, P = 0.053). Shorter admission durations were observed for those offered CBTp, particularly if they received it (incidence rate ratio 0.73, 95% CI 0.59–0.89, P = 0.002), although reduced durations were also seen in those that refused it (incidence rate 0.84, 95% CI 0.66–0.96, P = 0.037).
Clozapine recipients had lower emergency department attendance rates than those who were ineligible for clozapine (incidence rate ratio 0.75, 95% CI 0.66–0.86, P < 0.001). There was suggestive evidence that those who had an identified carer might also have lower rates of emergency department attendance than those who had not – including those who received a carer intervention (incidence rate ratio 0.90, 95% CI 0.85–0.96, P = 0.002) and those who did not (incidence rate ratio 0.90, 95% CI 0.84–0.96, P = 0.002). Rates of attendance were increased for those who received interventions for alcohol (incidence rate ratio 1.37, 95% CI 1.23–1.54, P < 0.001), smoking (incidence rate ratio 1.18, 95% CI 1.10–1.26, P < 0.001) and substance use (incidence rate ratio 1.37, 95% CI 1.26–1.48, P < 0.001).
For ‘general hospital admission’, no components of care were associated with improved incidence rates. Increased rates were observed for those who received interventions for smoking (incidence rate ratio 1.22, 95% CI 1.12–1.32, P < 0.001), alcohol (incidence rate ratio 1.29, 95% CI 1.13–1.47, P < 0.001) and substance use (incidence rate ratio 1.24, 95% CI 1.13–1.36, P < 0.001).
Missing data and sensitivity analyses
Our chosen outcomes are mandatory submissions for NHS England. The absence of data for an outcome was interpreted by NHS England as the patient not having experienced the outcome. There is a possibility that. in some cases, missing data were due to incomplete or incorrect recording rather than to a true absence of the outcome. If the likelihood of recording was associated with patient characteristics or outcomes, this could introduce a risk of informative censoring. However, due to the nature of the data, it was not possible to test for this.
The data supplied by NCAP were extremely comprehensive, with very few missing values – the only exceptions were the service-level exposure variables (care coordinator case-load per service and proportion meeting waiting time standard per service), which had been compiled from sources other than the case-note audit. For a more detailed breakdown of missing data see ‘Missing Data’ and Tables A5 and 6 in the Appendix.
Our sensitivity analyses examining the effects of different approaches to imputing missing data for service-level exposure variables (care coordinator case-load size and waiting times) resulted in comparable findings (full results are available in the Appendix).
Discussion
This study is the first to examine outcomes from components of EIP care using population-level routine health outcome data in the UK. Our findings suggest that smaller care coordinator case-loads and clozapine use are associated with improved relapse rates, while highlighting the potential benefits of other care components including CBTp and physical health interventions. These results offer actionable pathways to optimise EIP services, and important principles for supporting people with psychosis across clinical settings.
Smaller care coordinator case-loads were strongly associated with reduced relapse rates, with a 2% increase in hazard per additional patient. Over 3 years, this equated to a difference of nearly 50% in relapse probability between the smallest and largest case-loads. This finding aligns with recent evidence highlighting the importance of quality care coordination in EIP, where it is viewed as a significant advantage compared with other settings such as Community Mental Health Teams. Reference Rickett, Kingstone, Gupta, Shiers, French and Lennox22 Smaller case-loads may facilitate more frequent patient contact but also provide capacity for other activities, such as care coordinator involvement in group-based interventions and liaison with family and carers. These benefits may promote the formation of a stronger therapeutic relationship, as well as allowing for enhanced monitoring and timely intervention. This, in turn, may better enable care coordinators to prevent deterioration, or to detect and avert the early signs of relapse.
This finding does contrast with the results of a previous clinical trial – the UK700 study – which concluded that reduced case-load sizes in intensive case management did not confer significant benefits over standard care. Reference Burns23 However, UK700 investigated a population with chronic psychosis and prior hospitalisations and was conducted before the widespread implementation of EIP services. This might have diluted the benefits of smaller case-loads, which may be more impactful during the critical early stages of psychosis. Our findings may reflect the greater importance of intensive, personalised treatment in EIP, and we feel they are more valid in the context of modern psychosis care. They align with recent studies identifying care coordination as one of the most impactful elements of an EIP package of care Reference Williams, Ostinelli, Agorinya, Minichino, De Crescenzo and Maughan14 and associating smaller case-loads with improvements in patient-reported outcomes. Reference Williams, Morris, Gupta, Penington, Cullen and Quirk24
Current standards for EIP implementation in the UK recommend a case-load of 15 per full-time care coordinator 17 – we would note that the median case-load at services in this study exceeded this (17.4). Published NCAP reports have previously advised that case-loads >25 are ‘likely to adversely impact on EIP outcomes’, and suggested that directors of operations should ‘work to ensure [case-loads] remain appropriate’, 16 but this figure was also exceeded for around 15% of our sample. Given that our analysis associated a reduction from a case-load of >25 to 15 with a roughly 25% reduction in relapse probability over 3 years, we would suggest that the lower end of this range remains a more appropriate target for EIP case-loads, although economic cost–benefit analyses are required to provide concrete recommendations.
Our findings reaffirm clozapine’s established efficacy for the management of psychotic disorders, Reference Siskind, McCartney, Goldschlager and Kisely25 including early psychosis. Reference Thien, Bowtell, Eaton, Bardell-Williams, Downey and Ratheesh26 Although the number of eligible patients was small, we found benefits in reducing rates of relapse, detention under the Mental Health Act, bed days in hospital and emergency department attendance. Eligible individuals not offered or declining clozapine had worse outcomes, while recipients had risks comparable to, or lower than, those ineligible for clozapine – which is striking in light of historically poor outcomes for people with treatment-resistant psychosis. Reference Kennedy, Altar, Taylor, Degtiar and Hornberger27 Treatment resistance is common even in the early stages of psychosis and may be under-identified. Reference Siskind, Orr, Sinha, Yu, Brijball and Warren28 We note that the majority even of those patients identified as eligible for clozapine (i.e. treatment resistant) in this cohort did not receive it (987/1842, 54%). For some of these, clozapine may have been unsuitable for other reasons not evident from our data (e.g. contraindications related to physical health). However, data from the latest round of NCAP and regional studies show large ongoing variations in the proportion of eligible patients who receive clozapine treated by different service providers. 16,Reference Beattie, Nott and Krishnadas29,Reference Stokes, Griffiths, Jones, Everard, Jones and Fowler30 Addressing underuse of clozapine through clinician training, enhanced monitoring infrastructure and patient education campaigns remains a critical priority, particularly in EIP. Reference Howes, Vergunst, Gee, McGuire, Kapur and Taylor31–Reference Oloyede, Blackman, Mantell, Harris, Williams and Taylor33
There were few deaths in the cohort, but the association between targeted physical health interventions and reduced mortality emphasises the importance of integrating these (including addiction services) into EIP infrastructure. Those receiving interventions for reducing alcohol use or promoting weight loss had mortality rates comparable to those not requiring them, while refusal or non-offer of interventions was associated with higher mortality risks. These findings highlight the potential for tailored physical health interventions to address the disproportionate physical health burden faced by individuals with psychosis. People who received these interventions did have increased rates of emergency department attendance, but this may reflect improved knowledge about accessing healthcare services rather than poorer health outcomes.
There was weak evidence that CBTp was linked to reductions in hospital admissions and bed days, although the influence of ‘confounding by indication’ should be considered (with evidence that those who refused CBTp also had shorter admissions than those who were not offered it, indicating that CBTp may be offered disproportionately to those likely to have shorter admissions, whether they then accept or refuse it). It was not significantly associated with relapse overall, and the relatively small effect sizes are consistent with previous studies. Reference Jauhar, McKenna, Radua, Fung, Salvador and Laws34 However, there was somewhat stronger evidence that the receipt of CBTp was associated with reduced hazard rates for detention under the Mental Health Act (compared with both those who refused or otherwise did not receive it). In the context of the more modest improvement in overall admission rates, this suggests that those who received CBTp may have a greater proportion of voluntary admissions, possibly due to improved collaboration and an increased likelihood of understanding and accepting the need for in-patient care without detention.
The lack of clear improvements with other NICE-recommended interventions, such as family interventions and vocational support, is consistent with a recent meta-analysis of components of EIP care. Reference Williams, Ostinelli, Agorinya, Minichino, De Crescenzo and Maughan14 While these components have demonstrated efficacy in focused studies, Reference Rodolico, Bighelli, Avanzato, Concerto, Cutrufelli and Mineo35–Reference Bird, Premkumar, Kendall, Whittington, Mitchell and Kuipers37 evidence of additional benefit when combined with other EIP components is limited. This may reflect variability in implementation quality, and further research is needed to optimise their delivery in routine clinical practice and bridge the gap to consistent effectiveness.
Finally we would highlight that, even controlling for the components received, people from non-White ethnic groups had increased hazard rates of relapse, and higher still rates of detention under the Mental Health Act. This finding unfortunately underscores ongoing systemic inequalities in mental healthcare, Reference Barnett, Mackay, Matthews, Gate, Greenwood and Ariyo38,Reference Bansal, Karlsen, Sashidharan, Cohen, Chew-Graham and Malpass39 highlighting the need for targeted outreach, culturally tailored interventions and initiatives to address racial disparities in EIP and mental health services generally. Continued measurement of demographic-specific outcomes is needed to ensure that these measures are effective.
Strengths and limitations
We used a large, nationally representative cohort and conducted robust statistical modelling of real-world outcomes using high-quality data-sets. Although data quality was assured through NCAP’s rigorous validation processes, the study does have some important limitations. The definition of relapse for our primary outcome relied on health service use rather than symptoms or self-defined recovery. As an observational study, residual confounding remains a possibility. For example, those services able to maintain lower case-loads may also have other advantageous characteristics (e.g. resources, staff continuity), which influence outcomes. Associations may also have been influenced by other unmeasured variables such as overall symptom severity, duration of untreated psychosis or the use of other medications besides antipsychotics, if these differed between groups (i.e. between those who received versus refused clozapine). This is particularly evident in some results – while antipsychotic medication use overall was associated with increased relapse hazard, this probably reflects the fact that those who do not receive antipsychotic medication (a small proportion of EIP patients) have less severe baseline symptoms, rather than an effect of treatment itself.
Time-to-event variables were measured from the NCAP audit date rather than by using exact exposure times, introducing potential variability. For instance, some individuals may have completed CBTp before the NCAP while others may have still been receiving it at the time of the audit period and continued afterwards. However, given the large size of our cohort, these variations are likely to attenuate through random distribution across the sample, and we would not expect them to differ systematically between exposure variables. Our categories for demographic variables may not capture more specific differences for groups within these categories (e.g. specific ethnicities or people who were unemployed for differing reasons). Finally, generalisability may be limited to contexts with similar policy and funding frameworks to England.
Implications
Smaller case-loads and increased use of clozapine and physical health interventions should be prioritised in EIP service design and delivery. Policymakers and commissioners should consider adopting case-load size as a quality metric supported by adequate funding, particularly in high-demand areas. Initiatives to improve clozapine uptake and engagement with physical health interventions among eligible individuals could further enhance outcomes. These could include training programmes for clinicians, improved infrastructure for monitoring and education campaigns for patients and carers. Closer collaboration with primary care may also be particularly helpful in regard to improving the delivery of interventions for physical health. Future research should examine optimal case-load thresholds and explore the mechanisms underlying the associations we have identified. For instance, qualitative research could explore perspectives of care coordinators and patients on how lower case-loads might impact the quality and frequency of therapeutic interactions. Real-world testing of optimal EIP case-load thresholds through randomised or quasi-experimental studies, and cost–benefit analyses balancing the costs of increased staffing against improved outcomes, would provide concrete evidence to guide policy and service design. Further research exploring the possible link between CBTp and reductions in compulsory treatment would also be beneficial. Our findings offer a foundation for developing more effective and equitable EIP models, ultimately improving outcomes for individuals with psychosis.
Supplementary material
The supplementary material can be found online at https://doi.org/10.1192/bjp.2025.126
Data availability
R.W., E.P., A.T. and A.B. had access to the full study data-set. The data-set and code script for analysis are held in the Office for National Statistics (ONS) Secure Research Service and, as such, these are unfortunately not possible to share on request. Statistical data from the ONS are subject to Crown copyright. The use of ONS statistical data in this work does not imply the endorsement of the ONS in relation to the interpretation or analysis of the statistical data. This work uses research data-sets that may not exactly reproduce National Statistics aggregates.
Acknowledgements
We thank all members of the NCAP team at the Royal College of Psychiatrists, all members of the EXTEND-InG service user and carer advisory group, the Healthcare Quality Improvement Partnership, NHS England and the ONS for their support with this study.
Author contribution
R.W., B.L., P.F. and M.J.C. formulated the presented research question. R.W., A.B. and M.J.C. designed the study. V.G. and D.S. provided feedback on the design from a lived-experience perspective. R.W. and E.P. accessed and verified the data for the study. R.W. and E.P. analysed the data. R.W., A.B. and M.J.C. interpreted the results. R.W. wrote the first draft of the manuscript. All authors provided critical input and revisions to the draft manuscripts and approved the final manuscript. M.J.C. had final responsibility for the decision to submit for publication.
Funding
R.W. is supported by a National Institute for Health Research (NIHR) Doctoral Fellowship (reference no. NIHR302320), with additional support from the NIHR Imperial Biomedical Research Centre. The linked data-set was produced as part of the EXTEND: Personalised Care for Early Psychosis Study, which is support by a NIHR Programme Grant for Applied Research (reference no. NIHR203277). The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
Declaration of interest
None.
Ethics
Ethical approval for the analysis necessary for this study was granted by the London Queens Square Research Ethics Committee (REC), part of the NHS Health Research Authority (HRA) – REC reference no. 22/PR/0602. Approval has also been obtained from the NHS HRA Confidentiality Advisory Group (CAG) for the additional requirements related to sharing personal identifiable information from NCAP with NHS England for the purposes of matching and production of the pseudonymised data-set – CAG reference no. 22/CAG/0078. Only the pseudonymised data-set was accessed directly by the research team. We maintained compliance with NHS England data security requirements for use of the Secure Research Service.







eLetters
No eLetters have been published for this article.