Introduction
The order Caryophyllidea Van Beneden in Carus, 1863 represents a unique group of tapeworms, distinguished from other ‘true tapeworms’ within the subclass Eucestoda by a number of distinct characteristics, especially their monozoic body (i.e. non-proglottized, unsegmented). Phylogenetic analyses indicate that caryophyllidean tapeworms form the earliest diverging lineage within the Eucestoda (Waeschenbach et al., Reference Waeschenbach, Webster and Littlewood2012). Tapeworms of the order Caryophyllidea primarily parasitize fish of the orders Cypriniformes and Siluriformes and are found across all zoogeographical regions, with the exception of the Neotropical region and Antarctica (Mackiewicz, Reference Mackiewicz1972; Scholz and Oros, Reference Scholz and Oros2017).
Since its establishment in 1935, a total of 21 species have been assigned to the genus Khawia Hsü, 1935, which is among the most species-rich genera within the order Caryophyllidea. However, the validity of many of these species was reassessed in a comprehensive taxonomic revision by Scholz et al. (Reference Scholz, Brabec, Kráľová-Hromadová, Oros, Bazsalovicsová, Ermolenko and Hanzelová2011). This revision resulted in the synonymy of several taxa and, together with the reclassification of K. baltica by Barčák et al. (Reference Barčák, Oros, Hanzelová and Scholz2017), resulted in a substantially reduced number of valid species. Currently, only seven species are recognized as valid (Table 1). Although the genus Khawia was historically classified under the family Lytocestidae due to the cortical distribution of vitelline follicles in the parenchyma, this morphology-based classification has recently been reassessed (Scholz et al., Reference Scholz, Waeschenbach, Oros, Brabec and Littlewood2021) and the genus is currently placed in the family Caryophyllaeidae.
Table 1. List of species of Khawia Hsü, 1935 (Cestoda: Caryophyllidea) currently recognized as valid, with their synonyms and fish hosts
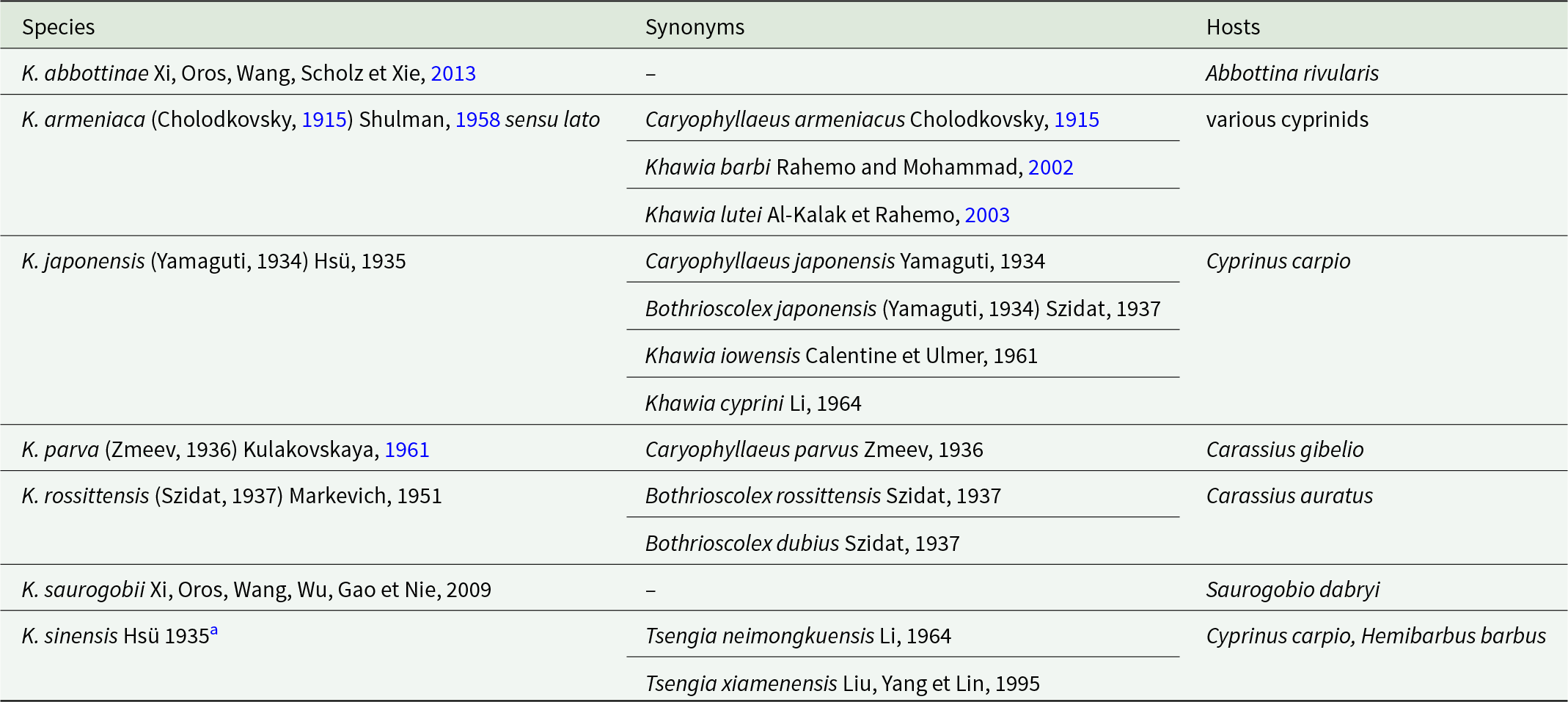
a Type species.
Species of the genus Khawia are found in Europe, Asia, Africa and North America (introduced with common carp), primarily in cypriniform fish. They typically infect one or a few closely related fish species and are thus considered host-specific (Scholz et al., Reference Scholz, Brabec, Kráľová-Hromadová, Oros, Bazsalovicsová, Ermolenko and Hanzelová2011). A notable exception is K. armeniaca, which has been recorded in several species of barbels, particularly of the genera Barbus, Capoeta, Carasobarbus and Luciobarbus. Khawia armeniaca was first discovered in Lake Sevan, Armenia, and described as Caryophyllaeus armeniacus by Cholodkovsky (Reference Cholodkovsky1915). The original description is relatively brief and lacks any illustrations, with the host species identified only as Capoeta. According to Popov (Reference Popov1924), this host was almost certainly C. sevangi (the taxonomy of the genus Capoeta herein follows the classification by Levin et al. (Reference Levin, Freyhof, Lajbner, Perea, Abdoli, Gaffaroğlu, Özuluğ, Rubenyan, Salnikov and Doadrio2012) and Zareian et al. (Reference Zareian, Esmaeili, Gholamhosseini, Japoshvili, Özuluğ and Mayden2018)). Furthermore, the type material of the new species has not been deposited and does not exist.
A more detailed description of K. armeniaca was provided by Popov (Reference Popov1924), who found the parasite in the intestines of Varicorhinus capoeta sevangi (= C. sevangi) and Salmo ischchan at the type locality (Lake Sevan) and the Hrazdan River (Zanga), which flows into the lake. Shulman (Reference Shulman1958) later moved this species to the genus Khawia, although he mentioned this taxonomic change only in a footnote. Subsequently, Kulakovskaya (Reference Kulakovskaya1961), in her extensive revision of the order Caryophyllidea in the USSR, also classified this species under the genus Khawia. Kulakovskaya (Reference Kulakovskaya1961) assumed that this species was endemic to Lake Sevan, but Mikailov (Reference Mikailov1975) found it in Azerbaijan in V. capoeta capoeta (= C. capoeta), and Paperna (Reference Paperna1964) reported it in Israel in Barbus longiceps. Williams et al. (Reference Williams, Gibson and Sadighian1980) later documented its presence in Iran, providing a description with illustrations and were the first to suggest that K. armeniaca has a much broader range than previously thought.
Khawia armeniaca is the species with the widest geographical distribution within its genus, spanning three continents and two zoogeographical regions: the Palearctic and Afrotropical zones (Scholz et al., Reference Scholz, Brabec, Kráľová-Hromadová, Oros, Bazsalovicsová, Ermolenko and Hanzelová2011). Recorded occurrences include the Transcaucasus, where the type locality is situated, southwestern Europe, and parts of Africa. This extensive distribution is also associated with a broad host range, encompassing 20 host species across eight genera of fish. This tapeworm species exhibits remarkable morphological variability in characteristics associated with the anterior part of the body, including the shape of the scolex, the anterior extent of the testes and vitelline follicles, the number of vitelline follicles lateral to the ovary, and the number and extent of post-ovarian vitelline follicles (Oros et al., Reference Oros, Scholz, Hanzelová and Mackiewicz2010; Scholz et al., Reference Scholz, Brabec, Kráľová-Hromadová, Oros, Bazsalovicsová, Ermolenko and Hanzelová2011; Kibet et al., Reference Kibet, Wen-Ting, Huda and Pin2021). However, the authors note that these characteristics are not consistent within individual localities and cannot be used to unequivocally distinguish separate morphotypes. Therefore, these features are considered intraspecific variability within a morphologically polymorphic species, likely due to its broad host range and geographical distribution.
In the revision of the genus Khawia, Scholz et al. (Reference Scholz, Brabec, Kráľová-Hromadová, Oros, Bazsalovicsová, Ermolenko and Hanzelová2011) considered a typical feature of K. armeniaca, differentiating it from other congeners, a butterfly-shaped ovary with short, broad, almost oval lateral wings, a low number of post-ovarian follicles with few or no follicles alongside the posterior arms of the ovary, an oval to spherical cirrus sac, and a simple scolex with a smooth anterior margin.
Considering the remarkable host and geographical range of K. armeniaca, and high putative intraspecific morphological variability in important taxonomic features, the aims of this study were (1) to examine the magnitude of intraspecific morphological variability in K. armeniaca from different fish hosts from two distant geographical regions, (2) to assess genetic diversity among these specimens and (3) to evaluate the host range and geographical distribution of K. armeniaca in the western Palearctic.
Material and methods
Material collection
Tapeworms used in this study were obtained from 15 host species collected at 16 localities in seven countries (Table 2, Figure 1) by the present authors and their collaborators. The material originating prior to year 2011 was retrieved from the Helminthological collection of the Institute of Parasitology of the Biology Centre of the Czech Academy of Sciences in České Budějovice (IPCAS). Later material was collected from either freshly caught fish specimens or from fish specimens obtained from the market. Prior to parasitological dissection, live fish were kept in aerated holding barrels with river water from the collection site. Fish were anaesthetized and then sacrificed by severing the spinal cord. Fish were dissected using the standard method (Chervy, Reference Chervy2024), and intestinal tracts were examined under a stereomicroscope in order to collect Khawia specimens. The tapeworms collected by the present authors were rinsed, killed with boiling saline and then fixed in 70% ethanol for subsequent morphological and molecular analyses.
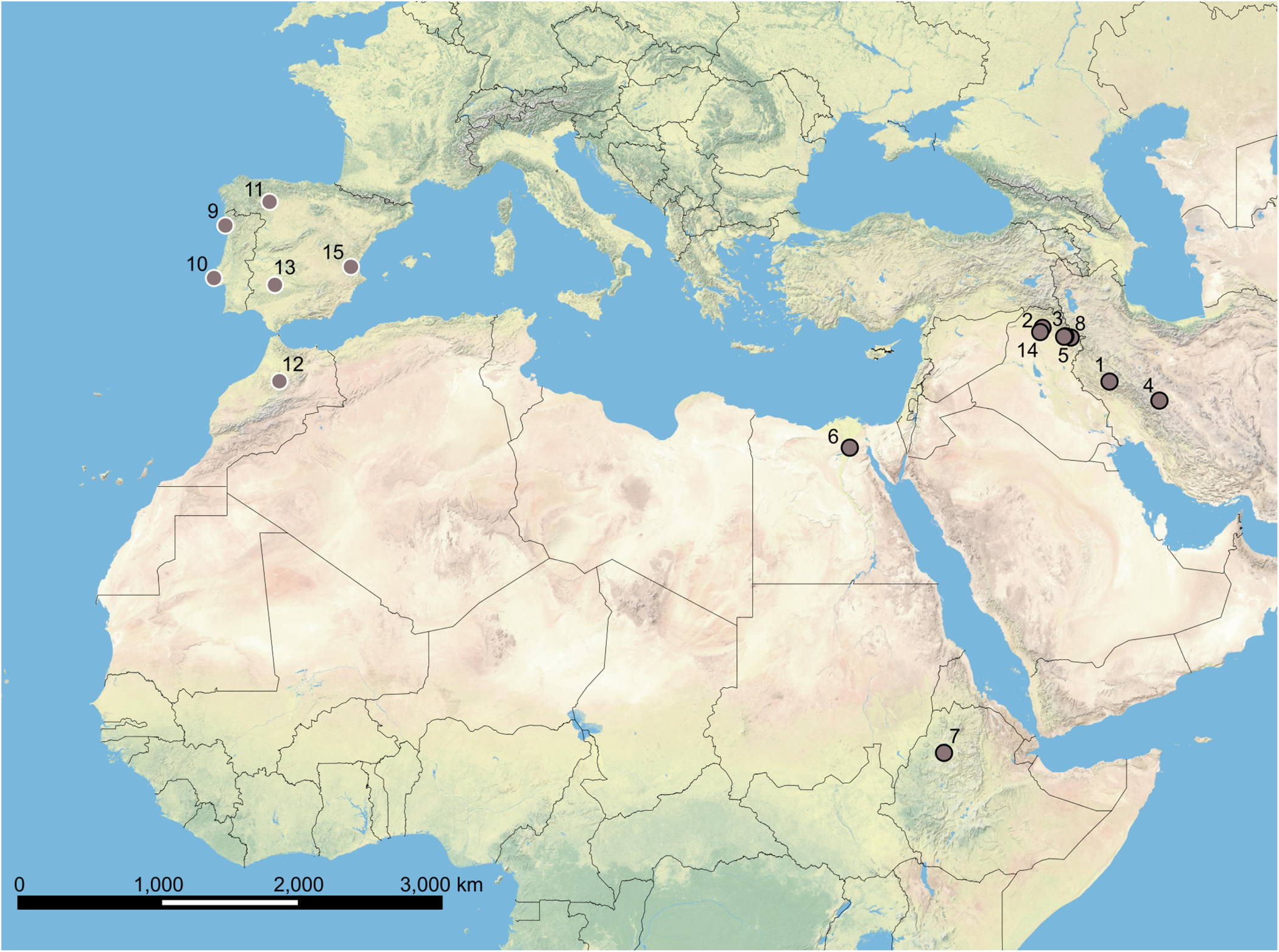
Figure 1. Collection sites of investigated ‘Khawia armeniaca’ specimens. Numbers on the map correspond to locality numbers in Table 2
Table 2. List of specimens studied with their hosts, countries of origin and localities

a Excluded due to poor condition.
b Postcyclic host.
Due to poor quality which prevented reliable measurements (see Remarks to K. armeniaca below), some of the mounted specimens were excluded from the study, therefore reducing the available dataset to 11 host species from 14 localities and 4 countries (i.e. Iran, Iraq, Spain and Portugal). In total, 35 specimens were analysed morphologically and 22 molecularly.
DNA amplification, sequence analysis and phylogenetic analyses
The extraction of genomic DNA was performed using a commercially produced extraction kit (DNeasy Blood & Tissue Kit, Qiagen, Hilden, Germany) following the manufacturer’s protocol. Three genomic DNA regions were amplified: partial gene coding large ribosomal subunit (hereinafter referred to as 28S), partial gene coding small ribosomal subunit (18S) and entire ITS2 region (ITS2). A list of primers and thermocycling conditions are provided in Supplementary Table S1. Polymerase chain reactions (PCRs) were carried out in a total volume of 20 μL containing 10 μL Taq DNA PCR MasterMix Kit (Qiagen, Hilden, Germany), 0.5 μM of each primer, 8 μL nuclease-free water, and 1 μL of DNA template (corresponding to approximately 20 ng of DNA). The PCR products were checked on 1% agarose gel and purified using EPPiC Fast (Amplia, Bratislava, Slovakia) following the standard protocol. Sequencing was then performed in both directions using PCR primers specific for each region. Commercial services provided by Macrogen Europe (Amsterdam, Netherlands) were used for Sanger sequencing of newly obtained amplicons.
In order to assess phylogenetic relationships and genetic diversity among Khawia spp. additional ortholog 18S and 28S sequences of Khawia spp. were retrieved from GenBank (accession numbers are included within phylogenetic trees). The sequence alignments for each genomic region were built using the Fast Fourier transform algorithm implemented in MAFFT software (Katoh et al., Reference Katoh, Misawa, Kuma and Miyata2002), using the G-INS-i refinement method and then manually trimmed to unify the length of all sequences. Final alignment for phylogenetic tree reconciliation was built from concatenated DNA sequences of 18S and 28S, and concatenated ortholog sequences of Atractolytocestus sagittatus Anthony, 1958 (Caryophyllaeidae) were included and used for rooting of phylogenetic trees. Since ortholog 18S and 28S sequences were available only for six out of seven currently recognized Khawia species, non-complete data were discarded from the final alignment. The data were treated as partitioned, and a general time-reversible model (Lanave et al., Reference Lanave, Preparata, Saccone and Serio1984) was applied for each gene segment individually, also including a gamma distribution.
Phylogenetic trees were constructed using Bayesian inference (BI) and Maximum likelihood (ML) approaches in MrBayes 3.2 (Ronquist et al., Reference Ronquist, Teslenko, van der Mark, Ayres, Darling, Höhna, Larget, Liu, Suchard and Huelsenbeck2012) and RAxML 8.1.12 (Stamatakis, Reference Stamatakis2006; Reference Stamatakis2014), respectively. BI analysis used the Metropolis-coupled Markov chain Monte Carlo algorithm with two parallel runs of one cold and three hot chains and was run for 106 generations, sampling trees every 100 generations. The initial 30% of all saved trees were discarded as a ‘burn-in’ period after checking that the standard deviation split frequency fell below 0.01. The convergence of the runs and the parameters of individual runs were checked using Tracer v. 1.7.1 (Rambaut et al., Reference Rambaut, Drummond, Xie, Baele and Suchard2018). Posterior probabilities for each tree node were calculated as the frequency of samples recovering a given clade. The clade bootstrap support for ML trees was assessed by simulating 103 pseudoreplicates.
The ITS2 sequences were used to assess the intraspecific genetic variability among a priori delineated K. armeniaca populations. Uncorrected pairwise distances (p-distances) were calculated using the dist.dna function from the ‘ape’ package in R statistical environment, version 4.1.3 (R Core Team, 2022).
Morphological study
For light microscopy, specimens were stained with Mayer’s carmine, dehydrated in an ethanol series, cleared with clove oil (eugenol), and mounted in Canada balsam. Line drawings were made using an Olympus BX51 microscope equipped with phase contrast and differential interference contrast (Olympus Inc., Tokyo, Japan). For scanning electron microscopy, scoleces of selected specimens were processed as described by Oros et al. (Reference Oros, Uhrovič, Choudhury, Mackiewicz and Scholz2020) and examined with a Jeol JSEM 7401F electron microscope (Jeol Ltd., Tokyo, Japan). Voucher specimens and types of the new species are deposited in the IPCAS and Natural History Museum in London (NHMUK). To comply with the regulations set out in Article 8.5 of the amended 2012 version of the International Code of Zoological Nomenclature (2012), details of the new cestode species have been submitted to ZooBank.
Results
Molecular study
The final alignment for assessing the phylogenetic relationships among investigated Khawia spp. built from 24 concatenated 18S and 28S sequences spanned 3,490 unambiguously aligned nucleotide positions (1,945 bp long for 18S and 1,545 bp for 28S). The ML and BI analyses generated trees with congruent topologies and therefore only the BI tree is presented in Figure 2. The Khawia species were divided into three well-supported clades, with two well-differentiated and strongly supported lineages formed by novel Khawia sequences within clade A.

Figure 2. Phylogenetic tree of 23 concatenated sequences of seven Khawia species resulting from BI analysis. The tree is based on concatenated partial sequences of genes coding for 18S rDNA and 28S rDNA and is rooted with Atractolytocestus sagittatus as an outgroup. The numbers at the nodes represent posterior probabilities and bootstrap support values, resulting from BI and ML analyses, respectively. Specimen accession numbers (formatted as 18S;28S) and host species are provided in parentheses, when available.
In clade A, the lineage A1 encompassed all sequences from Khawia individuals collected from Luciobarbus species in the Iberian Peninsula. It also included the sequences retrieved from GenBank, originating from a specimen of Khawia cf. armeniaca from Portugal. Lineage A2 in clade A included four sequences of Khawia armeniaca collected from cyprinids in the Middle East (i.e. from Arabibarbus grypus, Barbus lacerta, Capoeta birunii and Luciobarbus kersin) and two sequences retrieved from GenBank, from specimens collected from C. capoeta and Coregonus lavaretus in Lake Sevan (Armenia). Clade B included all sequences of K. sinensis, and a single sequence of K. saurogobii, nested within this group. The last clade C occupied a well-supported sister position to clade B and consisted of specimens of K. parva, K. rossittensis and K. japonensis.
The novel sequences of ITS2 region were used to assess intraspecific genetic variability among Khawia specimens initially recognized as Khawia armeniaca. A final sequence alignment included 18 sequences and spanned 807 unambiguously aligned nucleotide positions. Uncorrected p-distances ranged from 0.000 to 0.203 (Supplementary Figure 1). The highest genetic divergence was recorded between Khawia individuals from L. bocagei (Portugal – new species) and C. birunii (Iran – K. armeniaca).
Morphological study
Examination of stained specimens of both genetic lineages revealed some slight but consistent differences between specimens of individual clades, i.e. the ‘true’ Khawia armeniaca and another described below as new (Figures 3–8). The validity of both species is supported by molecular data, their own unique host association and distribution range. Therefore, Khawia armeniaca is redescribed based on new material from Iraq and the new species from Spain and Portugal is described.
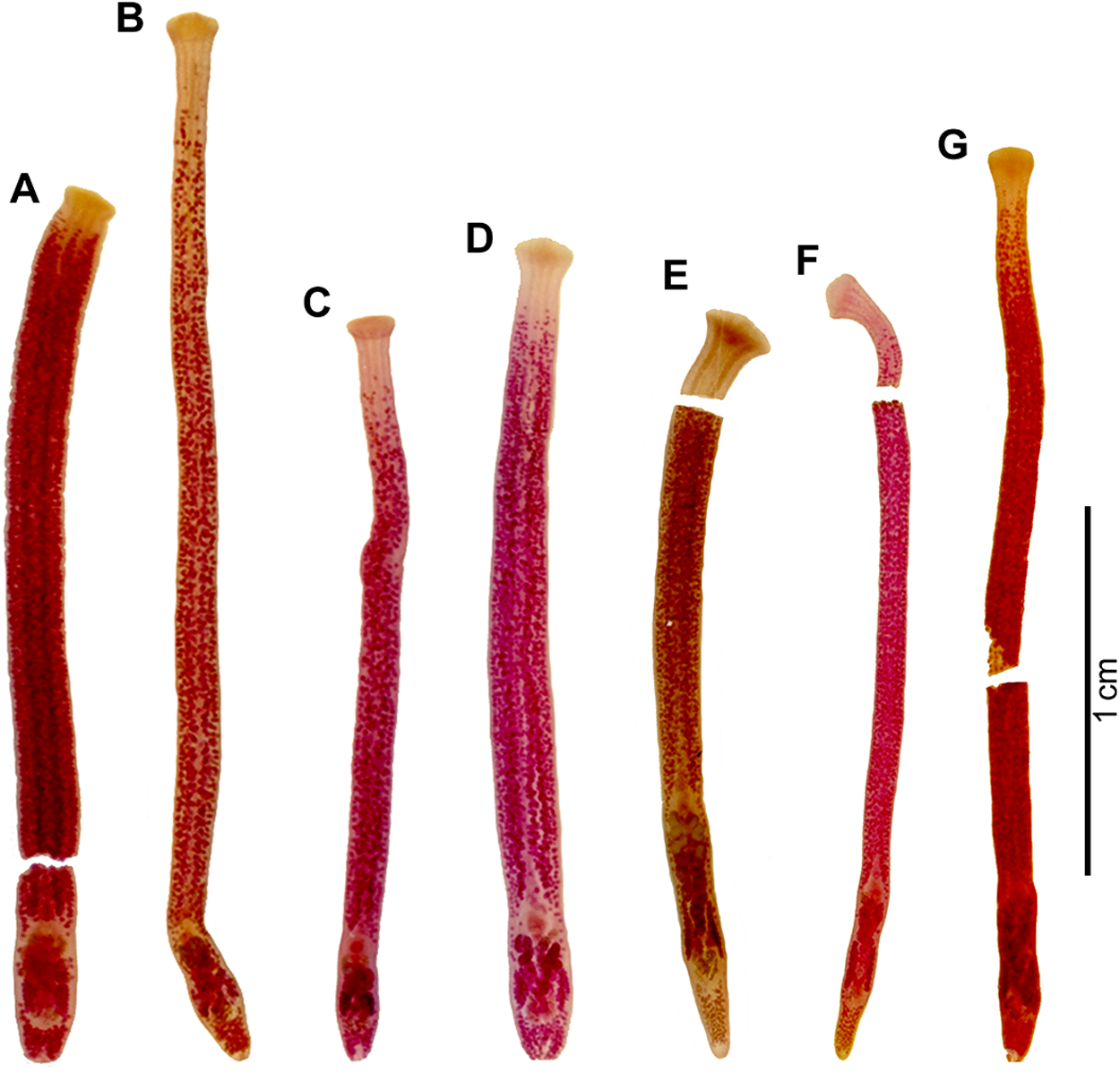
Figure 3. Whole, stained specimens of Khawia armeniaca s. str. (A–D) from various hosts in Iraq and Khawia iberica n. sp. (E–G) from various hosts in Spain. (A) Luciobarbus esocinus; (B) Arabibarbus grypus; (C) Mastacembelus mastacembelus (postcyclic host); (D) L. kersin; (E) L. bocagei (type host); (F) L. comizo; (G) L. guiraonis.
Redescription of Khawia armeniaca
(Cholodkovsky, Reference Cholodkovsky1915) Figures 3A–D, 4, 6A, 8C
Material studied: See Table 2.
Description: (based on total of 19 adult specimens from the following hosts: A. grypus – 5 specimens measured, B. lacerta – 3, L. barbulus – 1, L. esocinus – 2, L. kersin – 8; measurements in micrometres unless otherwise stated): Caryophyllidea, Caryophyllaeidae sensu Scholz et al. (Reference Scholz, Waeschenbach, Oros, Brabec and Littlewood2021). Body elongate, robust, rod-like, i.e. more or less of same width throughout (Figure 3A–D), 15–36 mm long, with maximum width 818–2446 at level of anterior vas deferens; width at level of cirrus sac 806–2,127, at level of ovarian isthmus 677–2,130. Posterior end of body widely rounded. Body surface covered with acicular fillitriches (filiform microtriches).
Scolex spatulate, dorsoventrally flattened, 917–2,175 wide, variable in shape (Figure 4), slightly (Figure 4A) to markedly (Figure 4C) wider than neck; neck 715–1,844 wide at level of anteriormost vitelline follicles. Anterior margin of scolex slightly convex, entire or with few, shallow notches (incisions); anterior part of dorsal and ventral surface of scolex slightly concave, with shallow longitudinal wrinkles (Figures 4, 6A). Inner longitudinal musculature formed by relatively few, irregular and small bundles of muscle fibres. Osmoregulatory canals well developed, sinuous and anastomosing; number of canals varies greatly due to numerous anastomoses, but usually two wider canals situated medially on ventral and dorsal sides; narrower canals lateral or beneath median canals.
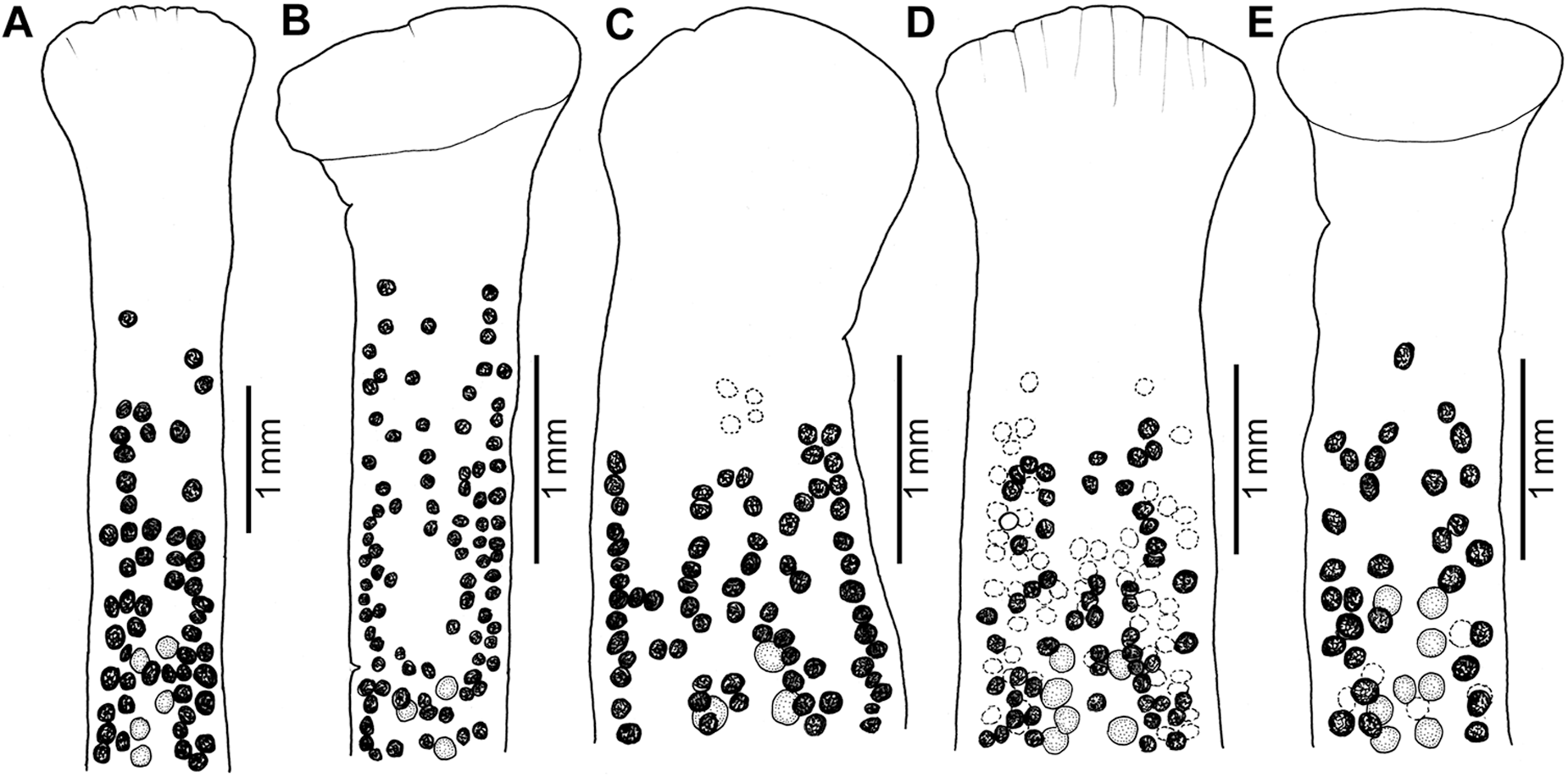
Figure 4. Morphological variation of scoleces of Khawia armeniaca s. str. from different hosts in Iraq. (A) Arabibarbus grypus; (B) Barbus lacerta; (C) Carasobarbus luteus; (D) Luciobarbus kersin; (E) Mastacembelus mastacembelus (postcyclic host). The image illustrates the relative position of anteriormost testes and vitelline follicles. Vitelline follicles (C, D) and testes (E) on the other (lower) side of the body are dotted (dotted open circles).
Testes medullary, subspherical to widely oval, variable in size, 85–335 long and 75–235 wide. Anteriormost testes begin posterior to anteriormost vitelline follicles (0.42–2.35 mm), 0.60–4.29 mm from anterior end of body (distance between first testes and anterior extremity represents 4.0–19.9% of total length of body) (Figure 4). First 10 testes occupy region 473–2,735 long. Posteriorly, testes reach cirrus sac.
Cirrus sac thick-walled, spherical to subspherical, 208–649 long and 252–658 wide. Width of cirrus sac represents 22–44% of body width. External seminal vesicle absent. Male genital duct opens to genital atrium with female genital duct (uterovaginal duct), corresponding to 5.24 of Mackiewicz (Reference Mackiewicz, Khalil, Jones and Bray1994).
Ovary follicular, grape-like, with deep lobes, H-shaped, with slightly concave (U-shaped) isthmus (Figure 8C). Ovary 614–1,730 wide at level of isthmus. Ovarian arms (wings) 528–1855 long and 212–658 wide, represent 30–55% of length of uterine region and 3.0–7.4% of total length of body. Length/width ratio of ovary 0.57–1.09. Ovarian isthmus pre-equatorial, equatorial to post-equatorial (ratio of length of ovarian arms anterior to isthmus to length of ovarian arms posterior to isthmus 0.41–2.52). Vagina tubular, slightly sinuous, widened to form elongate, narrow seminal receptacle anterior to ovarian isthmus, joins with uterus to form uterovaginal canal, opens separately from gonopore into distinct genital atrium. Gonopore (opening of common genital atrium) situated 541–2,282 anterior to ovary.
Preovarian vitelline follicles numerous, cortical, variable in size, 58–190 long and 54–179 wide. First (anteriormost) vitelline follicles situated 577–2,603 from anterior margin of body (distance to anterior extremity represents 3.1–9.8% of total length of body), much anterior to first (anteriormost) testes; number of pretesticular vitelline follicles 7–194. Preovarian vitelline follicles reach posteriorly to level of cirrus sac, with few follicles alongside preovarian uterine region; follicles may reach to ovary; distance of last preovarian follicles to ovarian arms very variable, 0–585. Number of vitelline follicles alongside uterus 0–24; number of follicles alongside ovary 0–8. Post-ovarian vitelline follicles numerous, 58–172 in number; anteriormost post-ovarian follicles 0–79 posterior to ovary.
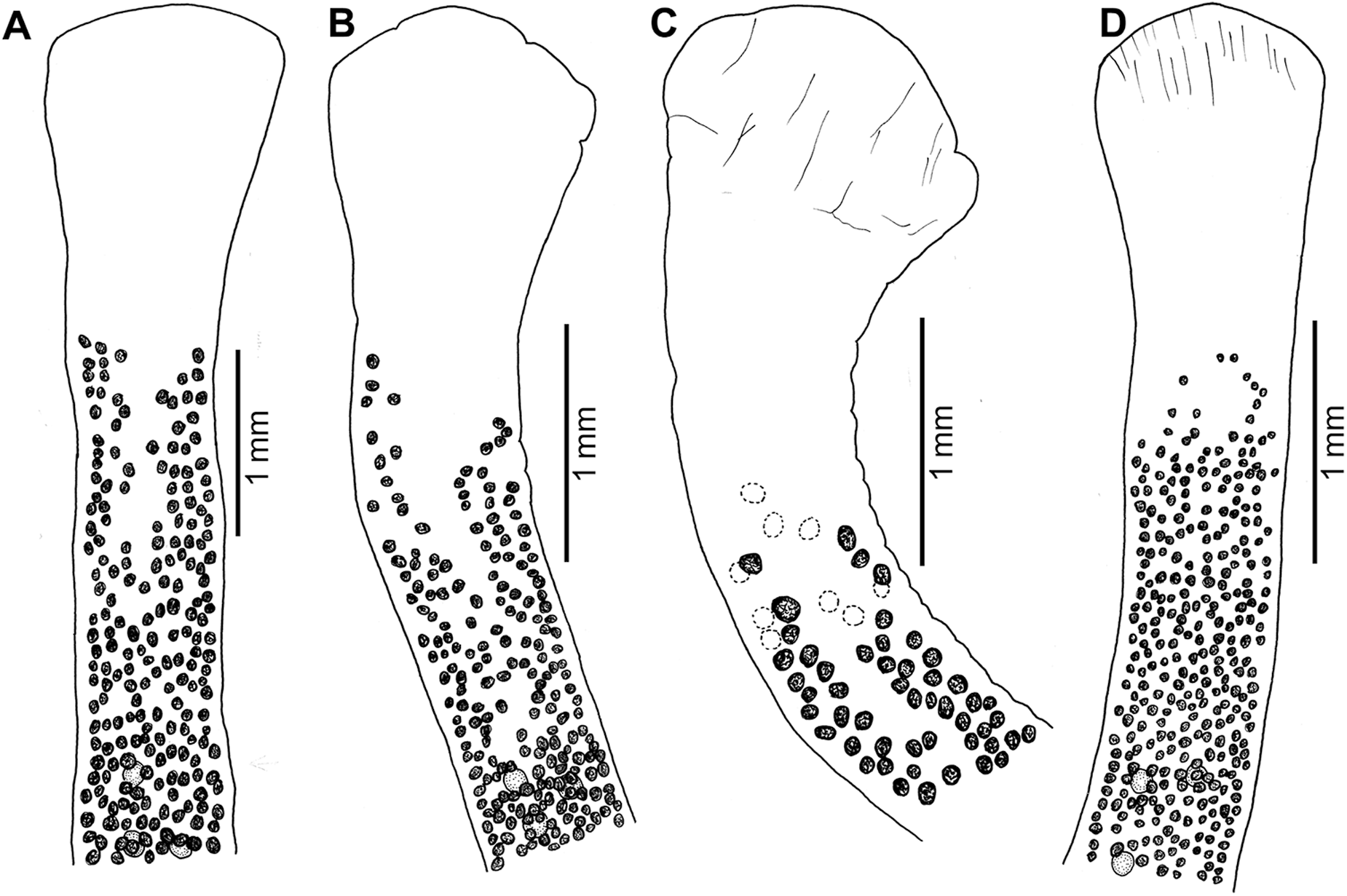
Figure 5. Morphological variation of scoleces of Khawia iberica n. sp. from different hosts in Spain. (A, B) Luciobarbus bocagei (type host); (C) L. comizo; (D) L. guiraonis. The image illustrates the relative position of anteriormost testes and vitelline follicles. Vitelline follicles on the other (lower) side of the body in C are dotted (dotted open circles).
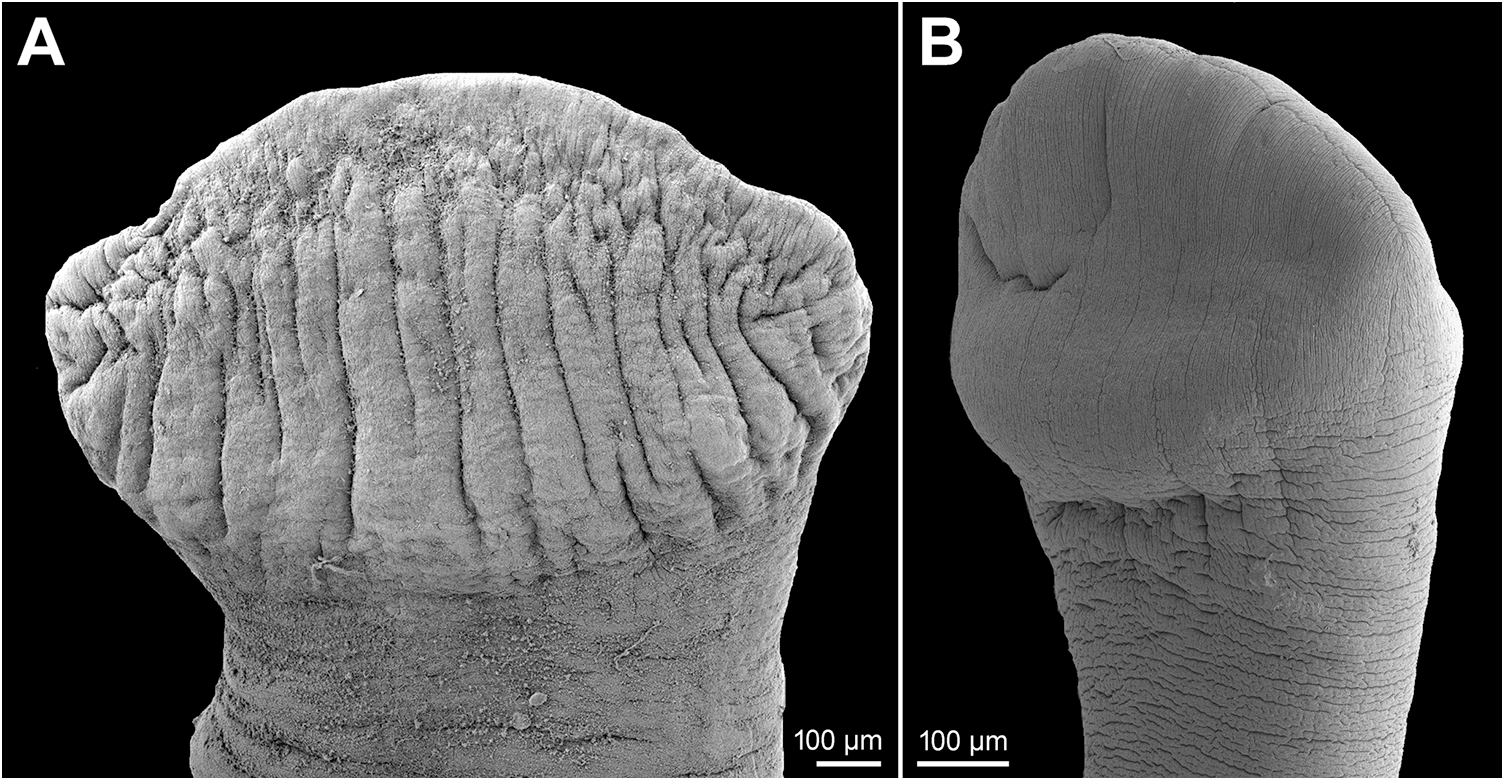
Figure 6. Scanning electron micrographs of scoleces. (A) Khawia armeniaca s. str. from Luciobarbus kersin, Iraq; (B) Khawia iberica n. sp. from Luciobarbus bocagei, Spain.
Uterus tubular, forms several preovarian loops, extending to posterior margin of cirrus sac. Uterine region 1.12–4.34 mm long, i.e. 2.8–6.9% of total length of body; region occupied by uterus between cirrus sac to ovary 442–2,142. Uterine glands well developed, absent only in most distal and proximal parts of uterus. Eggs operculate, without fully formed oncosphere in utero, 51–75 long and 31–45 wide.
Taxonomic summary
Type host: Capoeta capoeta (Güldenstädt, 1773).
Additional hosts (*confirmed genetically): *Arabibarbus grypus (Heckel, 1843) (vouchers IPCAS C-48/2); *Barbus lacerta Heckel, 1843 (IPCAS C-48/11); *Capoeta birunii Zareian et Esmaeili, 2017; Capoeta damascina (Valenciennes, 1842); Carasobarbus luteus (Heckel, 1843) (IPCAS C-48/6); Luciobarbus barbulus (Heckel, 1847) (IPCAS C-48/4); L. esocinus Heckel, 1843 (IPCAS C-48/10); *L. kersin Heckel, 1843 (IPCAS C-48/9); Coregonus lavaretus (Lavaretus, 1758); Salmo ischchan Kessler, 1877; Silurus triostegus Heckel, 1843, and Mastacembelus mastacembelus (Banks et Solander, 1794) (IPCAS C-48/12), four latter host species are atypical (postcyclic or accidental) hosts.
Site of infection: Anterior intestine.
Type locality: Lake Sevan, Armenia.
Distribution (* confirmed genetically): Armenia*, Azerbaijan, Georgia, Iran*, Iraq*, Israel, Turkey.
Records: Cholodkovsky (Reference Cholodkovsky1915), Popov (Reference Popov1924), Dinnik (Reference Dinnik1933), Mikailov (Reference Mikailov1975), Williams et al. (Reference Williams, Gibson and Sadighian1980), Rahemo & Al-Kalak (Reference Rahemo and Al-Kalak1993), Protasova et al. (Reference Protasova, Kuperman, Roitman and Poddubnaya1990); present study.
Type material: Does not exist.
Representative DNA sequences: See above and following provided from the host B. lacerta: 18S rDNA – PV558932; 28S rDNA – PV558942; ITS2 – PV558921. In addition, Scholz et al. (Reference Scholz, Brabec, Kráľová-Hromadová, Oros, Bazsalovicsová, Ermolenko and Hanzelová2011) provided sequences of tapeworms from Coregonus lavaretus (probably accidental host), Lake Sevan, Armenia (JN004246 – 18S rDNA; JN004257 – 28S rDNA; JN004235 – nad3; JN004224 – cox1).
Remarks: Khawia armeniaca is a common and widespread parasite of barbels in the Middle East. It has been found in a wide range of fish hosts (10 host species, excluding atypical hosts). The species exhibits morphological and biometrical variability, most likely reflecting intraspecific variability of individual parasite populations from different definitive hosts (Scholz et al., Reference Scholz, Brabec, Kráľová-Hromadová, Oros, Bazsalovicsová, Ermolenko and Hanzelová2011). Furthermore, the specimens examined were fixed by different methods, including severe flattening, they are deformed or contracted, with the scolex unnaturally shortened, and some were obviously dead and therefore decomposed at the time of fixation, which may have affected their appearance and subsequent measurements.
Scholz et al. (Reference Scholz, Brabec, Kráľová-Hromadová, Oros, Bazsalovicsová, Ermolenko and Hanzelová2011) also reported K. armeniaca from the ripon barb, Labeobarbus altianalis (Boulenger, 1900), in Uganda; the Niger barb, Labeobarbus bynni (Fabricius, 1775), in Egypt; Luciobarbus callensis (Valenciennes, 1842), in Morocco; and Labeobarbus tropidolepis (Boulenger, 1900), in Tanzania. However, the species affiliation of these tapeworms is unclear as no molecular data are available. It is possible that some of these specimens are another new species, especially the specimens from Tanzania and Uganda, which are very large and considerably exceed the maximum size of K. armeniaca and K. iberica n. sp. The species identification of ‘K. armeniaca’ from barbels in Lake Tana is also uncertain and molecular data are needed.
Khawia iberica n. sp
Synonyms: Khawia baltica Szidat, 1942 of Chubb et al. (Reference Chubb, Eiras and Saraiva1997); K. armeniaca (Cholodkovsky, Reference Cholodkovsky1915) of Scholz et al. (Reference Scholz, Brabec, Kráľová-Hromadová, Oros, Bazsalovicsová, Ermolenko and Hanzelová2011) (partim – specimens from Portugal)
Material studied: See Table 2.
Description (based on total of 16 adult specimens from the following hosts: Luciobarbus bocagei – 11 specimens measured, L. guiraonis – 4, L. comizo – 1; measurements in micrometres unless otherwise stated; measurements of the holotype in brackets): Caryophyllidea, Caryophyllaeidae sensu Scholz et al. (Reference Scholz, Waeschenbach, Oros, Brabec and Littlewood2021). Body elongate, slender, rod-like (Figure 3E–G), i.e. more or less of same width throughout 13–29 mm [15.5 mm] long, with maximum width 787–1369 [937] at level of anterior vas deferens; width at level of cirrus sac 760–1,275 [854], at level of ovarian isthmus 689–1,264 [786]. Posterior end of body widely rounded posteriorly (Figures 7, 8A). Body surface covered with acicular fillitriches (filiform microtriches).
Scolex spatulate, dorsoventrally flattened, 860–2,200 [1,126] wide, slightly (Figure 5A, B, D) to markedly (Figure 5C) wider than neck; neck 656–1,266 [746] wide at level of anteriormost vitelline follicles. Anterior margin of scolex slightly convex, entire or with few, shallow notches (incisions); anterior part of dorsal and ventral surface of scolex slightly concave, with narrow longitudinal wrinkles (Figure 6B). Inner longitudinal musculature formed by relatively small bundles of muscle fibres. Osmoregulatory canals well developed, sinuous and anastomosing; number of canals varies greatly due to numerous anastomoses, but usually two wider canals situated medially on ventral and dorsal sides; narrower canals lateral or beneath median canals.
Testes medullary, subspherical to widely oval, 96–186 [106–123] long and 91–171 [91–108] wide. Anteriormost testes begin posterior to anteriormost vitelline follicles (0.90–5.92 mm [2.39 mm]), 1.62–7.50 mm [3.88 mm] from anterior end of body (distance between first testes and anterior extremity represents 7.6–31.9% [25.0%] of total length of body) (Figure 5). First 10 testes occupy region 170–1,798 [693] long. Posteriorly, testes reach cirrus sac (Figures 7, 8A).
Cirrus sac thick-walled, spherical to subspherical, 249–560 [264] long and 263–523 [263] wide. Width of cirrus sac represents 23–47% [28%] of body width (Figure 8A). External seminal vesicle absent. Male genital duct opens to joint genital atrium with female genital duct (uterovaginal duct), corresponding to 5.24 of Mackiewicz (Reference Mackiewicz, Khalil, Jones and Bray1994) (Figures 7, 8A).
Ovary non-follicular, with deep lobes, H-shaped, with concave (U-shaped) isthmus (Figures 7, 8A, B). Ovary 446–1,048 [673] wide at level of isthmus. Ovarian arms (wings) 535–1639 [789] long and 184–477 [238] wide. Ovarian arms represent 29–58% [48%] of length of uterine region and 4.1–7.9% [5.1%] of total length of body. Length/width ratio of ovary 0.89–1.85 [1.17]. Ovarian isthmus usually post-equatorial (ratio of length of ovarian arms anterior to isthmus to length of ovarian arms posterior to isthmus 0.74–3.40 [1.74]. Vagina tubular, slightly sinuous, widened to form elongate, narrow seminal receptacle anterior to ovarian isthmus. Isthmus joins with uterus to form uterovaginal canal, opens separately from gonopore into distinct genital atrium (Figures 7, 8A). Gonopore (opening of common genital atrium) situated 516–2,282 [644] anterior to ovary.
Preovarian vitelline follicles numerous, cortical, variable in size, 58–133 [66–72] long and 50–125 [53–69] wide. First (anteriormost) vitelline follicles situated 900–3,321 [1487] from anterior margin of body (distance to anterior extremity represents 3.1–11.7% of total length of body), much anterior to first (anteriormost) testes; number of pretesticular vitelline follicles 57–627 [244] (Figure 5). Preovarian vitelline follicles reach posteriorly to level of cirrus sac, with few follicles alongside preovarian uterine region; follicles may reach to ovary; distance of last preovarian follicles to ovarian arms variable, 0–263 [41]. Number of vitelline follicles alongside uterus 0–25 [4]; number of follicles alongside ovary 0–7 [0]. Post-ovarian vitelline follicles numerous, 31–125 [125] in number; anteriormost post-ovarian follicles 0–543 [9] posterior to ovary (Figures 7, 8A).
Uterus tubular, forms several preovarian loops, extending to posterior margin of cirrus sac (Figures 7, 8A, B). Uterine region 1.56–4.77 mm [1.66 mm] long, i.e. 2.8–8.7% [4.2%] of total length of body; region occupied by uterus between gonopore to ovary 586–2,510 [651]. Uterine glands well developed, absent only in most distal and proximal parts of uterus. Eggs operculate, without fully formed oncosphere in utero, 53–64 [mean 53] long and 30–37 [mean 31] wide.
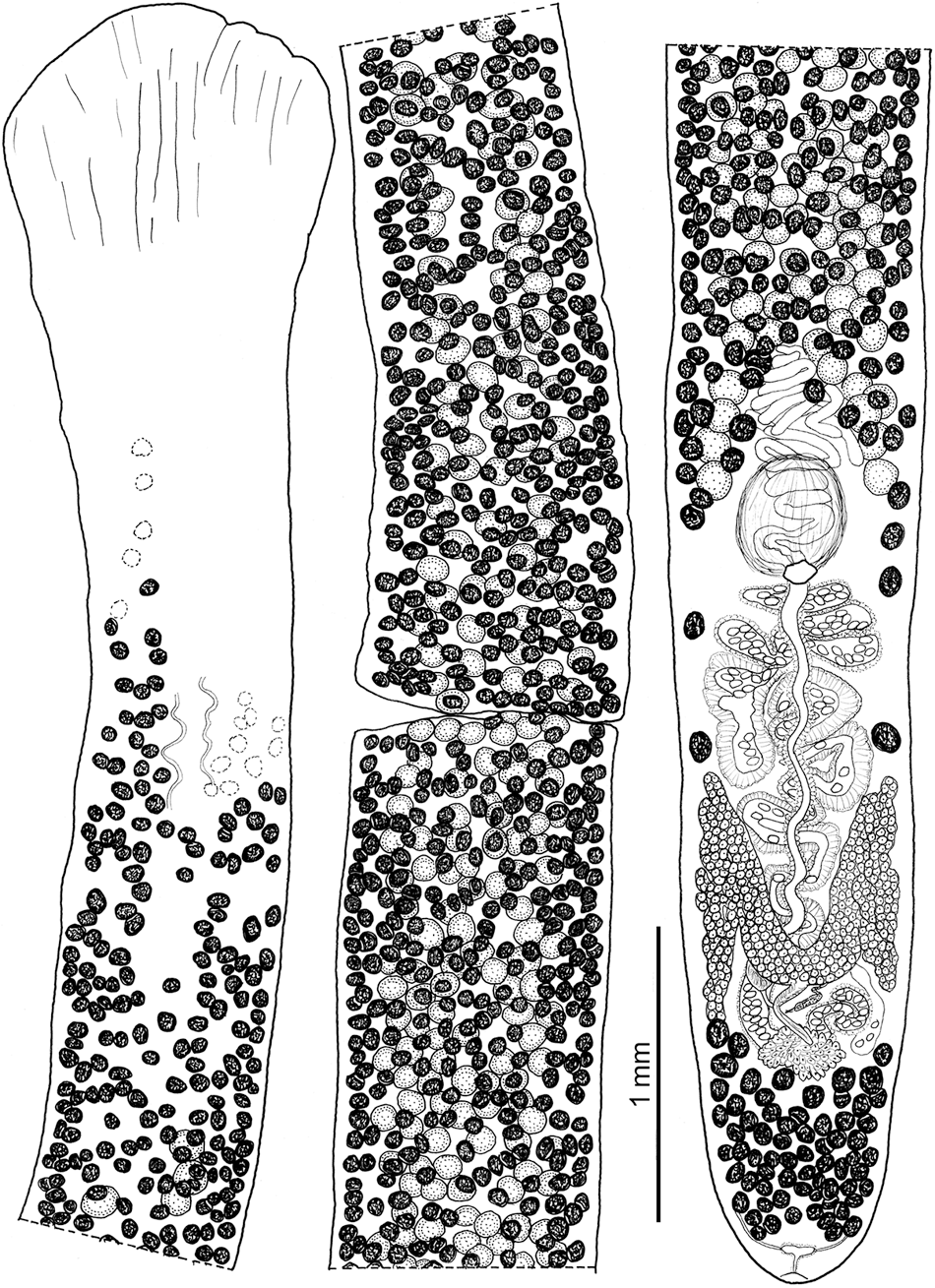
Figure 7. Line drawing of Khawia iberica n. sp., holotype from Luciobarbus bocagei, Spain. Note the elongated lobes of the ovary with a U-shaped isthmus. Vitelline follicles on the other (lower) side of the body are dotted (dotted open circles).
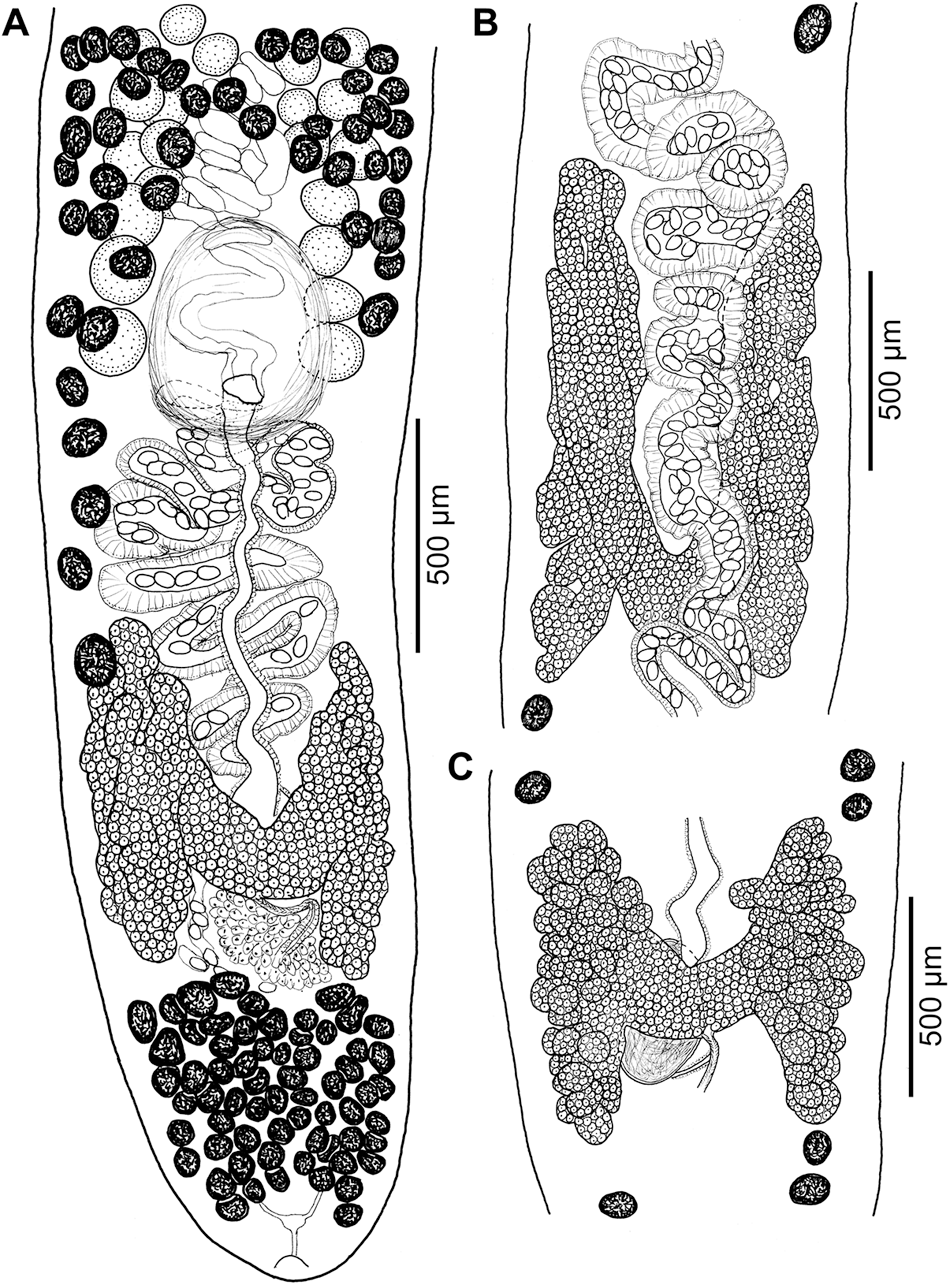
Figure 8. Line drawings of ovaries. (A) Khawia iberica n. sp. from Luciobarbus bocagei (type host), Spain; (B) K. iberica n. sp. from L. comizo, Spain; (C) Khawia armeniaca s. str. from Arabibarbus grypus, Iraq.
Taxonomic summary
Type host: Luciobarbus bocagei (Steindachner, 1864).
Additional hosts: Luciobarbus comizo (Steindachner, 1864); Luciobarbus guiraonis (Steindachner, 1866).
Site of infection: Anterior intestine.
Type locality: Colares, Portugal.
Additional localities: Este River, Portugal; Orbigo River, Spain; Magro River; Spain; Zújar River, Spain.
Type material: Holotype (IPCAS C-1014/1) and two paratypes (IPCAS C-1014/1) – whole-mounted specimens from Luciobarbus bocagei (host code LUBO 1), Colares, Portugal, collected by A. Šimková on 22 June 2016; one paratype – whole-mounted specimen from L. bocagei (LUBO 10), Colares, Portugal, collected by Andrea Vetešníková Šimková on 22 June 2016; one paratype (IPCAS C-48/6) from L. bocagei (PT 69), Este River near Porto, Portugal, collected by Tomáš Scholz on 21 September 2009; two paratypes (IPCAS C-1014/2) – whole-mounted specimens from Luciobarbus comizo (LUMI 1b, LUMI 3), Peraleda de Zancejo, Zújar River, Spain, collected by Kateřina Čermáková Vyčítalová on 25 June 2017; two paratypes (IPCAS C-1014/3) – whole-mounted specimens from Luciobarbus guiraonis (LUGU 4, LUGU 11), Magro River, Spain, collected by Andrea Vetešníková Šimková on 22 June 2016; and one paratype (NHMUK 2025.6.9.1) – whole-mounted specimen from L. guiraonis (LUGU 11), Magro River, Spain, collected by Andrea Vetešníková Šimková on 22 June 2016;.
Representative DNA sequences: 18S rDNA – PV558936; 28S rDNA – PV558946; ITS2 – PV558925
Etymology: The specific name refers to the recorded distribution of the species, which is Iberian Peninsula.
ZooBank registration (LSID): urn:lsid:zoo bank.org:act:225DBA46-DAC4-49F9-8439-9FA0675D2AF8
Differential diagnosis: The new species differs from K. armeniaca in the structure, shape and size of the ovary and the anterior extension of the vitelline follicles and testes. The ovary of K. iberica n. sp. is compact and consists of elongated lobes (Figure 8A, B), whereas the ovary of K. armeniaca is follicular (Figure 8C). The ovary of the former species (K. iberica n. sp.) has longer, slender lateral arms (wings), and the isthmus is more concave (U-shaped) and located further back in most specimens (Figures 7, 8A, B).
Scholz et al. (Reference Scholz, Brabec, Kráľová-Hromadová, Oros, Bazsalovicsová, Ermolenko and Hanzelová2011) described the ovary of K. armeniaca sensu lato (s. lat.) as butterfly-shaped, but an ovary of this shape is almost exclusively typical for tapeworms from the Middle East, i.e. K. armeniaca sensu stricto (s. str.). A follicular ovary is also described in the first description of K. armeniaca by Cholodkovsky (Reference Cholodkovsky1915).
The two species also differ in the anterior extension of vitelline follicles and testes (Figures 4, 5), especially in the distance between them, which is usually greater in K. iberica n. sp. (up to 2.5 times). In this species, the first (anteriormost) testes start at a greater distance from the anterior end compared to K. armeniaca (Figure 4) (usually twice as far).
In addition, the new species is a specific parasite of Luciobarbus spp. and has only been found in Portugal and Spain (the species identification of Khawia tapeworms from barbels in North Africa is uncertain).
Remarks: Khawia iberica was previously conflated with K. armeniaca, although Scholz et al. (Reference Scholz, Brabec, Kráľová-Hromadová, Oros, Bazsalovicsová, Ermolenko and Hanzelová2011) found a striking genetic divergence between specimens from Portugal (= K. iberica n. sp.) and those of K. armeniaca s. str. from the type locality (Lake Sevan in Armenia). The poor quality of the available material and the lack of molecular data on tapeworms from different host species and geographical regions prevented the proposal of a new species at that time. The new material, suitable for comparative molecular and morphological analysis, allowed us to distinguish the two species parasitising barbels in Europe and the Middle East.
Chubb et al. (Reference Chubb, Eiras and Saraiva1997) reported caryophyllidean tapeworms identified as K. baltica in L. bocagei in northwestern Portugal (Este River). However, a re-examination of vouchers (IPCAS C-48/6) confirmed that these tapeworms belong to K. armeniaca s. lat. (see Scholz et al., Reference Scholz, Brabec, Kráľová-Hromadová, Oros, Bazsalovicsová, Ermolenko and Hanzelová2011), specifically to K. iberica n. sp.
At the beginning of the 21st century, two new species of Khawia were described from the yellow barbell, Barbus luteus (= Carasobarbus luteus [Heckel, 1843], a known fish host for K. armeniaca from the Tigris) at the same locality, the Tigris River in Iraq: Khawia barbi Rahemo et Mohammad (Reference Rahemo and Mohammad2002) and Khawia lutei Al-Kalak et Rahemo (Reference Al-Kalak and Rahemo2003). However, both species were inadequately described, based on poorly preserved material, and the original descriptions contained almost no morphometric data. Scholz et al. (Reference Scholz, Brabec, Kráľová-Hromadová, Oros, Bazsalovicsová, Ermolenko and Hanzelová2011) synonymized these species as they are morphologically indistinguishable from K. armeniaca and share the same host range and geographic distribution. The available molecular data fully support this synonymy.
Discussion
Previous studies documented remarkable intraspecific variability in Khawia armeniaca (see Scholz et al., Reference Scholz, Brabec, Kráľová-Hromadová, Oros, Bazsalovicsová, Ermolenko and Hanzelová2011), although only a small number of properly fixed individuals had been investigated. The variability was apparent at both the morphological and the molecular level, suggesting the existence of two separate species with specific geographic distribution and host ranges. Nonetheless, previously available material was not sufficient to fully describe and delimit the new taxon, and therefore herein 35 specimens were morphologically analysed, and molecular analyses were performed on 22 specimens, in order to provide a proper taxonomic description.
The examined specimens were collected in Spain, Portugal, Iran and Iraq, with hosts including the cyprinid fish species of the genera Arabibarbus, Barbus, Capoeta, Carasobarbus, Labeobarbus and Luciobarbus, and Mastacembelus mastacembelus, which is considered to be an accidental or postcyclic host due to predation. Congruently with the aforementioned review, analysed specimens of Khawia were found to be clearly divided into two molecularly well-supported lineages, each associated with the different geographical region: the Iberian Peninsula lineage and the Middle Eastern lineage. Considering both morphological and molecular differences, the Middle Eastern lineage is identified as Khawia armeniaca s. str., corresponding to the original description and the genetically characterized isolate from the type locality (Lake Sevan, Armenia – see Scholz et al., Reference Scholz, Brabec, Kráľová-Hromadová, Oros, Bazsalovicsová, Ermolenko and Hanzelová2011). The Iberian lineage, on the other hand, is considered a new species, Khawia iberica n. sp., based on molecular and morphological distinctiveness.
After the split of K. armeniaca s. lat. into two separated species, both taxa still exhibit a remarkably wide host range. In the case of K. iberica n. sp. this includes three endemic Luciobarbus species; however, it cannot be ruled out that this species may also infect other congeneric hosts in the region. The host range of K. armeniaca s. str. appears to be even wider, including 10 species belonging to six cyprinid genera. Other representatives of the genus Khawia generally exhibit much narrower host specificity (Scholz et al., Reference Scholz, Brabec, Kráľová-Hromadová, Oros, Bazsalovicsová, Ermolenko and Hanzelová2011; Xi et al., Reference Xi, Oros, Wang, Scholz and Xie2013), which is typical for the majority of fish tapeworms. Notably, 61% of cestodes of bony fish utilize a single definitive host (Scholz and Kuchta, Reference Scholz and Kuchta2017). One of the few genera exhibiting similarly variable levels of host specificity is Caryophyllaeus; while C. laticeps has a broad host range encompassing more than 30 fish species, C. balticus is considered a specialist, occurring in only a single definitive host species (Scholz et al., Reference Scholz, Brabec, Kráľová-Hromadová, Oros, Bazsalovicsová, Ermolenko and Hanzelová2011; Barčák et al., Reference Barčák, Oros, Hanzelová and Scholz2017; Kuchta et al., Reference Kuchta, Řehulková, Francová, Scholz, Morand and Šimková2020).
As with many other parasites, the classification of the order Caryophyllidea historically relied primarily on morphological traits (Mackiewicz, Reference Mackiewicz2003). However, molecular methods have introduced an invaluable tool for studying intraspecific variability and interspecific differences. In the case of the present study, the molecular data reinforced morphologically evident differences. These methods have also been instrumental in identifying so-called cryptic and sibling species – genetically distinct but morphologically nearly indistinguishable species (Nadler and Pérez-Ponce de León, Reference Nadler and Pérez-Ponce de León2011). For instance, cryptic or sibling species have recently been identified within the genera Paracaryophyllaeus (Caryophyllidea) and Bothriocestus (previously placed in Bothriocephalus; Bothriocephalidea) (Scholz et al., Reference Scholz, Oros, Bazsalovicsová, Brabec, Waeschenbach, Xi, Aydoğdu, Besprozvannykh, Shimazu, Králová-Hromadová and Littlewood2014; Choudhury and Scholz, Reference Choudhury and Scholz2020). The integration of molecular approaches into the study of parasite taxonomy and diversity has become almost indispensable. However, this approach was not used in earlier studies on K. armeniaca, such as those by Rahemo and Mohammad (Reference Rahemo and Mohammad2002), Al-Kalak and Rahemo (Reference Al-Kalak and Rahemo2003), and Kibet et al. (Reference Kibet, Wen-Ting, Huda and Pin2021), which significantly complicated efforts to explore the true diversity of these tapeworms and their phylogenetic relationships.
The reliability of classical morphometric criteria has also been questioned in the study by Bazsalovicsová et al. (Reference Bazsalovicsová, Králová-Hromadová, Brabec, Hanzelová, Oros and Scholz2014), which revealed substantial intraspecific plasticity in tapeworms of the genus Caryophyllaeus, members of the same family as Khawia. This study uncovered morphological plasticity that led to the division of C. laticeps and C. brachycollis into morphotypes, usually typical of individual fish hosts. However, genetic analysis did not provide conclusive evidence that these morphotypes represented distinct species. As a result, these species are considered polymorphic parasites infecting a broad range of hosts (Barčák et al., Reference Barčák, Oros, Hanzelová and Scholz2017). The findings of Bazsalovicsová et al. (Reference Bazsalovicsová, Králová-Hromadová, Brabec, Hanzelová, Oros and Scholz2014) highlight that species identification in the genus Caryophyllaeus based solely on morphology can be sometimes misleading due to their phenotypic plasticity. This observation is relevant to the present study, where morphology alone provided only weak evidence to differentiate the two genetically well-defined lineages because of high degree of intraspecific variability of both species. The observed differences in ovarian structure and the anterior part of the tapeworm’s body may be considered manifestations of intraspecific variability.
It is clear that morphology alone may not provide adequate taxonomic resolution for closely related species (Nadler and Pérez-Ponce de León, Reference Nadler and Pérez-Ponce de León2011). This is particularly true for caryophyllidean tapeworms, which, compared to other orders, appear to lack prominent taxonomic traits. Based on the results of this study, several principles emerge as critical: proper and careful fixation methods, limiting tissue sampling for molecular purposes to the middle body segment (Chervy, Reference Chervy2024), and focusing morphological analyses on traits unaffected by fixation or the individual’s age. This approach necessitates the use of only sexually mature, adult specimens. For widespread species and/or species with a broad host range, it is also important to include an appropriate number of specimens from different localities and/or host species. This will ensure that the data used cover the full range of potential variability, thereby increasing the reliability of the results.
The results of the phylogenetic analyses placed a clade encompassing K. armeniaca and K. iberica n. sp. in a basal position to the other congeners, consistent with phylogenetic trees presented by Scholz et al. (Reference Scholz, Brabec, Kráľová-Hromadová, Oros, Bazsalovicsová, Ermolenko and Hanzelová2011) and Scholz et al. (Reference Scholz, Waeschenbach, Oros, Brabec and Littlewood2021). Although Khawia baltica was identified as basal in the study by Scholz et al. (Reference Scholz, Brabec, Kráľová-Hromadová, Oros, Bazsalovicsová, Ermolenko and Hanzelová2011), this species was later reassigned to the genus Caryophyllaeus as Caryophyllaeus balticus by Barčák et al. (Reference Barčák, Oros, Hanzelová and Scholz2017). Our phylogenetic analysis similarly places K. iberica n. sp. lineage as sister to K. armeniaca, aligning with the findings of Scholz et al. (Reference Scholz, Brabec, Kráľová-Hromadová, Oros, Bazsalovicsová, Ermolenko and Hanzelová2011). The resulting phylogenetic trees do not show significant intraspecific variability in ribosomal subunit (18S and 28S) sequences within either lineage at the molecular level, indicating that representatives of these lineages can be considered conspecific.
Within the K. iberica n. sp. lineage, analyses based on concatenated data of two ribosomal markers reveal an apparent genetic divergence between specimens from the host Luciobarbus comizo and those parasitizing L. bocagei. This separation is more pronounced in the ITS2 sequences, suggesting that even within K. iberica n. sp. conspecifics, there is host-dependent intraspecific structuring. Khawia armeniaca also exhibits a degree of structure, notably separating a single specimen from the host Capoeta birunii in Iran. This specimen originates from a highly isolated locality in comparison to other studied individuals, located approximately 400 km from the nearest other recorded occurrence. Furthermore, it is the only parasite recovered from this host species. To further investigate the differences between these isolates, additional material will be required.
From a geographic perspective, a key finding is the distribution of K. iberica n. sp., which, based on the available data, appears to be restricted to the Iberian Peninsula. These tapeworms, prior to this study considered to be K. armeniaca, have not been reported from other southern European peninsulas or elsewhere in Europe. This absence of findings cannot be simply attributed to insufficient sampling in these regions. Such a restricted distribution suggests that the dispersal of these tapeworms to the Iberian Peninsula likely occurred via North Africa and Gibraltar, rather than through the Apennine or Balkan Peninsulas or Central Europe. While several records of ‘K. armeniaca’ exist from North Africa (Scholz et al., Reference Scholz, Brabec, Kráľová-Hromadová, Oros, Bazsalovicsová, Ermolenko and Hanzelová2011), no genetic data are available from this region, posing a major obstacle to understanding the presumed historical dispersal. The lack of genetic data also hinders the study of speciation and/or the origin of this species on the Iberian Peninsula and its subsequent dispersal. As such, it remains speculative how closely the Iberian Khawia iberica n. sp. resembles individuals from North Africa.
It is notable that the Iberian Peninsula harbours no other representatives of the order Caryophyllidea besides the genus Caryophyllaeus and the invasive genus Atractolytocestus Anthony, 1958 (Cordero del Campillo et al., Reference Cordero del Campillo, Castañon and Reguera1994). The host range of K. iberica n. sp. based on the findings of this study appears to be restricted to the genus Luciobarbus, specifically the endemic and sympatric species L. comizo, L. bocagei and L. guiraonis (Kottelat and Freyhof, Reference Kottelat and Freyhof2007; Gante et al., Reference Gante, Doadrio, Alves and Dowling2015; Froese and Pauly, Reference Froese and Pauly2025). The genus Luciobarbus is widely distributed across Europe, Asia and North Africa, with its historical dispersal being the subject of many biogeographical hypotheses (e.g. Doadrio, Reference Doadrio1990; Oellermann and Skelton, Reference Oellermann and Skelton1990; Tsigenopoulos et al., Reference Tsigenopoulos, Kasapidis and Berrebi2010; Yang et al., Reference Yang, Sado, Hirt, Pasco-Viel, Arunachalam, Li, Wang, Freyhof, Saitoh, Simons, Miya, He and Mayden2015).
One such hypothesis (Doadrio, Reference Doadrio1990; Perea et al., Reference Perea, Böhme, Zupančič, Freyhof, Šanda, Özuluğ, Abdoli and Doadrio2010) proposes that these fish dispersed to the Iberian Peninsula from North Africa. Given the sympatric occurrence of both the parasite and its host in North Africa and the Iberian Peninsula, it can be hypothesized (though not confirmed) that K. iberica n. sp. followed this dispersal route of its hosts. Nevertheless, a critical prerequisite for testing the hypothetical phylogeographic association of the two species is an analysis of the genetic diversity of the North African lineage of these tapeworms. If a close molecular phylogenetic relationship with K. iberica n. sp. is confirmed, this model would align with the known relationships of North African and Iberian Luciobarbus species (Machordom and Doadrio, Reference Machordom and Doadrio2001; Tsigenopoulos et al., Reference Tsigenopoulos, Durand, Unlu and Berrebi2003; Casal-López and Doadrio, Reference Casal-López and Doadrio2018), supporting the reconstruction of the dispersal history of both host and parasite.
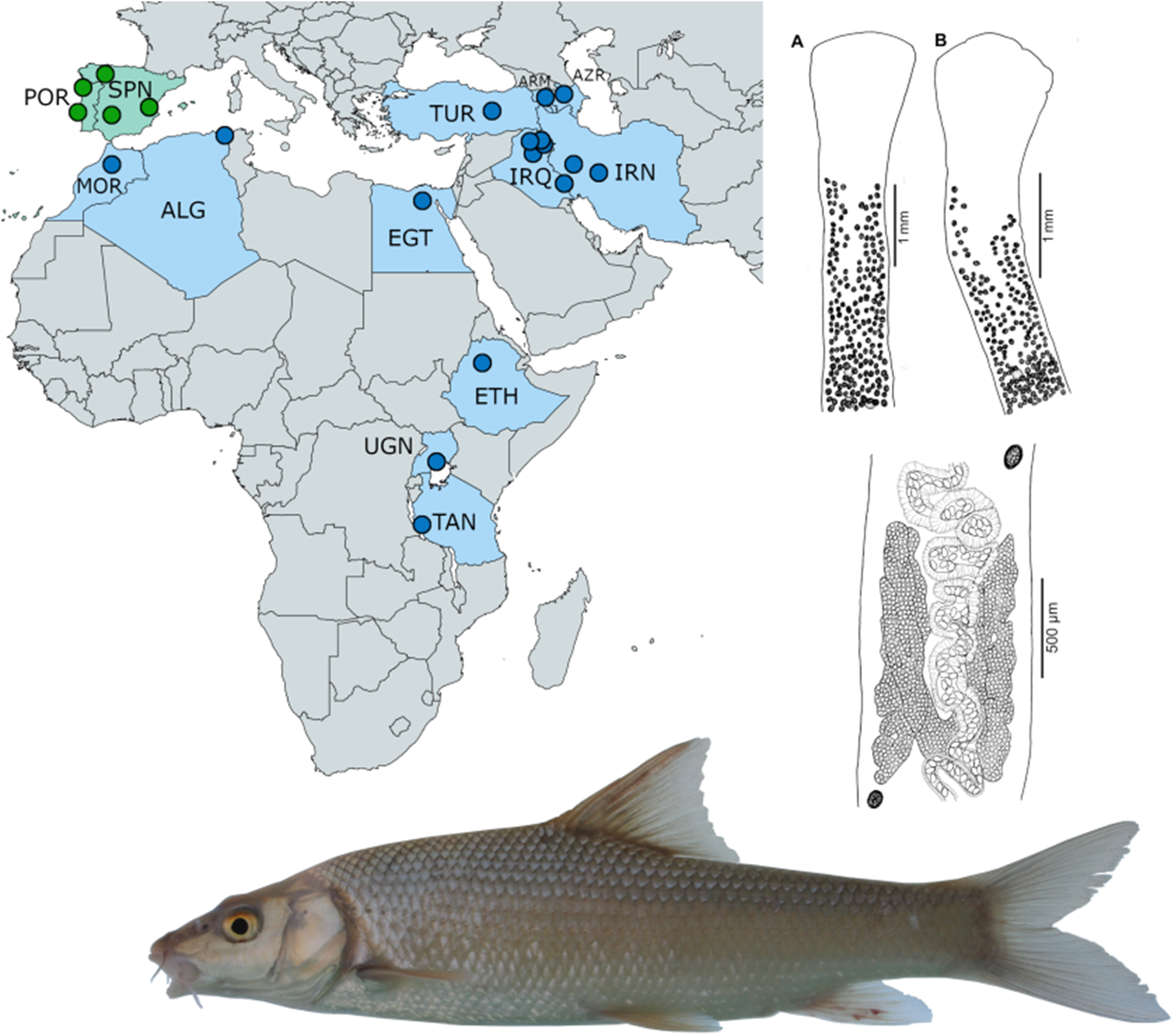
The occurrence of K. armeniaca s. lat. (or ‘K. armeniaca’; in previous literature referred to as K. armeniaca, nonetheless without molecular confirmation) in sub-Saharan Africa, specifically in Lake Tana, is remarkable and adds fish of the species Labeobarbus intermedius and L. tsanensis as definitive hosts (Scholz et al., Reference Scholz, Brabec, Kráľová-Hromadová, Oros, Bazsalovicsová, Ermolenko and Hanzelová2011; Kibet et al., Reference Kibet, Wen-Ting, Huda and Pin2021). According to previous morphological data (see Scholz et al., Reference Scholz, Brabec, Kráľová-Hromadová, Oros, Bazsalovicsová, Ermolenko and Hanzelová2011), the distribution of ‘K. armeniaca’ also encompasses other equatorial African countries (i.e. Tanzania and Uganda), further highlighting the disjunctive distribution of this species (see also Figure 9). As was previously hypothesized (e.g. Tang et al., Reference Tang, Getahun and Liu2009; Yang and Mayden, Reference Yang and Mayden2010; Yang et al., Reference Yang, Sado, Hirt, Pasco-Viel, Arunachalam, Li, Wang, Freyhof, Saitoh, Simons, Miya, He and Mayden2015) cyprinids (specifically labeonins and labeonin-like species) migrated into Africa via the historical connections with the Middle East and then through the Nile basin dispersed into other river systems. As these fish species appear to be suitable hosts for Khawia cestodes, it is likely that their dispersal patterns are intertwined, considering the north-African presence of ‘K. armeniaca’ in Egypt (Scholz et al., Reference Scholz, Brabec, Kráľová-Hromadová, Oros, Bazsalovicsová, Ermolenko and Hanzelová2011). It is therefore reasonable to assume that the African ‘K. armeniaca’ lineage will also be present in other eastern African countries. Nonetheless, understanding the phylogenetic relationship of this entirely separate lineage is again hindered by the lack of genetic data and the limited material available for morphological analysis in this study.
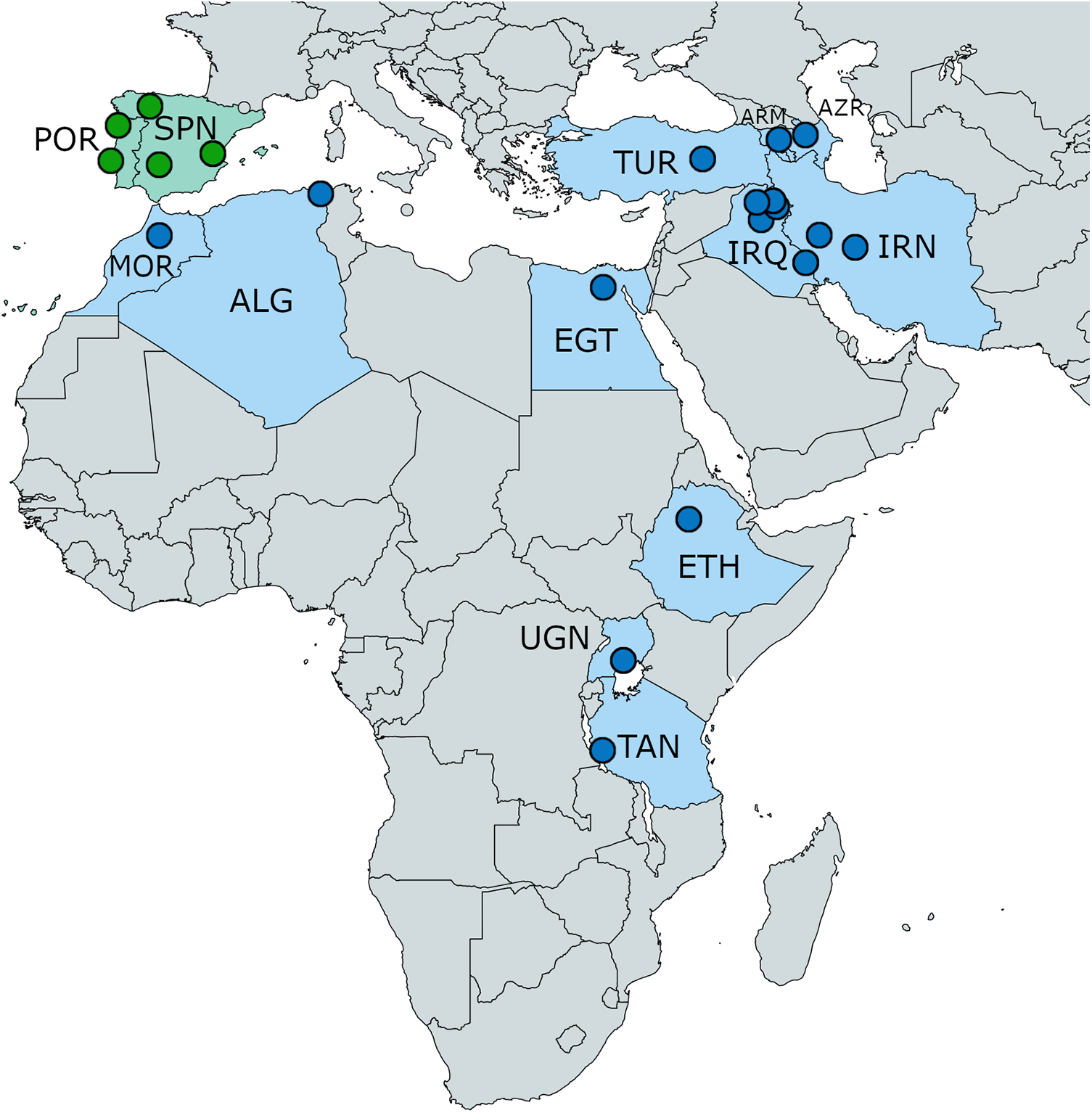
Figure 9. Distribution of Khawia armeniaca s. lat. (in blue colour) and Khawia iberica n. sp. (in green) based on Popov (Reference Popov1924), Özdemir and Sarieyyüpoğlu (Reference Özdemir and Sarıeyyüpoğlu1993), Abderrafik et al. (Reference Abderrafik, Khedidja, Amel, Eddine and Smail2010), Scholz et al. (Reference Scholz, Brabec, Kráľová-Hromadová, Oros, Bazsalovicsová, Ermolenko and Hanzelová2011) and present study. Abbreviations: ALG, Algeria; ARM, Armenia; AZR, Azerbaijan; EGT, Egypt; ETH, Ethiopia; IRQ, Iraq; IRN, Iran; MOR, Morocco; POR, Portugal; SPN, Spain; TAN, Tanzania; TUR, Turkey; UGN, Uganda.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0031182025100206.
Data availability statement
The data supporting the conclusions of this study are included in the article. All new DNA sequences of Khawia armeniaca and K. iberica n. sp. obtained during this study were deposited into the GenBank and are available under accession numbers PV558914–PV558951. Type material of Khawia iberica n. sp. was deposited in IPCAS under voucher number IPCAS C-48/6 and IPCAS C-1014, and in NHMUK under voucher number NHMUK 2025.6.9.1.
Acknowledgements
The following persons kindly provided specimens for the present study or helped in their sampling: Shamall Abdullah, Suncus N. Al-Kalak, Samir Bilal (all Iraq), Larisa Poddubnaya, Alexander Zhokhov (Russia), Aurelia Saraiva (Portugal), Andrea Vetešníková Šimková, Kateřina Čermáková, Maria Lujza Červenka Kičinja, Tomáš Pakosta (all Czech Republic). Roman Kuchta (Czech Republic) provided SEM micrographs of Khawia tapeworms (Figure 6) and prepared Figure 3 with Blanka Škoríková (Czech Republic).
Author contributions
T.S. and M.B. conceived and designed the study and collected parasitological material. K.K. stained and mounted the specimens, when necessary, conducted molecular analyses and provided morphological measurements. T.S. provided line drawings and differential diagnosis. M.B. performed phylogenetic analysis. T.S., M.B. and KK wrote the respective parts of the draft of the manuscript. All authors read and approved the final version of the manuscript.
Financial support
The field sampling for this study was supported by the Czech Science Foundation (20-13539S) and laboratory procedures by the Institute of Parasitology (RVO: 60077344) and Grant Agency of Ministry of Education, Research, Development and Youth of the Slovak Republic VEGA, project no. 1/0583/22.
Competing interests
The authors declare no conflicts of interest.
Ethical standards
All applicable institutional, national and international guidelines for the care and use of animals were followed.