Introduction
Increasing evidence suggests that interactions between plants and their soil are important determinants of the structure of plant communities through plant-soil feedbacks (PSFs). PSFs arise through plants changing the biotic and abiotic properties of the soils they inhabit, which may then influence plant growth (Bever Reference Bever2003, Van Der Putten et al. Reference Van Der Putten, Bardgett, Bever, Bezemer, Casper, Fukami, Kardol, Klironomos, Kulmatiski and Schweitzer2013). A plant species may influence soil conditions in a way that supports its growth (positive feedback) or hinders its development (negative feedback). Positive PSFs arise through the accumulation of soil nutrients (Berendse Reference Berendse1990, Chapman et al. Reference Chapman, Langley, Hart and Koch2006, Wardle Reference Wardle1999) or symbiotic mutualists (Klironomos Reference Klironomos2002, Van Der Putten et al. Reference Van Der Putten, Bradford, Pernilla Brinkman, Van De Voorde and Veen2016) and may eventually lead to clumped distribution patterns or monodominance (Crawford & Knight Reference Crawford and Knight2017, Dickie et al. Reference Dickie, Koele, Blum, Gleason and Mcglone2014, Teste et al. Reference Teste, Kardol, Turner, Wardle, Zemunik, Renton and Laliberté2017). Positive PSFs could be used to explain conspecific facilitation when a plant modifies its soil environment in a way that has positive effects on its conspecifics (Reinhart et al. Reference Reinhart, Bauer, McCarthy-Neumann, MacDougall, Hierro, Chiuffo, Mangan, Heinze, Bergmann, Joshi, Duncan, Diez, Kardol, Rutten, Fischer M van der Putten, Bezemer and Klironomos2021). Negative feedbacks are generated through nutrient depletion (Berendse, Reference Berendse1994) or changes in litter quality and nutrient cycling (Bennett & Klironomos Reference Bennett and Klironomos2018), or the accumulation of soil pathogens (Bever et al. Reference Bever, Mangan and Alexander2015, Chung & Rudgers Reference Chung and Rudgers2016, Smith-Ramesh & Reynolds Reference Smith-Ramesh and Reynolds2017, Van Der Putten et al. Reference Van Der Putten, Bradford, Pernilla Brinkman, Van De Voorde and Veen2016,). Soil microbial effects tend to be much stronger than nutrient effects (Ke et al. Reference Ke, Miki and Ding2015) and are most pronounced at the seedling stage. Negative soil feedbacks occur when a plant modifies its soil biota in a way that inhibits conspecific more than heterospecific neighbours (Bever et al. Reference Bever, Mangan and Alexander2015, Mangan et al. Reference Mangan, Schnitzer, Herre, Mack, Valencia, Sanchez and Bever2010).
Evidence suggests that rare species are more prone to negative PSFs than abundant species (Klironomos Reference Klironomos2002, Maron et al. Reference Maron, Laney, Ortega, Pearson and Callaway2016, Thakur et al. Reference Thakur, Van Der Putten, Wilschut, Veen, Kardol, Van Ruijven, Allan, Roscher, Van Kleunen and Bezemer2021). Plants’ traits may also help predict the direction of PSFs; fast growth rates and larger specific leaf areas are associated with more negative values (Maron et al. Reference Maron, Laney, Ortega, Pearson and Callaway2016, Xi et al. Reference Xi, Adler, Chen, Wu, Catford, Van Bodegom, Bahn, Crawford and Chu2021, Xi et al. Reference Xi, Mccarthy-Neumann, Feng, Wu, Wang and Semchenko2023) while larger seed size is associated with more positive (or less negative) values (Xi et al. Reference Xi, Adler, Chen, Wu, Catford, Van Bodegom, Bahn, Crawford and Chu2021). Thus, fast-growing, small-seeded plants investing less in traits associated with natural enemy defence are predicted to have more negative PSFs than slower-growing, large-seeded species (Xi et al. Reference Xi, Adler, Chen, Wu, Catford, Van Bodegom, Bahn, Crawford and Chu2021). Shade tolerance can also influence the direction of PSFs; shade-tolerant species tend to be slower growing and allocate more resources towards enemy defence, thus having a less negative PSF (Xi et al. Reference Xi, Adler, Chen, Wu, Catford, Van Bodegom, Bahn, Crawford and Chu2021).
In this study, we explored the relative contribution of soil biota in generating negative feedback across six plant species with different abundances, seed traits and shade tolerance in a West African montane forest. A shade-house experiment was used to assess PSFs on plant performance. Our specific question was: Does the performance of conspecific seedlings differ when grown in the presence of soil biota associated with their adult trees compared to the soil biota of heterospecific adults? Earlier studies in this forest demonstrated weak or non-existent negative density/distance effects associated with Janzen-Connell effects (Abiem et al. Reference Abiem, Dickie, Kenfack and Chapman2021, Matthesius et al. Reference Matthesius, Chapman and Kelly2011), which were outweighed by facilitation (Abiem et al. Reference Abiem, Dickie, Kenfack and Chapman2021). This study is the first, to our knowledge, to investigate PSFs in an Afromontane forest.
Methods
Study site
Our study was based in the Ngel Nyaki Forest Reserve on the Mambilla Plateau in southeast Nigeria (7.0876°N, 11.0534°E, ∼1650 m a.s.l.). The 46 km2 reserve comprises mainly degraded, grazed savannah but includes two forest fragments, the Ngel Nyaki fragment being approximately 5.2 km2 in area (Yadok et al. Reference Yadok, Forget, Gerhard and Chapman2019). The forest is a sub montane to mid altitude forest (Chapman et al. Reference Chapman, Olson and Trumm2004). The climate is characterised by an average annual precipitation of ∼2300 mm, which falls between the months of April and October and a mean monthly maximum and minimum temperatures for the wet and dry seasons as 25.6 and 15.4°C, and 28.1 and 15.5°C respectively (Unpublished data from Nigerian Montane Forest Project). The soil is volcanic and has a high clay content with a pH ranging between 6 and 6.5 (Chapman & Chapman Reference Chapman and Chapman2001).
Seedling species
We conducted a multispecies shade house experiment using six target species which vary in density and represent a range of ecological characteristics (Table 1). We included the most common species in the 20.28 ha ForestGeo plot Garcinia smeathmanii (608 individuals/ha) and Deinbollia pinnata (159 individuals/ha); not so common species, Entandrophragma angolense (5 individuals/ha); and a rare species, Ekebergia capensis (<0.1 individuals/ha). G. smeathmanii is a small understory tree forming monospecific stands with evidence of clonal reproduction through root suckers (Tembe & Deodhar Reference Tembe and Deodhar2011).
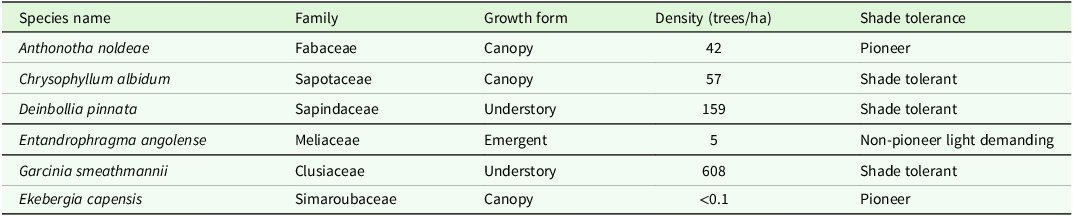
Table 1. Plant species used in the experiments and their families, growth form, shade tolerance and the density of adult trees/hectare in the 20.28 ha ForestGEO Plot. Details of species shade tolerance extracted from Abiem et al. Reference Abiem, Dickie, Kenfack and Chapman2021
Experimental setup, data collection and analysis
We collected fruits from at least three individual trees each of Entandophragma angolense (Meliaceae), Chrysophyllum albidum (Sapotaceae), Deinbolia pinnata (Sapindaceae), Anthonotha noldeae (Fabaceae), Ekerbergia sp (Meliaceae) and Garcinia smeathmannii (Clusiaceae). Seeds were removed from the fruits of plants and surface sterilised for 1 minute in 50% commercial bleach solution (sodium hypochlorite) and then washed thoroughly with distilled water. These seeds were planted in seed trays filled with steam-sterilised sand and raised for two months before they were transplanted.
Our inoculum for each species was soil collected from 2 m away from the base of three adult trees per species and homogenised (Adopted from Mangan et al. Reference Mangan, Schnitzer, Herre, Mack, Valencia, Sanchez and Bever2010). All the soil (a combination of 1 part forest soil and 3 parts sand) for the experiment was steam sterilised to remove biotic differences among the treatments. We filled 192 polypots (10 cm diameter × 20 cm depth) with this sterilised soil mixture and then added to each pot about 50 ml (5% of the total soil volume) of inoculum, each polypot being inoculated with the inoculum of one tree species. One seedling of each of the six tree species in the experiment was planted in each pot. Our experiment included conspecific combinations where we planted the seedling of each species in inoculum from conspecific adults, and heterospecific combinations where seedlings of each species were planted with inoculum of each of the five other species individually. The conspecific combination was replicated five times for each species and the heterospecific combination was replicated three times for each species. As a control, two replications of the plant-inoculum combinations were set up using sterilised inoculum, to account for differences from abiotic factors. The pots were placed randomly on two benches in the middle of the shade house and watered every other day for six months. The shade house comprised a wooden frame lined with mosquito netting and covered with a grass-thatched roof. Seedling growth and survival were recorded every two weeks for six months, after which all the plants were harvested, and their total biomass was estimated. We calculated the relative growth rate (RGR) for each individual for the six-month period as log(size t + 1)-log(size t)/time, where size t + 1 and size t corresponds to the height of seedlings at the end of the six-month period of experiment and the start of the experiment respectively.
We analysed the data using the Two-way Analysis of Variance (ANOVA) test to examine if seedling biomass differed between sterile and live soil inoculum sources (conspecific and heterospecific). We also carried out ANOVA test at the species level to test if biomass differed across inoculum sources (conspecific and heterospecific species).
Results
Effects of plant-soil feedback on plant biomass and growth rate
A total of 165 seedlings survived the experiment and for these, we measured biomass and relative growth rates. We found little evidence for plant-soil feedback; across five of the six species used in the experiment (Figure 2). Seedling biomass did not differ among those grown in inocula from conspecific adults versus heterospecific adults, or between the live biota and sterile inocula (Figure 1).
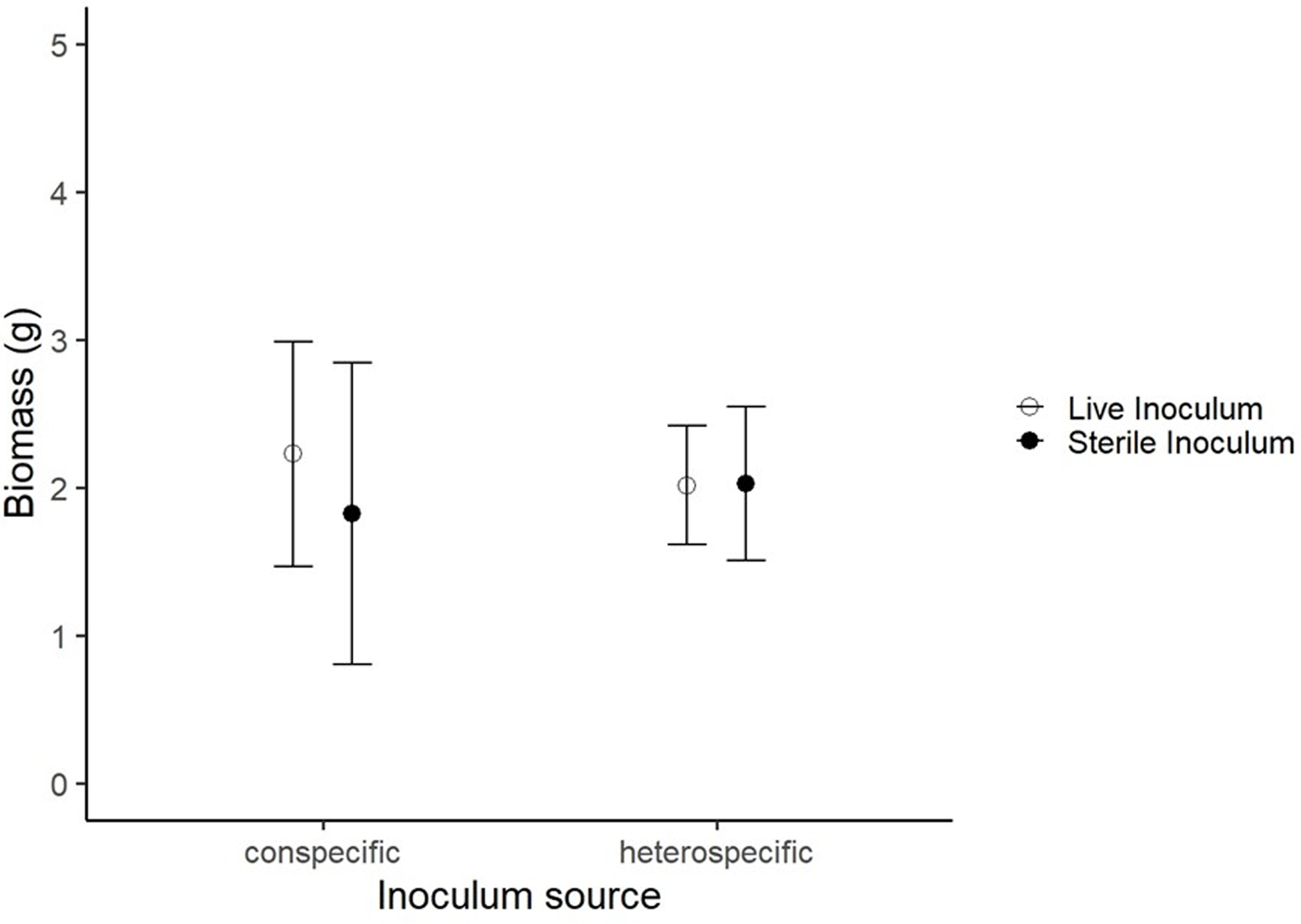
Figure 1. Mean (±SE) seedling biomass across live and sterile inocula from conspecific and heterospecific adults.
The biomass of G. smeathmannii seedlings did show significant variation across live inocula (F 5,19 = 4.387, P = 0.013, Table 2; Figure 2).
Table 2. Statistical results from the shade house experiment evaluating the biomass in seedlings of Anthonotha noldeae, Chrysophyllum albidum, Deinbolia pinnata, Entandophragma angolense, Garcinia smeathamannii and Ekebergia sp. in potted soils containing live inoculum from conspecific or heterospecific adult trees
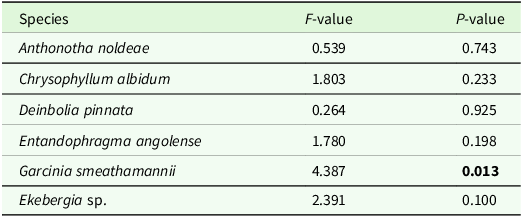
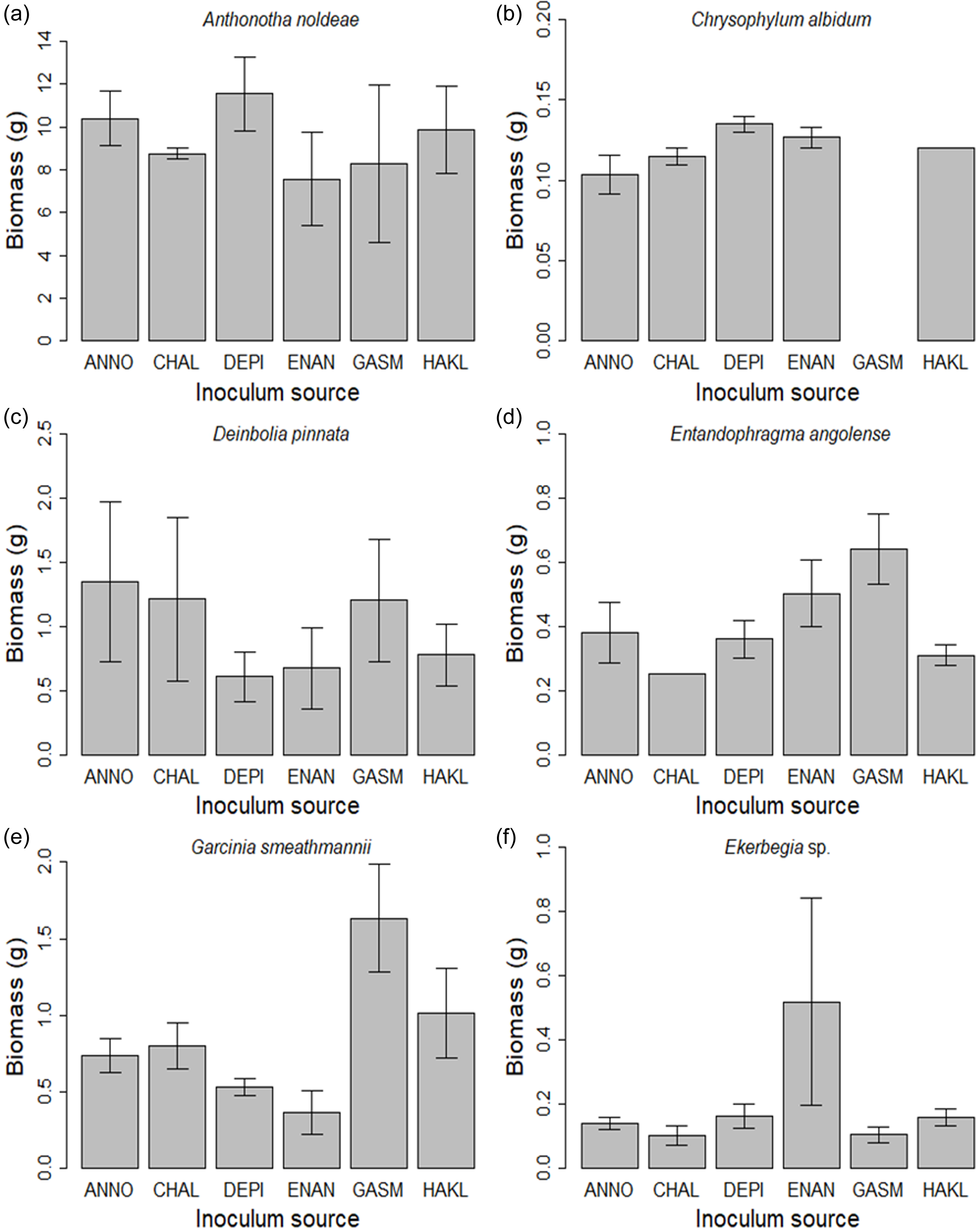
Figure 2. Mean (±SE) biomass of (a.) Anthonotha noldeae (ANNO), (b.) Chrysophyllum albidum (CHAL), (c.) Deinbolia pinnata (DEPI), (d.) Entandophragma angolense (ENAN), (e.) Garcinia smeathamannii (GASM) and (f.) Ekebergia sp (HAKL) estimated from shade house experiment comparing growth in response to six inoculum sources (one conspecific and five heterospecific). The ANOVA F and P values are reported in Table 2. (where error bars are absent, there was only one data point for the groups because the replicates died during the experiment).
Discussion
Despite the variation among species in abundance, shade tolerance and seed sizes, we found no evidence of PSFs in five of the six species used in our experiment. This is contrary to the findings from many other studies across a range of locales and vegetation types which report negative (Bezemer et al. Reference Bezemer, Jing, Bakx-Schotman and Bijleveld2018, Klironomos Reference Klironomos2002, Kulmatiski et al. Reference Kulmatiski, Beard, Stevens and Cobbold2008, Mangan et al. Reference Mangan, Schnitzer, Herre, Mack, Valencia, Sanchez and Bever2010) and sometimes positive (Bauer et al. Reference Bauer, Mack and Bever2015, Smith & Reynolds Reference Smith and Reynolds2015) conspecific PSFs. The only significant PSF we observed was in Garcinia smeathmannii. G. smeathmannii is the most abundant species in the Ngel Nyaki ForestGEO plot, so theoretically, if any species did show a positive PSF it should be G. smeathmannii; because being an abundant tree species could mean that it is less susceptible to the harmful effects of its associated soil community than a rare tree species (Mangan et al. Reference Mangan, Schnitzer, Herre, Mack, Valencia, Sanchez and Bever2010). In a study in Barro Colorado Island (BCI), Mangan et al. (Reference Mangan, Schnitzer, Herre, Mack, Valencia, Sanchez and Bever2010) reported that less common species showed stronger negative feedbacks than common species. Again, in a meta-analysis of results from 22 experiments, Reinhart et al. (Reference Reinhart, Bauer, McCarthy-Neumann, MacDougall, Hierro, Chiuffo, Mangan, Heinze, Bergmann, Joshi, Duncan, Diez, Kardol, Rutten, Fischer M van der Putten, Bezemer and Klironomos2021) showed that harmful soil biota impacted rare species more. However, the strength and direction of PSFs are often context-dependent (Bennett and Klironomos Reference Bennett and Klironomos2019, De Long et al. Reference De Long, Fry, Veen and Kardol2019, Xi et al. Reference Xi, Mccarthy-Neumann, Feng, Wu, Wang and Semchenko2023) and the results of experiments in glasshouses and shade houses may not be applicable to field conditions. Schittko et al. (Reference Schittko, Runge, Strupp, Wolff and Wurst2016) argue that negative PSFs are more likely to be found under glasshouse conditions than in nature. Also, G. smeathmannii is an understory species in Ngel Nyaki Forest and studies have shown that light levels can influence the strength of PSFs in understory species (Smith & Reynolds Reference Smith and Reynolds2015, Xi et al. Reference Xi, Bloor and Chu2020, Xi et al. Reference Xi, Mccarthy-Neumann, Feng, Wu, Wang and Semchenko2023). Plus, positive PSFs in these shaded plants may be facilitated by ectomycorrhizal fungal communities, which improve their nutrient acquisition. There is however very little that is known about the link between ectomycorrhizal fungi and seedlings of monodominant trees in the tropics (Delevich et al. Reference Delevich, Koch, Aime and Henkel2021).
Given the context and the small sample size of our experiment, we cannot adequately conclude that there are positive effects; however, results from a community-wide study on density dependence (Abiem et al. Reference Abiem, Dickie, Kenfack and Chapman2021) suggested the possibility of facilitation (Goldenheim et al. Reference Goldenheim, Irving and Bertness2008), or pathogen suppression by mycorrhizal fungi (Liang et al. Reference Liang, Liu, Etienne, Huang, Wang and Yu2015). An alternative explanation may be that the relatively drier and colder environment of Ngel Nyaki Forest does not support as many species-specific pathogens that generate strong negative feedback as in more tropical climates. Pathogens are especially abundant in warmer and wetter environments (Comita & Engelbrecht Reference Comita and Engelbrecht2014, Inman-Narahari et al. Reference Inman-Narahari, Ostertag, Hubbell, Giardina, Cordell and Sack2016) such as lowland tropical forests which have reported strong negative conspecific density dependence (Lamanna et al. Reference Lamanna, Mangan, Alonso, Bourg, Brockelman, Bunyavejchewin, Chang, Chiang, Chuyong and Clay2017). Alternatively, there is the possibility that factors other than soil biota may be causing the conspecific negative effects that are observed in Ngel Nyaki Forest; for instance, distance-dependent herbivory was reported on some plant species by Matthesius et al. (Reference Matthesius, Chapman and Kelly2011).
Plant-soil feedbacks (PSFs) are a mechanism through which soil microbes influence species diversity in plant communities. However, the consistency of PSFs among plant species remains unclear.
Conclusions
This study showed that for a range of Afromontane species, negative plant-soil feedback does not influence seedling performance. While our results suggest that this mechanism may be present in some species, it is not sufficiently widespread to regulate plant populations and drive community dynamics. Further work on G. smeathmanii as well as more species is needed to confirm our interpretation of these results.
Acknowledgements
We wish to thank the management and staff of the Nigerian Montane Forest Project for helping with field work and logistics support. This study is a part of IA’s PhD thesis that was funded by the University of Jos NEEDS assessment programme, Nigeria and a grant from the A. G. Leventis Foundation.
Financial Support
IA received a research grant from the Smithsonian Institute’s Forest Global Earth Observatory (ForestGEO) for this project (Project ID 341243).
Competing interests
Authors declare none.







