Introduction
Caudal anaesthesia for paediatric cardiac surgical procedures is a described technique for improving analgesia, allowing for potential early extubation, decreasing hospital length of stay, avoiding positive pressure ventilation, and minimising exposure to opioids.Reference Leyvi, Taylor, Reith, Stock, Crooke and Wasnick1–Reference Gaies, Tabbutt and Schwartz6 Studies to date differ in the combination of medications utilised, all with diverse spinal pharmacokinetics, study numbers, patient age, and underlying pathophysiology.Reference Maharramova and Taylor3–Reference Beamer, Ferns, Edwards, Gunther and Nelson4 Furthermore, concerns related to potential complications from dural puncture and the risk of bleeding, haemodynamic instability, risk of airway compromise, and the need for reintubation have limited the use of caudal anaesthesia in paediatric cardiac surgery.Reference Preisman, Lembersky and Yusim2–Reference Gaies, Tabbutt and Schwartz6 Given the lack of data on morphine and clonidine caudals, we sought to describe a regimen for analgesia in paediatric cardiac surgery.
Methods
This study was approved by the Johns Hopkins Medicine Institutional Review Board (IRB00389283), and the requirement for informed consent was waived.
Study design
We reviewed all paediatric patients less than 6 years of age who underwent cardiac surgery requiring cardiopulmonary bypass and received caudal analgesia according to an institutional clinical care algorithm from October 2020 through April 2023 were included (Supplemental Figure 1). Patients with any of the following were excluded: documented allergy, contraindication to neuraxial opioid, cellulitis or infection at sacrum, history of chronic pain syndrome or opioid dependence, previous sacral surgery or violation of epidural space, obscured superficial landmark anatomy, or known deep anatomical variation. Surgical case complexity was stratified using the Society of Thoracic Surgeons-European Association for Cardiothoracic Surgery Congenital Heart Surgery Mortality (STAT) Categories.Reference Jacobs, Jacobs and Thibault7
Outcomes
Outcomes of interest included the morphine and clonidine caudal dose, reported adverse outcomes, extubated in the operating room after the cardiac surgery case, rate of patients reintubated in the first 48 hours, and length of stay.
Statistical analysis
Continuous variables were reported using medians and interquartile range; categorical variables were described using frequencies and percentages.
Results
Among the 200 patients included in the final sample, 101 (51%) were infants, and most cases were STAT 1 (n = 84, 42%) (Table 1). Types of cardiac operations included: Norwood (n = 7, 3.5%), ventricular septal defect (n = 32, 16%), truncus (n = 5, 2.5%), aorta/arch repair (n = 18, 9%), cortriatriatum (n = 2, 1%), Fontan (n = 7, 3.5%), Glenn (n = 16, 8%), tetrology/pulmonary artery plasty (n = 30, 15%), atrioventricular canal (n = 20, 10%), arterial switch (n = 8, 4%), interrupted aortic arch ± ventricular septal defect (n = 5, 2.5%), total anomalous pulmonary venous return (n = 5, 2.5%), unifocalization of major ortopulmonary collateral arteries (n = 4, 2%), atrial septal defect (n = 9, 4.5%), valvuloplasty (n = 19, 9.5%), myomectomies (n = 4, 2%), anomalous left coronary artery from the pulmonary artery (n = 1, 0.5%), and other cardiac procedures (n = 8, 4%).
Table 1. Patient characteristics, intraoperative medications, and caudal medications
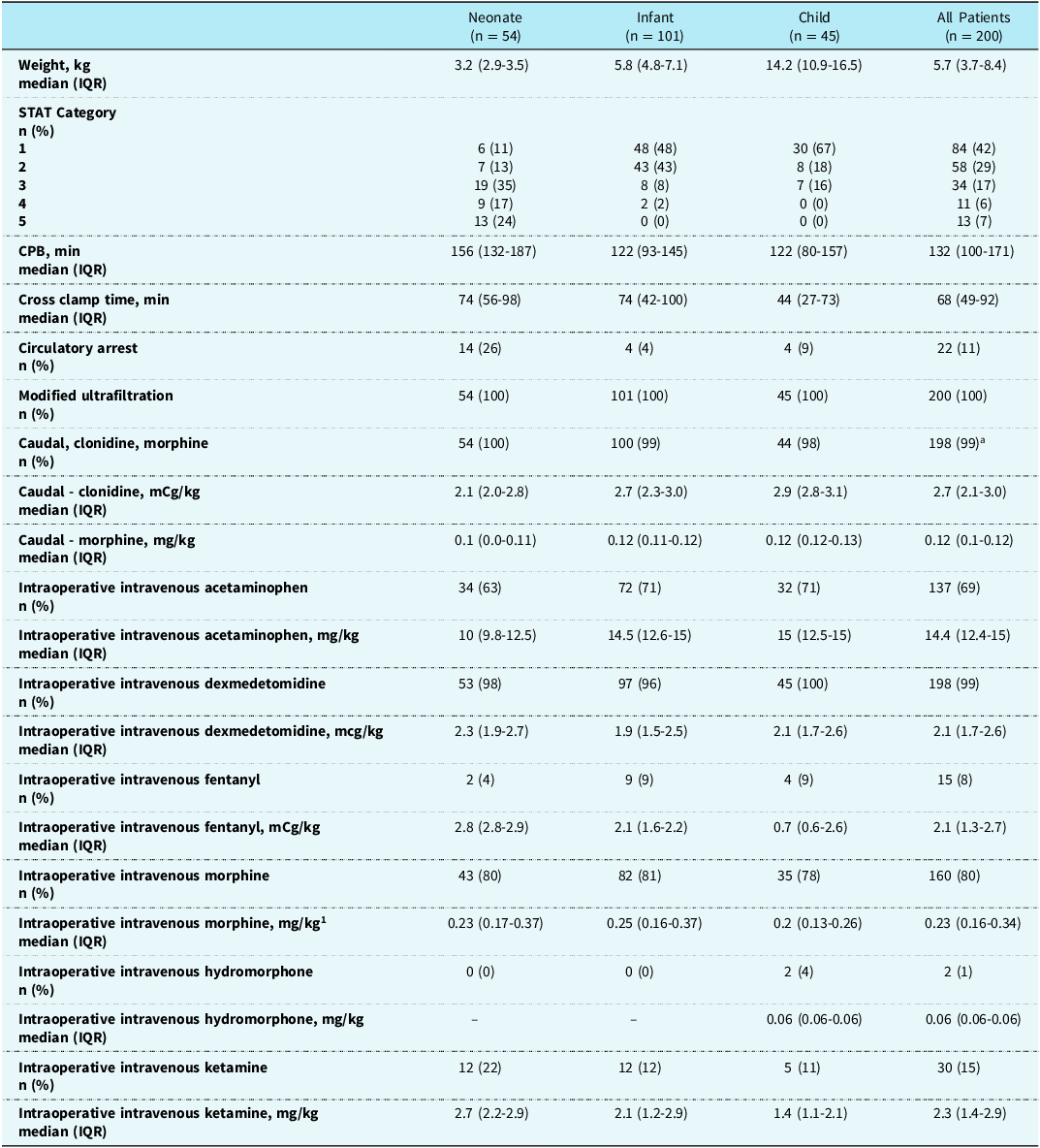
a 1 patient had only clonidine, and 1 patient had only morphine for caudal. IQR = interquartile range; STAT = Society of Thoracic Surgeons-European Association for Cardiothoracic Surgery Congenital Heart Surgery Mortality.
The median (interquartile range) doses of caudal clonidine and morphine used were 2.7 (2.1–3) mcg/kg and 0.12 (0.1-0.12) mg/kg (Table 1). Most patients received other analgesic agents during the case, with morphine being administered to 80% of patients (Table 1).
There were no reports of neurological injury, infection, or neuraxial haematoma in patients who received caudal morphine and clonidine. Of the 200 patients, 162 (81%) underwent extubation at the end of the case in the OR, including 5/13 (38%) STAT 5 cases and 29/54 (54%) neonatal cases (Table 2). Within 48 hours of extubation, 10/165 (6 %) patients were re-intubated (Supplemental Table 2). The median (interquartile range) opioid requirements in intravenous morphine milligramme equivalents within the first 24 hours following ICU were 0.2 (0.1–0.35) mg/kg/day. The median (interquartile range) length of stay for the cohort was 8 (8–17) days (Table 2).
Table 2. Outcomes

IQR = interquartile range; MME = morphine milligram equivalents.
Discussion
We report 200 paediatric patients undergoing cardiac surgery employing a morphine and clonidine caudal at higher doses than previously reported. Nguyen et al described in a retrospective study the use of clonidine up to 2 mcg/kg with morphine up to 0.04 mg/kg.Reference Nguyen, Byrd and Tan5 Reported complications include epidural haematoma, neurological sequalae, epidural abscess, and cutaneous infections.Reference Nguyen, Byrd and Tan5 In our study, we did not note any of the above complications or any concerns related to bleeding from anticoagulation.
Beamer et al in a retrospective cohort study reported that a caudal consisting of 0.05–0.1 mg/kg morphine if < 5 years of age or 0.005–0.01 mg/kg morphine and 1–2 mcg/kg clonidine facilitated early extubation and shorter length of stay in patients undergoing STAT 1, 2, 3, and 4 cardiac operations.Reference Nguyen, Byrd and Tan5 While there is not a comparison group in our study, we report a rate of extubation to be 81% in all patients, including 38% of STAT 5 cases and 54% of neonatal cases, with 6% of the 162 extubated patients requiring re-intubation (Supplemental Table 1).
Leyvi et al reported that paediatric patients randomised to receive caudal epidural morphine 0.07 mg/kg and 0.25% bupivacaine at 1 mL/kg resulted in a 24-hour postoperative opioid requirement of 0.3 ± 0.1 mg/kg.Reference Leyvi, Taylor, Reith, Stock, Crooke and Wasnick1 We report a low median opioid requirement of 0.2 mg/kg/day IV morphine in the first 24 hours following surgery compared to other published studies.Reference Amula, Vener and Pribble8–Reference Walker, Long and Sathyamoorthy10 Beamer et al. reported a mean hospital length of stay of 6 days in patients who underwent early extubation.Reference Beamer, Ferns, Edwards, Gunther and Nelson4 We report a similar overall length of stay of 8 days (Table 2).
Limitations of this study include its single-centre, retrospective study design and outcomes that are not patient-centric. Because any effort to construct a concomitant or historical control group would engender substantial selection bias, we have contextualised our findings regarding published literature in similar or relevant paediatric populations. Also, most patients in this study were either STAT 1 or 2 cases, which may limit generalisability to more complex cardiac cases. Patients also received intraoperative opioids before extubation, which could impact overall postoperative opioid requirements. While we collected reasons for re-intubation, it is difficult to know for certain to what extent the caudal morphine and clonidine may have impacted re-intubation. Despite these limitations, there is an opportunity to prospectively measure specific outcome metrics in a programme confident in an early extubation strategy, perhaps identifying specific criteria to determine a patient population that truly benefits from this strategy.
This study demonstrates that the use of high-dose caudal analgesia consisting of clonidine and morphine was tolerated in paediatric cardiac surgical patients. Prospective studies of this approach are needed to evaluate the use of caudal analgesia in this patient population.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S1047951125100620
Acknowledgements
Grace Flurry.
Financial support
No funding to report.
Competing interests
The authors have nothing to disclose.
Ethical standard
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008, and have been approved by the institutional committees (Johns Hopkins).




