Introduction
Palmer amaranth is the most troubling weed in domestic cotton production (WSSA 2017). Morgan et al. (Reference Morgan, Baumann and Chandler2001) reported populations as low as 10 plants 9 m−1 of row reduced cotton yield by up to 54%. Similarly, Rowland et al. (Reference Rowland, Murray and Verhalen1999) reported 8 weeds 10 m−1 of row at one site-year led to a 92% reduction in cotton lint yield. The development of Palmer amaranth with resistance to glyphosate has caused producers to shift to high utilization of herbicides with alternative modes of action (MOAs), notably glufosinate and protoporphyrinogen oxidase (PPO) inhibitors (Sosnoskie and Culpepper Reference Sosnoskie and Culpepper2014). Palmer amaranth populations with resistance to PPO inhibitors have already been identified (Heap Reference Heap2019; Salas et al. Reference Salas, Burgos, Tranel, Singh, Glasgow, Scott and Nichols2016).
The use of herbicide mixtures with multiple MOAs can help mitigate the development of herbicide resistance (Beckie and Reboud Reference Beckie and Reboud2009; Gressel and Segel Reference Gressel and Segel1990; Norsworthy et al. Reference Norsworthy, Ward, Shaw, Llewellyn, Nichols, Webster, Bradley, Friswold, Powles, Burgos, Witt and Barrett2012). Combinations of glyphosate and glufosinate with auxin herbicides such as 2,4-D or dicamba can effectively control glyphosate-resistant (GR) weeds, including Palmer amaranth (Cahoon et al. Reference Cahoon, York, Jordan, Everman, Seagroves, Culpepper and Eure2015b; Chahal and Johnson Reference Chahal and Johnson2012; Inman et al. Reference Inman, Jordan, York, Jennings, Monks, Everman, Bollman, Fowler, Cole and Soteres2016; Merchant et al. Reference Merchant, Sosnoskie, Culpepper, Steckel, York, Braxton and Ford2013, Reference Merchant, Culpepper, Eure, Richburg and Braxton2014; Meyer et al. Reference Meyer, Norsworthy, Young, Steckel, Bradley, Johnson, Loux, Davis, Kruger, Bararpour, Ikley, Spaunhorst and Butts2015; Vann et al. Reference Vann, York, Cahoon, Buck, Askew and Seagroves2017). However, while glufosinate, glyphosate, and dicamba are labeled for use in XtendFlex® (Bayer, Whippany, NJ 07981), cotton (Behrens et al. Reference Behrens, Mutlu, Chakraborty, Dumitru, Jiang, LaVallee, Herman, Clemente and Weeks2007; Feng and Brinker Reference Feng and Brinker2014), the area utilizing this technology may already contain GR weeds. Furthermore, Palmer amaranth exhibits a wide emergence window in the southern United States (Jha and Norsworthy Reference Jha and Norsworthy2009), which can increase selection pressure on postemergence herbicides if multiple weed generations are present at sequential applications (Norsworthy et al. Reference Norsworthy, Ward, Shaw, Llewellyn, Nichols, Webster, Bradley, Friswold, Powles, Burgos, Witt and Barrett2012). Additional herbicide MOAs should be included with dicamba to help mitigate resistance development (Norsworthy et al. Reference Norsworthy, Ward, Shaw, Llewellyn, Nichols, Webster, Bradley, Friswold, Powles, Burgos, Witt and Barrett2012).
Residual herbicides are recommended and increasingly used in weed control programs along with postemergence herbicides (Neve et al. Reference Neve, Norsworthy, Smith and Zelaya2011; Norsworthy et al. Reference Norsworthy, Ward, Shaw, Llewellyn, Nichols, Webster, Bradley, Friswold, Powles, Burgos, Witt and Barrett2012; Sosnoskie and Culpepper Reference Sosnoskie and Culpepper2014). Multiple applications of residual herbicides can contribute to control of species such as Palmer amaranth and common waterhemp [Amaranthus tuberculatus (Moq.) Sauer] (Everman et al. Reference Everman, Clewis, York and Wilcut2009; Meyer et al. Reference Meyer, Norsworthy, Young, Steckel, Bradley, Johnson, Loux, Davis, Kruger, Bararpour, Ikley, Spaunhorst and Butts2015; Steckel et al. Reference Steckel, Sprague and Hager2002). Chloroacetamide herbicides such as S-metolachlor and acetochlor are Group 15 herbicides that function by inhibiting the biosynthesis of very-long-chain fatty acids (Senseman Reference Senseman2007a, Reference Senseman2007b) and can be used both preemergence and postemergence in cotton. The fate of acetochlor and S-metolachlor in the soil depends largely on weather and soil conditions and includes microbial degradation in the soil and photodegradation in the case of S-metolachlor (Senseman Reference Senseman2007a, Reference Senseman2007b).
S-metolachlor is efficacious in controlling weed emergence and is a viable residual option for mixing with dicamba. Geier et al. (Reference Geier, Stahlman and Frihauf2006) reported up to 94% control of Palmer amaranth at 75 d after preemergence in corn (Zea mays L.) by 1,420 g S-metolachlor ha−1 and Steele et al. (Reference Steele, Porpiglia and Chandler2005) observed 98% control of Palmer amaranth at 9 wk after preemergence application of S-metolachlor in corn. Similarly, Clewis et al. (Reference Clewis, Wilcut and Porterfield2006) found the addition of S-metolachlor co-applied with glyphosate early postemergence improved control of Palmer amaranth by up to 47%. At one site-year, Whitaker et al. (Reference Whitaker, York, Jordan and Culpepper2011) reported S-metolachlor increased late-season control of Palmer amaranth by up to 11% when co-applied with glyphosate and by up to 8% when co-applied with glufosinate relative to glyphosate or glufosinate alone, respectively. S-metolachlor may also help protect crop yields. Clewis et al. (Reference Clewis, Miller, Koger, Baughman, Price, Porterfield and Wilcut2008) observed a 5% to 9% increase in control of various annual broadleaf weeds and a 420 kg ha−1 increase in cotton lint yield following co-application of S-metolachlor with glyphosate early postemergence as opposed to glyphosate alone. Similarly, Whitaker et al. (Reference Whitaker, York, Jordan and Culpepper2010) reported 7% and 6% greater control of Palmer amaranth at 30 and 90 d after postemergence and 7% greater soybean [Glycine max (L.) Merr.] yield following use of S-metolachlor compared with pendimethalin.
Similarly, acetochlor and combinations of acetochlor and postemergence herbicides demonstrate efficacy in controlling Palmer amaranth. Cahoon et al. (Reference Cahoon, York, Jordan, Everman, Seagroves, Culpepper and Eure2015b) reported 13% to 17% greater early-season Palmer amaranth control when dicamba was co-applied preemergence with acetochlor compared with acetochlor alone. In a bare ground study, Meyer et al. (Reference Meyer, Norsworthy, Young, Steckel, Bradley, Johnson, Loux, Davis, Kruger, Bararpour, Ikley, Spaunhorst and Butts2015) reported 80% control of GR Palmer amaranth at 6 to 7 wk after application (WAA) preemergence by acetochlor and dicamba alone and numerically improved control at 3 to 4 WAA late postemergence by treatments that contained 2,4-D or dicamba compared with those that did not. Wiggins et al. (Reference Wiggins, Hayes and Steckel2016) documented reduced Palmer amaranth density at 28 d after preemergence (DAPRE) following application of acetochlor relative to fluometuron or no herbicide in a study on GR Palmer amaranth control by cover crops and herbicides. Steckel et al. (Reference Steckel, Sprague and Hager2002) described improved season-long control of common waterhemp in corn by encapsulated acetochlor applied preemergence relative to acetochlor EC or S-metolachlor, although this did not result in statistically different grain yields. However, acetochlor may also help protect crop yields. Manuchehri et al. (Reference Manuchehri, Dotray and Keeling2017) found co-application of acetochlor with glufosinate early postemergence in Enlist™ cotton (Corteva AgriSciences, Indianapolis, IN 46268) resulted in an 889 kg ha−1 increase in seed cotton yield at one site-year relative to an early postemergence application of glufosinate alone.
One component of stewarding dicamba as a weed control tool is maximizing efficacy by mixing with residual herbicides. To determine the optimal application timing of chloroacetamide herbicides in a dicamba-based cotton production system, research was conducted to evaluate Palmer amaranth and cotton response to applications of S-metolachlor or acetochlor with dicamba at preemergence, preemergence fb early postemergence, preemergence fb late postemergence, early postemergence, late postemergence, and early postemergence fb late postemergence timings.
Materials and Methods
Research was conducted in Mississippi in 2017 and 2018 to investigate the effect of application timing of S-metolachlor and acetochlor for control of GR Palmer amaranth in dicamba-resistant cotton production. Four site-years of research were conducted from 2017 to 2018 in Dundee and Robinsonville, MS. Location, soil, herbicide application, and harvest date information are displayed in Table 1. The cotton cultivar ‘DP1725B2XF’ (DeltaPine®, Bayer, resistant to glyphosate, dicamba, and glufosinate) was seeded at 114,000 and 111,000 seeds ha−1 at a 2.5-cm depth in Robinsonville and Dundee, respectively. A two by six factorial arrangement of treatments in a randomized complete block with four replicates and a nontreated control (NTC) was utilized. Plots measured 12.2 m in length and consisted of four 97-cm rows. Seedbeds were prepared with conventional fall tillage and raised bed cultivation. Nitrogen fertilizer was applied as 28% or 32% urea ammonium nitrate at approximately 56 kg N ha−1 to 2- to 3-leaf cotton and again at 6- to 8-leaf cotton for a total of approximately 112 kg N ha−1 each year. Plots were managed according to local production recommendations from the Mississippi State University Extension Service (Anonymous 2018). This research was conducted under dryland production systems.
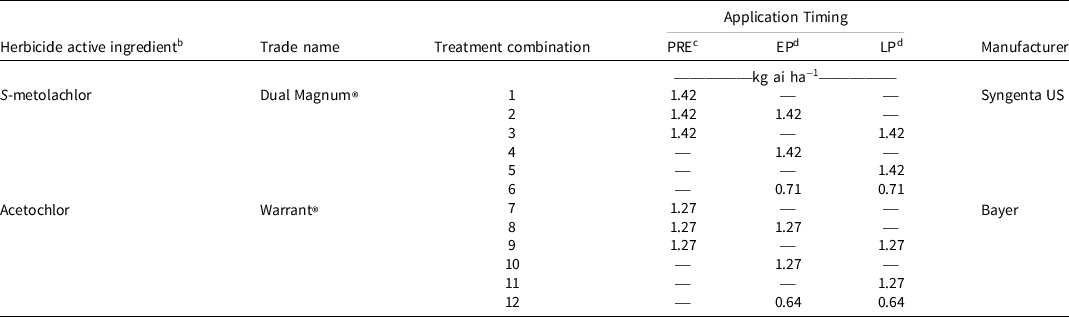
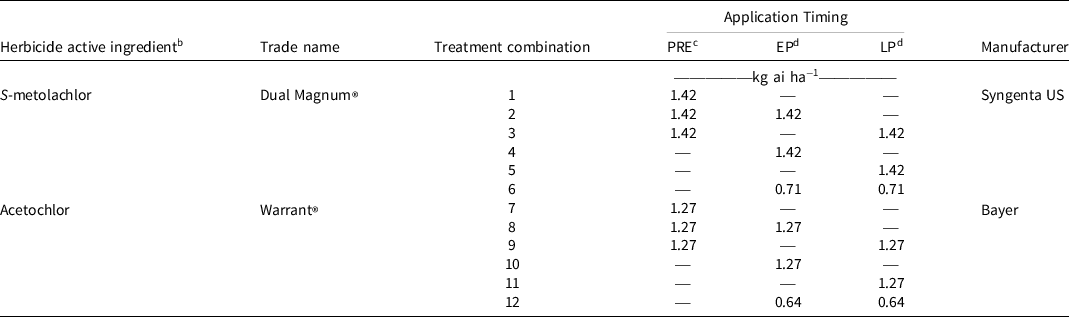
Table 1. Location, year, coordinates, elevation, soil type, planting and harvest dates, and application information for each experimental site-year used to investigate chloroacetamide herbicide timing in a dicamba-based weed control system in cotton production in Dundee and Robinsonville, MS, in 2017 and 2018. a

a Abbreviations: PRE, preemergence; EP, early postemergence; ht, height; LP, late postemergence.
b Source: USDA-NRCS (2021a).
c PRE applications occurred immediately after planting on the same day to a clean field.
d EP applications were made to cotton at the 3- to 4-leaf growth stage.
e LP applications were made to cotton at the pinhead square growth stage.
Chloroacetamide herbicide was either S-metolachlor (Dual Magnum®, Syngenta US, Greensboro, NC 27419) or acetochlor (Warrant®, Bayer Corporation). Application timings were: (1) preemergence immediately following planting, (2) preemergence followed by (fb) early postemergence at 3- to 4-leaf cotton (Ritchie et al. Reference Ritchie, Bednarz, Jost and Brown2004), (3) preemergence fb late postemergence at pinhead square cotton (Ritchie et al. Reference Ritchie, Bednarz, Jost and Brown2004), (4) early postemergence alone, (5) late postemergence alone, and (6) sequential postemergence. S-metolachlor applied preemergence or once postemergence (early postemergence or late postemergence) was applied at 1.42 kg ai ha−1, and acetochlor applied at these timings was applied at 1.27 kg ai ha−1 (Table 2). A concentration of 0.71 kg ha−1 S-metolachlor was utilized for each application in the sequential postemergence (early postemergence fb late postemergence) treatment (1.42 kg ha−1 total). Acetochlor was applied at a rate of 0.64 kg ha−1 per application for the sequential postemergence treatment (Table 2) and 0.56 kg ae ha−1 dicamba (XtendiMax®, Bayer) was included with all preemergence applications, and 1.12 kg glyphosate ae ha−1 plus 0.56 kg dicamba ae ha−1 (an experimental premix formulation) was included with all postemergence applications. A single application of 0.66 kg ai ha−1 glufosinate (Liberty® 280 SL, BASF, Florham Park, NJ 07932) was made to all plots at 28 d after late postemergence following data collection to prevent late-emerging Palmer amaranth from disrupting mechanical harvest (Morgan et al. Reference Morgan, Baumann and Chandler2001; Smith et al. Reference Smith, Baker and Steele2000). All chloroacetamide herbicide applications were made using a spray boom with TTI11002 spray tips (TTI, TurboTee Induction, TeeJet® Technologies, Glendale Heights, IL 60139) held 51 cm above the crop canopy. Herbicides were applied with a CO2-pressurized plot backpack sprayer at 4.8 km h−1 calibrated to deliver 140 L ha−1 at 276 kPa to the center two rows of each plot. Climate data by site-year are displayed as precipitation and temperature accumulation totals in Table 3. Air temperature accumulation values are presented as growing degree days (GDD). GDD were calculated via the formula:
where T max is the maximum daily air temperature, T min is the minimum daily air temperature, and T b is 16 C, the base threshold temperature for cotton (Reddy et al. Reference Reddy, Hodges, McCarty and McKinion1996).
Table 2. Concentrations of acetochlor or S-metolachlor applied PRE, EP, or LP immediately after planting, to 3- to 4-leaf cotton, or to pinhead square cotton, respectively, in experiments at Dundee and Robinsonville, MS, in 2017 and 2018. a
a Abbreviations: PRE, preemergence immediately after planting; EP, early postemergence to 3- to 4-leaf cotton; LP, late postemergence to pinhead square cotton.
b An application of 0.66 kg ai ha−1 glufosinate (Liberty® 280 SL, BASF) was made to all plots at 28 d after LP to facilitate harvest.
c All PRE applications included 0.56 kg ae ha−1 dicamba (XtendiMax®, Bayer).
d All postemergence applications included 1.12 kg glyphosate ae ha−1 plus 0.56 kg dicamba ae ha−1 (an experimental premix formulation).
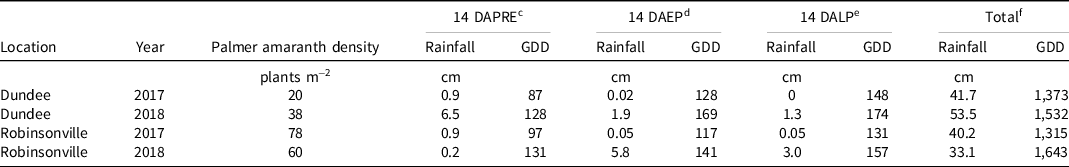
Table 3. Native early-season Palmer amaranth density and precipitation and heat accumulation totals 14 d after each application and season-long for each experiment conducted in Dundee and Robinsonville, MS, in 2017 and 2018. a, b
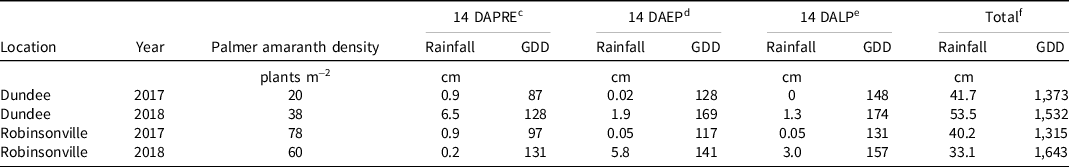
a Abbreviations: 14 DAPRE, 14 d after preemergence application made immediately following planting; 14 DAEP, 14 d after early postemergence application to cotton at the 3- to 4-leaf growth stage; 14 DALP, 14 d after late postemergence application to cotton at the pinhead square growth stage; GDD, growing degree days (calculated by subtracting 16 from the average daily temperature).
b Source: USDA-NRCS (2021b). Data for September 30, 2017, and September 30 and November 13, 2018, excluded due to suspected instrument error.
c Precipitation and GDD data in this section are cumulative totals from the 14 d immediately following PRE herbicide application immediately following planting.
d Precipitation and GDD data in this section are cumulative totals from the 14 d immediately following EP herbicide application to 3- to 4-leaf cotton.
e Precipitation and GDD data in this section are cumulative totals from the 14 d immediately following LP herbicide application to pinhead square cotton.
f Precipitation and GDD data in this section are cumulative totals from the entire growing season between the PRE application and harvest dates shown in Table 1.
Each site contained a native GR Palmer amaranth population with an estimated incidence of glyphosate resistance of 90%. Early-season native Palmer amaranth density for each site is shown in Table 3. Estimates of visible crop injury and Palmer amaranth control were made weekly (±4 d) up to 28 d after treatment (DAT) except for Robinsonville 2018, where weed control was recorded to 21 d after late postemergence (DALP) and crop injury to 16 DALP. Only 28 DAT data are presented herein, except for visible cotton injury reported at 14 DAT. The visible weed control and cotton injury evaluation scale ranged from 0 (no visible injury) to 100 (complete plant death) relative to the NTC (Frans et al. Reference Frans, Talbert, Marx, Crowley and Camper1986). Palmer amaranth density was recorded by counting all live plants in a randomly selected 0.25-m2 section of each plot at 28 ± 4 DALP. Late-season Palmer amaranth biomass was harvested at 28 ± 4 DALP from a randomly selected 0.25-m2 section of each plot by cutting all plants at ground level. Fresh biomass samples were then placed in a 40 C forced-air oven for 48 h, and dry biomass weights were recorded. Cotton height was recorded at 28 ± 4 DALP by randomly selecting six plants from the two center rows of each plot. Thidiazuron at 110 g ai ha−1 plus 1,680 g ai ha−1 ethephon was applied as a harvest aid defoliant and boll-opener when approximately 60% of cotton bolls in the NTC were open. Seed cotton yield (the total machine-harvested weight of cotton lint with seed; pre-ginning) was harvested using a two-row plot picker.
All data were analyzed with SAS 9.4 (SAS Institute, Cary, NC 27513). An ANOVA was conducted on visible cotton injury and height, seed cotton yield, and Palmer amaranth density, biomass, and visible control data using the PROC MIXED procedure. Means were separated using Fisher’s protected LSD at the α = 0.05 level of significance, and mean separations were visualized with the SAS 9.4 pdmix800 macro (Saxton Reference Saxton1998). The NTC was included in the field experiments for comparison purposes but was not included in the analyses. Year and location were combined as environment, and within each environment a randomized complete block design was run to provide four replicates per treatment combination of chloroacetamide herbicide and application timing. Environment was included as a random effect in the model (Blouin et al. Reference Blouin, Webster and Bond2011; Carmer et al. Reference Carmer, Nyquist and Walker1989; Yang Reference Yang2010), and replicates were nested within environment. Thus, all data were pooled over environment, and chloroacetamide herbicide and application timing were analyzed as fixed effects.
Results and Discussion
Visible Control, Weed Density, and Biomass of Palmer Amaranth
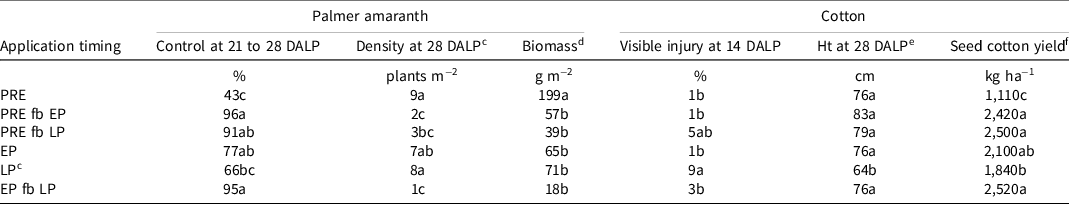
No interaction between chloroacetamide type and application timing was observed for any parameter analyzed (data not shown). Palmer amaranth control at 21 to 28 DALP and density at 28 DALP were affected by chloroacetamide application timing (P = 0.0024 and 0.0045, respectively). Maximum Palmer amaranth control at 21 to 28 DALP was achieved by any sequential chloroacetamide application or by early postemergence application alone (Table 4), although control following application early postemergence alone was numerically reduced relative to sequential applications. Similarly, Cahoon et al. (Reference Cahoon, York, Jordan, Everman, Seagroves, Culpepper and Eure2015b) reported improved control of Palmer amaranth following two applications of dicamba compared with one in a glyphosate-based system (each postemergence application in this experiment included dicamba plus glyphosate). The magnitude of visible control (91% to 96%) observed following sequential chloroacetamide application was consistent with previous research (Cahoon et al. Reference Cahoon, York, Jordan, Everman, Seagroves, Braswell and Jennings2015a; Steele et al. Reference Steele, Porpiglia and Chandler2005). Single postemergence applications resulted in 66% to 77% control, and a preemergence-only application provided only 43% control at 21 to 28 DALP (Table 4).
Table 4. Effect of chloroacetamide application timing on Palmer amaranth visible control, density, and biomass and cotton visible injury and height at 28 d after LP and seed cotton yield averaged over chloroacetamide herbicide from studies conducted in Dundee and Robinsonville, MS, in 2017 and 2018. a, b

a Abbreviations: DALP, days after late postemergence application to pinhead square cotton; EP, early postemergence application to 3- to 4-leaf cotton; fb, followed by; ht, cotton height; LP, late postemergence application to pinhead square cotton; PRE, preemergence application immediately following planting.
b Means within a column that share a letter are similar according to Fisher’s protected LSD (α = 0.05). Data are averaged over chloroacetamide herbicide.
c The mean Palmer amaranth density at 28 DALP in the nontreated control was 21 plants m−2.
d The mean late-season Palmer amaranth biomass in the nontreated control was 346 g m−2.
e The mean cotton height of the nontreated control was 53 cm.
f The mean seed cotton yield of the nontreated control was 160 kg ha−1.
Palmer amaranth density at 28 DALP was minimized by all sequential application timings: preemergence fb early postemergence, preemergence fb late postemergence, or early postemergence fb late postemergence (Table 4). Similarly, Meyer et al. (Reference Meyer, Norsworthy, Young, Steckel, Bradley, Johnson, Loux, Davis, Kruger, Bararpour, Ikley, Spaunhorst and Butts2015) reported 78% and 89% reductions in Palmer amaranth density relative to an NTC at 3 to 4 wk after late postemergence following applications of dicamba plus acetochlor preemergence fb dicamba plus glyphosate plus S-metolachlor early postemergence or late postemergence, respectively. Steckel et al. (Reference Steckel, Sprague and Hager2002) also observed reduced common waterhemp density in corn at 56 d after planting when sequential herbicide applications were made instead of preemergence alone. It is intuitive that postemergence applications containing dicamba plus glyphosate would lead to improved late-season control of emerged Palmer amaranth. However, dicamba has also been shown to provide some residual weed control. Norsworthy et al. (Reference Norsworthy, McClelland and Griffith2009) observed residual control of horseweed [Conyza canadensis (L.) Cronquist] emergence by addition of dicamba to glufosinate applied preplant. Similarly, Underwood et al. (Reference Underwood, Soltani, Hooker, Robinson, Vink, Swanton and Sikkema2017) reported improved late-season control of several species, including redroot pigweed (Amaranthus retroflexus L.) in soybean, by applications of glyphosate and dicamba relative to glyphosate alone. The residual control provided by dicamba may have played a role along with chloroacetamide activity in improved late-season Palmer amaranth control in the present study. Previous research by Cahoon et al. (Reference Cahoon, York, Jordan, Everman, Seagroves, Culpepper and Eure2015b) documented a 13% to 17% increased control at 18 to 23 d after planting following preemergence application of dicamba plus acetochlor relative to acetochlor alone. In Georgia, late-season Palmer amaranth density was reduced by including dicamba preemergence in glufosinate and glyphosate systems (Cahoon et al. Reference Cahoon, York, Jordan, Everman, Seagroves, Culpepper and Eure2015b).
Late-season Palmer amaranth dry biomass was affected by chloroacetamide application timing (P = 0.0002). Biomass ranged from 18 to 199 g m−2 and was minimized by any application timing other than preemergence alone (Table 4). While all application timings other than preemergence alone resulted in similar Palmer amaranth biomass, sequential applications (preemergence fb early postemergence, preemergence fb late postemergence, and early postemergence fb late postemergence) trended numerically less than those that included only a single application (early postemergence or late postemergence alone; Table 4), and these numeric trends may become more pronounced with heavier Palmer amaranth infestations or in scenarios in which a timely postemergence application cannot be made.
Cotton Response
While no interaction of main effects was detected for mean visible cotton injury at 14 DALP (data not shown), application timing (P = 0.0318) did affect cotton response and is shown in Table 4. However, injury severity was minor (<10%) and transient, consistent with previous research with chloroacetamide herbicides applied alone or in various mixtures (Cahoon et al. Reference Cahoon, York, Jordan, Everman, Seagroves, Braswell and Jennings2015a, Reference Cahoon, York, Jordan, Everman, Seagroves, Culpepper and Eure2015b; Clewis et al. Reference Clewis, Wilcut and Porterfield2006; Stephenson et al. Reference Stephenson, Bond, Landry and Edwards2013; Whitaker et al. Reference Whitaker, York, Jordan and Culpepper2011).
No interaction of main effects was detected for cotton height or seed cotton yield (data not shown). Cotton height at 28 DALP and seed cotton yield were each affected by chloroacetamide application timing (P = 0.003 and 0.0006, respectively). Cotton height was maximized following chloroacetamide application at any timing other than late postemergence alone (Table 4), which resulted in up to a 23% reduction. Stephenson et al. (Reference Stephenson, Bond, Landry and Edwards2013) reported no greater than 5% cotton height reduction at 21 and 42 DAT following postemergence S-metolachlor application, although these reductions were not accompanied by reductions in yield. Likewise, the low magnitude of injury in this study suggests that the height reduction reported here likely reflects the deleterious effect of allowing weed–crop competition to continue unchecked until late postemergence as opposed to any lingering cotton injury due to herbicide application.
Cotton yield was maximized by all sequential application timings and by an early postemergence application alone (Table 4). As with the biomass, control, and density results, the numerically reduced yield following chloroacetamide application early postemergence alone may become more pronounced with heavier weed infestations or in scenarios in which timely postemergence application is not possible. The efficacy of chloroacetamide herbicides in protecting crop yield has been documented previously (Clewis et al. Reference Clewis, Miller, Koger, Baughman, Price, Porterfield and Wilcut2008; Manuchehri et al. Reference Manuchehri, Dotray and Keeling2017; Whitaker et al. Reference Whitaker, York, Jordan and Culpepper2010). It is important to note that plots that received the postemergence-only sequential application timing (early postemergence fb late postemergence) received half of the total chloroacetamide rate of the application timings involving sequential preemergence and postemergence applications (Table 2). Conversely, if this same concentration was applied in a single late postemergence application with no preemergence application, yield was reduced by up to 680 kg ha−1, or 27% (Table 4). This result supports existing recommendations for timely postemergence applications (Norsworthy et al. Reference Norsworthy, Ward, Shaw, Llewellyn, Nichols, Webster, Bradley, Friswold, Powles, Burgos, Witt and Barrett2012).
Significance of Findings for Cotton Management
Best weed management practices regarding herbicide use and resistance management should be observed when developing a dicamba-based weed control plan. One component of resistance management and herbicide stewardship is the use of multiple herbicide MOAs in a weed control plan (Norsworthy et al. Reference Norsworthy, Ward, Shaw, Llewellyn, Nichols, Webster, Bradley, Friswold, Powles, Burgos, Witt and Barrett2012). This research sought to optimize the use of chloroacetamide herbicides as one part of a more comprehensive dicamba-based weed control plan. Acetochlor and S-metolachlor each can provide residual control of broadleaf weeds, including Palmer amaranth and smooth pigweed (Amaranthus hybridus L.) (Clewis et al. Reference Clewis, Miller, Koger, Baughman, Price, Porterfield and Wilcut2008; Geier et al. Reference Geier, Stahlman and Frihauf2006; Steele et al. Reference Steele, Porpiglia and Chandler2005), and dicamba has been shown to provide residual control as well (Cahoon et al. Reference Cahoon, York, Jordan, Everman, Seagroves, Culpepper and Eure2015b; Norsworthy et al. Reference Norsworthy, McClelland and Griffith2009; Underwood et al. Reference Underwood, Soltani, Hooker, Robinson, Vink, Swanton and Sikkema2017).
To reduce weed–crop competition and selection pressure on postemergence herbicides such as dicamba or glufosinate, a sequential application of either S-metolachlor or acetochlor is advisable in the context of an overall weed control program containing multiple herbicide MOAs and the use of multiple residual herbicides. In this context, the sequential chloroacetamide application would preferably contain one application preemergence so that more total active ingredient can be applied in season as opposed to the reduced total amount of active ingredient being applied in a postemergence-only program due to label restrictions. Further, the necessary reduction in concentration of each application in a sequential postemergence application (early postemergence fb late postemergence) relative to a single postemergence or sequential preemergence fb postemergence application may increase selection pressure for herbicide resistance. Utilizing additional residual herbicides with different MOAs is recommended to reduce selection pressure that may result following sequential applications of herbicides of the same MOA (chloroacetamides in this case) (Norsworthy et al. Reference Norsworthy, Ward, Shaw, Llewellyn, Nichols, Webster, Bradley, Friswold, Powles, Burgos, Witt and Barrett2012). Forgoing a preemergence application of either herbicide in favor of an early postemergence-only or sequential postemergence-only application may have resulted in yields similar to those seen with applications that included both a preemergence and postemergence application in this study, but this approach risks inducing additional selection pressure on postemergence herbicides such as dicamba and glyphosate and increases the potential for weed escapes if timely applications cannot be made. Further, in heavy infestation scenarios, delaying initial chloroacetamide application until postemergence could lead to increased weed biomass at harvest, which can impede mechanical cotton harvest (Morgan et al. Reference Morgan, Baumann and Chandler2001; Smith et al. Reference Smith, Baker and Steele2000). Additionally, Sarangi et al. (Reference Sarangi, Sandell, Kruger, Knezevic, Irmak and Jhala2017) observed improved late-season control of common waterhemp, reduced weed density, and improved soybean yield following sequential applications of herbicide treatments preemergence fb postemergence relative to postemergence alone, evidence of the value of preemergence fb postemergence treatments as opposed to postemergence-only treatments.
Utilizing multiple postemergence applications of residual herbicides may also provide overlapping control of weed emergence, which is necessary for weeds with extended emergence windows such as Palmer amaranth. A comprehensive GR Palmer amaranth management program should feature the use of multiple residual herbicides preemergence, which has been demonstrated as an effective approach for controlling Palmer amaranth (Cahoon et al. Reference Cahoon, York, Jordan, Everman, Seagroves, Braswell and Jennings2015a). Future inquiry may further investigate the efficacy and timing of chloroacetamide herbicides in combination with other residual and postemergence herbicides in a dicamba-based weed control system, especially given the identification of S-metolachlor-resistant Palmer amaranth in Arkansas (Brabham et al. Reference Brabham, Norsworthy, Houston, Varanasi and Barber2019). Efforts may also focus on the timing of chloroacetamide application in a dicamba plus glufosinate system, as Cahoon et al (Reference Cahoon, York, Jordan, Everman, Seagroves, Culpepper and Eure2015b) reported less benefit in adding dicamba to a glufosinate-based system as opposed to a glyphosate-based system in North Carolina. Likewise, Manuchehri et al. (Reference Manuchehri, Dotray and Keeling2017) observed statistically similar Palmer amaranth control at 28 d after mid-postemergence with the addition of acetochlor to glufosinate early postemergence relative to glufosinate alone. Chloroacetamide application timing may be further investigated in a dicamba plus glufosinate–based weed control system.
Acknowledgments
This publication is a contribution of the Mississippi Agricultural and Forestry Experiment Station. Material is based on work partially supported by the National Institute of Food and Agriculture, U.S. Department of Agriculture, Hatch project under accession number 230090. The Monsanto Company also supported this project as part of the Will D. Carpenter Distinguished Field Scientist Graduate Assistantship program. The authors would like to thank all the undergraduate and graduate research assistants at Mississippi State University for their assistance in conducting this research, especially Graham Oakley. No conflicts of interest have been declared.