Introduction
Wild Mexican sunflower [Tithonia tubaeformis (Jacq.) Cass., Asteraceae] is a summer annual troublesome weed. Even though sugarcane (Saccharum spp. hybrid) is the most affected crop, with reported yield losses close to 40% (Chaila et al. Reference Chaila2002; Sánchez Ducca et al. Reference Sánchez-Ducca, Vargas, Sabaté, López and Romero2019), its presence also reduces soybean [Glycine max (L.) Merr.], sunflower (Helianthus annuus L.), and bean (Phaseolus vulgaris L.) yields in the northwest of Argentina. In addition, this species is also found in Brazil (Zepeda-Bastida et al. Reference Zepeda-Bastida, Martínez and Simental2019), Mexico (Ramos-Patlán et al. Reference Ramos-Patlán, Salas-Araiza, Hernández-Hernández, Núñez-Palenius, Salcedo-Hernandez and Guzmán-Mendoza2023), Central America (Mendoza-Ramírez et al. Reference Mendoza-Ramírez, Ayala-Martínez, Soto-Simental, Zepeda-Bastida, Ocampo-López and García-Vázquez2021), and Zambia (Witt et al. Reference Witt, Shackleton, Beale, Nunda and Van Wilgen2019). The seedlings emerge at the end of spring and throughout summer, while flowering and seed sets are observed by the end of fall and early in winter (Ortiz Reference Ortiz1991). In Argentina, T. tubaeformis was declared a noxious weed in 1983, and since then, all attempts to reduce the size of the weed population have failed (Sánchez-Ducca et al. Reference Sánchez-Ducca, Vargas, Sabaté, López and Romero2019). Some of the traits that define T. tubaeformis as a problematic weed are its capacity to have several seedling cohorts with rapid growth in the same season; the large size of adult plants; and the rapid dispersal of achenes by wind, agricultural machinery, and water (DeLoach and Cordo Reference DeLoach and Cordo1983; Del Vitto and Petenatti Reference Del Vitto and Petenatti2015; Tovar-Sánchez et al. Reference Tovar-Sánchez, Rodríguez-Carmona, Aguilar-Mendiola, Mussali-Galante, López-Caamal and Valencia-Cuevas2012). Nowadays, chemical strategies to manage T. tubaeformis seedling emergence in Argentina rely on the use of amicarbazone, isoxaflutole + indaziflam, and a mix of dicamba + atrazine or 2,4D + dicamba (Sanchez Ducca et. al. Reference Sánchez-Ducca, Vargas, Sabaté, López and Romero2019). However, based on the sparse knowledge of the seed biology and ecology of T. tubaeformis, there is a need to provide additional management methods to develop a more sustainable approach for controlling its occurrence.
For many Asteraceae species such as T. tubaeformis, the dispersal unit is the achene, whose tissues, that is, the pericarp, surround the seed. For these species, the hard outer tissue could cause a coat-imposed dormancy that can be broken by excising the embryo out of the seed or even just scarifying the seed-covering layers (Penfield Reference Penfield2017). For instance, in species such as crown daisy [Glebionis coronaria (L.) Cass.] and cardoon (Cynara cardunculus L. var. sylvestris), the pericarp mechanically restrains the growth of the embryo (Huarte et al. Reference Huarte, Borlandelli, Varisco and Batlla2018, Reference Huarte, Puglia, Prjibelski and Raccuia2020; Puglia et al. Reference Puglia, Grimaldi, Carta, Pavone and Toorop2015). Also, for seeds that are physiologically dormant to fully germinate, a certain ratio of the phytohormones abscisic acid (ABA) and gibberellins (GAs) needs to be met (Yan and Chen Reference Yan and Chen2020). In sunflower achenes, the removal of pericarp tissues should be accompanied by ABA/GA reduction to complete germination (Rodriguez et al. Reference Rodríguez, Bodrone, Castellari and Batlla2018). More recently, reactive oxygen species (ROS) have been proposed as a key players acting together with the ABA and GAs, or independently, to facilitate embryo growth or weaken restrictive physical layers (Bailly Reference Bailly2019; Puglia Reference Puglia2024). Currently, no scientific study has been conducted on the germination ecophysiology of T. tubaeformis, nor has the seed physiological dormancy in this species been explored.
After dispersion, alleviation of dormancy depends on the interaction of the seeds with field environmental factors, mainly soil temperature and soil moisture content (Finch-Savage and Footit Reference Finch-Savage and Footitt2017). Two different processes explain the progressive release from the dormancy state: cold stratification or chilling and drying after ripening (Née et al. Reference Née, Xiang and Soppe2017). Cold stratification, frequently found in summer annual species, such as T. tubaeformis, requires that hydrated seeds remain for a period from a few days to several weeks at low temperatures (e.g., 4 to 6 C) (Batlla and Benech-Arnold Reference Batlla and Benech-Arnold2015). In contrast, winter annual species lose their dormancy when exposed to low humidity for several weeks at temperatures close to 20 to 25 C in a process called dry afterripening (DAR) (Chantre et al. Reference Chantre, Batlla, Sabbatini and Orioli2009; Iglesias-Fernández and Matilla Reference Iglesias-Fernández and Matilla2009). Exposure of seeds to cold stratification or DAR progressively increase the range of thermal and osmotic conditions under which germination occurs (Batlla and Benech-Arnold Reference Batlla and Benech-Arnold2010). However, once the chilling or DAR process has elapsed, many species still need exposure to environmental cues such as light and alternating temperatures for dormancy breakage (Arana et al. Reference Arana, Tognacca, Estravis-Barcalá, Sánchez and Botto2017; Tognacca and Botto Reference Tognacca and Botto2021). Light and alternating temperatures are crucial signals providing spatial information, indicating the seed’s position in the soil, whether buried or at the surface, as well as the presence of an aboveground canopy (Finch-Savage and Footitt Reference Finch-Savage and Footitt2017). Once the right signals are received, a complex set of physiological processes involving hormone and ROS signaling triggers the seed’s outer layer weakening and the completion of germination (Puglia et al. Reference Puglia, Balestrasse, Bustos and Huarte2022).
Several agronomic practices, such as tillage, no-till, sowing density, and irrigation, can alter these crucial environmental signals, thereby reducing or promoting the emergence of weed seedlings and improving subsequent management (Anderson Reference Anderson2008; Benvenuti and Mazzoncini Reference Benvenuti and Mazzoncini2021). For instance, in alfalfa (Medicago sativa L.), Huarte and Benech-Arnold (Reference Huarte and Benech-Arnold2003) showed that cultivar selection and a high sowing rate reduced seedling emergence of several weed species, including spiny plumeless thistle (Carduus acanthoides L.) and bull thistle [Cirsium vulgare (Savi) Ten.], by reducing soil thermal amplitude, and in curly dock (Rumex crispus L.) and tumble mustard (Sisymbrium altissimum L.) by reducing the red/far-red (R/FR) ratio. Moreover, Kruk et al. (Reference Kruk, Insausti, Razul and Benech-Arnold2006) and Ustarroz et al. (Reference Ustarroz, Kruk, Satorre and Ghersa2016) reported that crop presence and crop residues may impede seed dormancy breakage by reducing light amount and quality (i.e., low R/FR ratio) and narrowing the range of alternating temperatures. Likewise, crop residue could act as a mechanical barrier to seedling emergence (Nikolić et al. Reference Nikolić, Loddo and Masin2021).
Sugarcane harvest in Tucumán (Argentina) is performed with integral machines. Also, the burning of residues is avoided. Therefore, a range from 7 to 18 t of dry matter (DM) ha−1 of crop residues remains on the soil surface. The amplitude of this range depends on several factors, such as the variety cultivated, the productive level of the field, the time of harvest, and the configuration of the harvester (Digonzelli et al. Reference Digonzelli, Romero, Alonso, Fernández de Ullivarri, Rojas Quinteros, Scandaliaris and Fajre2011, Reference Digonzelli, Fernández de Ullivarri, Medin, Tortora, Romero and Rojas-Quinteros2015; Romero et al. Reference Romero, Scandaliaris, Digonzelli, Alonso, Leggio, Giardina, Casen, Tonatto and Fernández de Ullivarri2009). Therefore, the identification of specific environmental factors that modulate germination response in T. tubaeformis can be used as a key component in integrated agronomic practice to limit its emergence in crop fields. In the current study, our objectives were to (1) investigate the germination behavior of T. tubaeformis and (2) assess the impact of crop residues on seedling emergence and biomass.
Materials and Methods
Achene Sources and Preparation
We collected T. tubaeformis achenes during the first half of June in 2019 and 2021. For field experiments in Las Talitas (Tafí Viejo, Tucumán, 26.7758°S, 65.2006°W, 2,000 m asl), the achene lots from the two years were used independently, while for laboratory experiments, only the 2021 lot was used. The sugarcane crop area in Tucumán has a monsoon hydric regime where approximately 80% of total annual rainfall occurs during summer and fall. The average mean rainfall is 1101.9 mm, and the mean average annual temperature is 19.5 C (Estación Experimental Agroindustrial Obispo Colombres 2016). To confirm the general physiological behavior of achene germination in the T. tubaeformis species, tests were also performed on a second population (population 2) that was collected in June 2021 in San Andrés (Tucumán, 26.8919°S, 65.1931°W, 406 m asl). Achene collection for both populations was carried out on 20 random plants and bulked to get experimental samples. Once collected, the achenes were cleaned and exposed to dehumidification airflow for 24 h to reach a moisture content of approximately 4% to 5% on a fresh weight basis. Thereafter, until the beginning of each germination test, achenes were kept for 3 mo at −18 C in closed jars filled to 50% with silica gel. The silica gel was replaced as soon as a color change was observed.
Fresh Achene Germination Test
Three technical replicates of 30 achenes each were used for all germination tests and carried out in controlled-temperature chambers for 21 d, with a second run started 1 mo later. Achenes were placed in 9-cm-diameter petri dishes over two filter papers (Double Ring #102, Hangzhou Special Paper Industry, Hangzhou, China) wetted with 7 ml of distilled water and placed in plastic bags (Ziploc™, Johnson & Johnson, New Brunswick, NJ, USA) to prevent dehydration. The experiment had a completely randomized design with two factors: (1) light with two levels, 0 and 12 h; and (2) temperature with four levels, 20, 25, 30, and 20/30 C. Light treatment was performed by exposing achenes for 12 h (photoperiod light/dark 12 h/12 h) to six fluorescent Philips 40/15 40-W lamps (Philips, Eindhoven, The Netherlands) to obtain a calculated proportion of phytochrome (Pfr form) of 0.87 (Casal et al. Reference Casal, Sanchez, Benedetto and De Miguel1991). Dark treatment was carried out by covering the petri dishes with two layers of aluminum foil. For the alternating-temperature condition, the photoperiod (light/dark 12 h/12 h) was synchronized with the thermoperiod (30/20 C). We performed all the treatments in independent germination chambers, each with its own unique combination of light and temperature. Germination in light treatments was scored daily, and then germinated achenes were removed from the petri dishes. Germination in the dark treatment was scored only at the end of the test (i.e., day 21). The viability of fresh achene lots was evaluated via the tetrazolium test (ISTA 2007). Briefly, achenes were placed on petri dishes and then hydrated in distilled water for 2 h and then transferred to a 1% solution of 2,3,5 triphenyl tetrazolium chloride (Sigma-Aldrich, St Louis, MO, USA) for 3 h in the 25 C germination chamber in the dark. Achenes that displayed a completely stained embryo were classified as viable.
Effect of GAs, ABA, Fluridone, Trinexapac-Ethyl (TE), and ROS Compounds on Achene Dormancy Breakage
To evaluate the effect of different compounds on germination, independent experiments were carried out using a completely randomized design with different levels corresponding to the different concentration of a given compound. Tests were performed using three technical replicates of 30 achenes each at 20/30 C in the presence of light, which was the condition that showed the best performance. The experiment was repeated 1 month later. All the treatments were performed in independent germination chambers as described earlier, and, for the second run, light/temperature combinations were re-randomized to avoid imposing the same treatment within the same experimental unit. We used gibberellin (GA3) (Sigma Chemical Company) or ABA (Sigma Chemical Company) to test the effect of ABA:GA ratio alteration. In addition, we used fluridone (an inhibitor of ABA biosynthesis) (PhytoTechnology Laboratories, Shawnee Mission, KS, USA) to determine whether the maintenance of primary dormancy in this species was related to de novo ABA synthesis or TE (an inhibitor of GA biosynthesis) (Moddus 250 EC, Syngenta, Basel, Switzerland) to show how GA affects the balance of hormones in this species. Treatments were: 0, 10, 25, 50, 100, and 150 µM for GA3; 0, 32.5, 62.5, and 125 µM for TE; 0, 1, 2.5, 5, and 25 µM for ABA; and 0, 10, 25, and 50 µM for fluridone. To evaluate the effect of ROS compounds on dormancy breakage, achenes were immersed for 3 h in methyl viologen (MV) (0, 0.1, 0.25, 0.5, and 1 mM), an ROS donor (El-Maarouf-Bouteau et al. Reference El-Maarouf-Bouteau, Sajjad, Bazin, Cristescu, Balzergue, Baudouin and Bailly2015; Huarte and Benech-Arnold Reference Huarte and Benech-Arnold2010)). Afterward, achenes were rinsed in water and imbibed at 20/30 C in the presence of light. To corroborate the germination response to MV scored in population 1, a complementary test was done on population 2 using 0.5 mM of MV, which was the concentration exhibiting the highest germination percentages.
Effect of Pericarp on Achene Germination
To test the coat permeability to water, 20 achenes were individually weighed from dry condition to constant weight with an electronic balance (Acculab ALC210A, Sartorius Group, Gottingen, Germany). Weight measurements were performed at 2, 4, 6, 8, 10, 16, and 24 h from achene imbibition. Water uptake (%) for any of the stated times was plotted in relation to dry achene weight. To evaluate other effects of achene cover layers on germination, both mechanical and chemical scarification tests were carried out. These tests had a completely randomized design (three technical replicates of 30 achenes each), and the germination data were analyzed by ANOVA. Mechanical scarification was performed by sanding down the covering layers with sandpaper (grade 200, Abrasivos Argentinos SAIC, Buenos Aires, Argentina) for 4, 8, 16, and 32 s. Chemical scarification was performed through achene imbibition in concentrated sulfuric acid (H2SO4, 98.0%; J.T. Baker®, Center Vally, PA, USA) for 0, 0.5, 1, 10, and 30 min and then washed in tap water and dried on blotting paper. Scarified achenes were placed on petri dishes in germination chambers set at 20/30 C (12-h photoperiod and thermoperiod). The effect of the complete removal of pericarp was investigated by isolating embryos through a scalpel with the help of a magnifier lens and imbibing them at 25 or 20/30 C and in the continuous dark or with 12 h of light. The experiment was carried out in independent germination chambers using three technical replicates of 30 achenes each with a completely randomized design including two factors: (1) light with two levels, 0 and 12 h; and (2) temperature with two levels, 25 and 20/30 C.
Effect of Cold Stratification and DAR on Dormancy Release
Fresh achenes were exposed to cold stratification or DAR. Cold stratification was performed by imbibing achenes in darkness in a cold room at 5 C (air humidity content: 40%). To rule out a possible emergence of T. tubaeformis after summer droughts, we performed DAR treatment by storing the achenes at 20 ± 2 C (air humidity content: 10%). Each experiment had a completely randomized design with three factors: (1) duration of treatment with four levels, 0, 3, 6, and 9 wk; (2) temperature with two levels, 25 C and 20/30 C; and (3) light with two levels, 0 and 12 h. Each of the treatments was carried out in independent germination chambers with a unique combination of light and temperature using three technical replicates of 30 achenes each, and all possible interactions were evaluated.
Field Experiments
Field experiments were conducted during October, November, and December of 2019 and 2021 at Las Talitas, Tucumán, Argentina (26.7886°S, 65.202°W). Before the setup of our assays, this site was used for several years for sugarcane cultivation, and then a 2-yr chemical fallow was performed to control weeds. The soil was a silty loam with a pH of 7.1 and 2.1% organic matter. Tithonia tubaeformis achenes were collected in June of the same year of each experiment. A research area of 20 m2 was cultivated two times using a rolling disk cultivator in mid-September. The monthly mean temperatures were 21.5 and 22.9 C for October 2019 and 2021, respectively; 24.8 and 22.8 C for November, respectively; and 25.4 and 26.1 C for December, respectively. The monthly rain totals were 25 and 23.6 mm for October 2019 and 2021, respectively; 201.4 and 133.6 mm for November of 2019 and 2021, respectively; and 72.1 and 119.9 for December 2019 and 2021, respectively. Air temperature was registered at 600 m of the experimental site using a weather station, Davis Vantage Pro II (Davis Instruments, Hayward, CA, USA) (Estación Meterológica El Colmenar, Tucumán, Argentina). In addition, to measure the effect of crop residues on soil temperatures, a sensor (Thermochron DB 21, iButtonsLinks, Whitewater, WI, USA) was buried 5 cm beneath the soil surface at the center of each plot in all treatments during October 2021. Sensor outputs were logged at 4-h intervals. This experiment had a randomized complete design with four repetitions and was set up during mid-October 2019 and 2021. In each 1-m2 plot, 50 T. tubaeformis achenes were placed on the soil surface, and then sugarcane residue (leaves and tops) was spread to simulate residue left from a cane harvester (3520, Industrias John Deere Argentina S.A., Santa Fe, Argentina) at 0, 5, 10, and 20 Mg ha−1, equivalent to 0.5-, 1-, and 2-fold the typical amount of sugarcane crop residue collected per hectare in the northwest of Argentina (Digonzelli et al. Reference Digonzelli, Romero, Alonso, Fernández de Ullivarri, Rojas Quinteros, Scandaliaris and Fajre2011; Romero et al. Reference Romero, Scandaliaris, Digonzelli, Alonso, Leggio, Giardina, Casen, Tonatto and Fernández de Ullivarri2009). Around each plot, a wood framework was placed to prevent achene loss. Sugarcane ‘LCP 85-334’ harvested with a moisture content of 4% to 6% was used to create the residue treatments. The experiment was carried out using a completely randomized complete block design with four replicates. Block was considered as an aleatory effect, while year (2019 and 2021) and amount of sugarcane residue (0, 5, 10 and 20 Mg ha−1) were considered fixed effects. All interactions were considered. Comparisons between years were performed by means of t-test and did not provide any significant variation; therefore, the year effect was not included as a source of variation in the ANOVA test.
Data Analyses
ANOVA evaluation was performed using the Infostat P software package (Balzarini et al. Reference Balzarini, Gonzalez, Tablada, Casanoves, Di Rienzo and Robledo2011). Means were separated by the Tukey test (α = 0.05). Figures were plotted using GraphPad Prism v. 8.01 (GraphPad Software, Boston, Massachusetts USA).
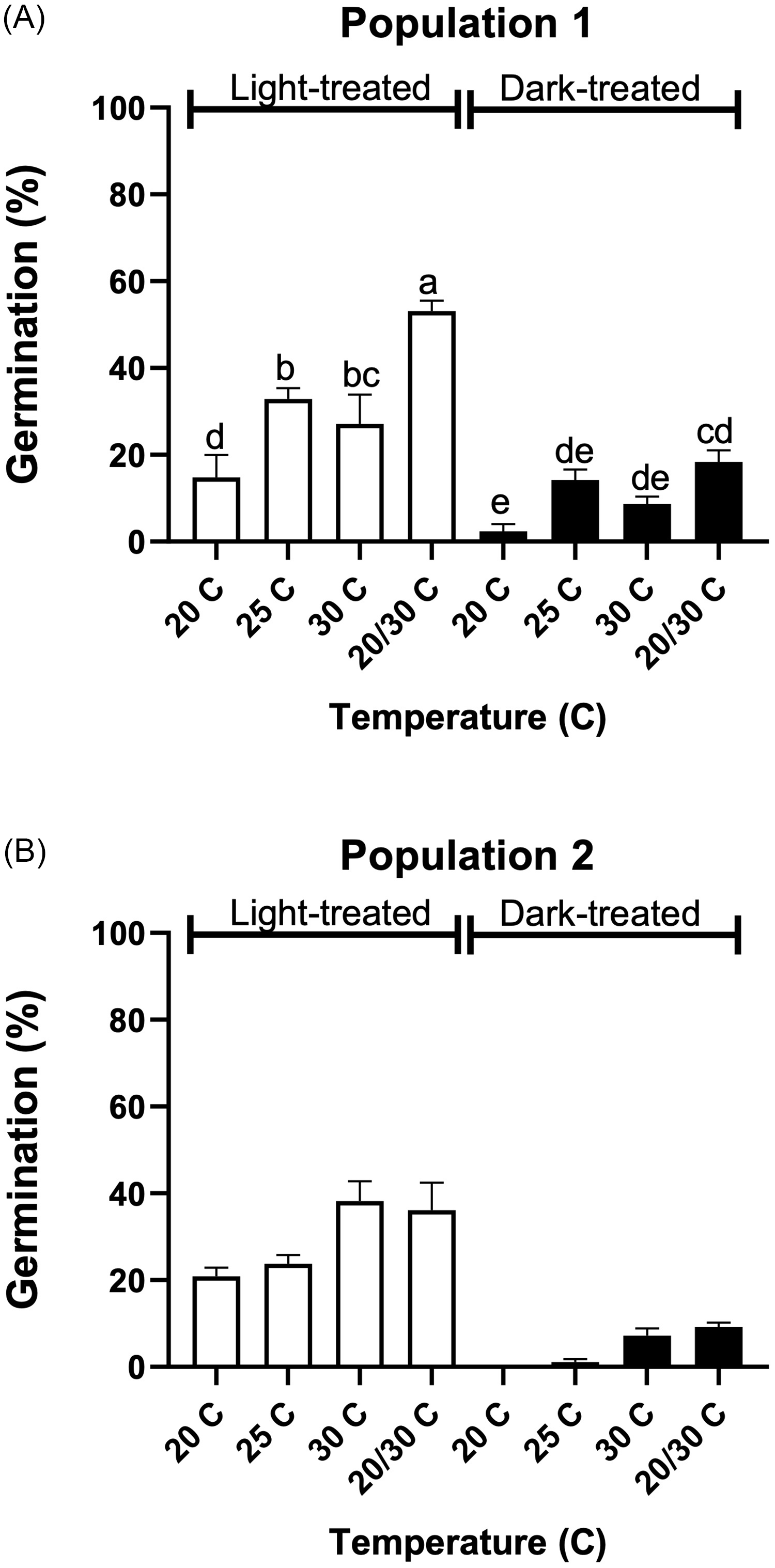
Figure 1. Tithonia tubaeformis achene germination of population 1 (A) and population 2 (B). Different letters at the top of each bar indicate significant differences according to Tukey’s test (α = 0.05).
Results and Discussion
Effect of Light and Temperatures on Achene Dormancy Breakage
Germination for population 1 was characterized by a nonsignificant effect between both experimental runs (P = 0.59), while the interaction between light and temperature was significant (P = 0.023) (Figure 1A). Germination scored at 20/30 C plus light was higher than for all other treatments, reaching 53.11% ± 2.41%. In contrast, the lowest germination was observed in darkness at constant temperatures of 20 and 30 C. That is, 2.33% ± 1.67% and 8.67% ± 1.22% for 20 C and 30 C, respectively. Population 2 showed no significant light by temperature interaction (P = 0.35), while germination in the presence of light had a significant effect on T. tubaeformis germination (P < 0.0001). Also, temperature had a significant effect on germination (P < 0.001), as total germination at 20/30 C and 30 C was higher than that at 20 C and 25 C. The tetrazolium tests showed that 98.17% ± 0.1% and 96.66% ± 1.66% of achene populations were viable for populations 1 and 2, respectively. The tetrazolium test results, as compared with the germination test scores in Figure 1A and B, demonstrated that achenes underwent primary dormancy during dispersal. Light and alternating temperatures partially disrupted this condition, a requirement that was consistent across all populations. The presence of dormancy is a frequently observed trait among non-domesticated, weedy, and invasive species (Qaderi Reference Qaderi2023) and lies in environmental factors that act on the plant during seed filling and maturation (Matilla Reference Matilla2020; Postma and Ågren Reference Postma and Ågren2015; Shu et al. Reference Shu, Meng, Shuai, Liu, Du, Liu and Yang2015).
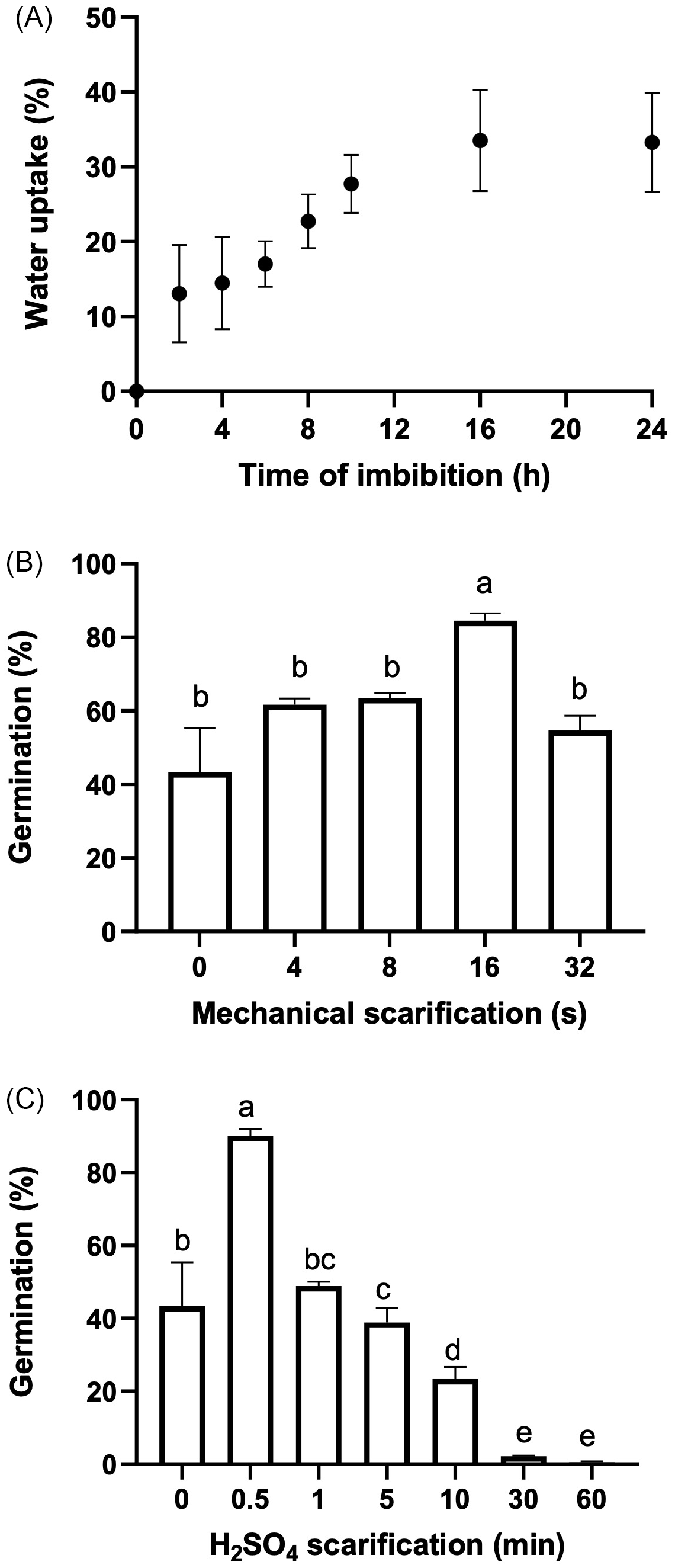
Figure 2. (A) Tithonia tubaeformis achenes water uptake (%). Data represent means ± SE (n = 20). (B) Final germination percentage of fresh achenes exposed to mechanical scarification with different treatment durations. (C) Final germination percentage of fresh achenes exposed to different immersion times in sulfuric acid. Data are means of three replicates ± SE. Different letters at the top of each bar indicate significant differences according to Tukey’s test (α = 0.05).
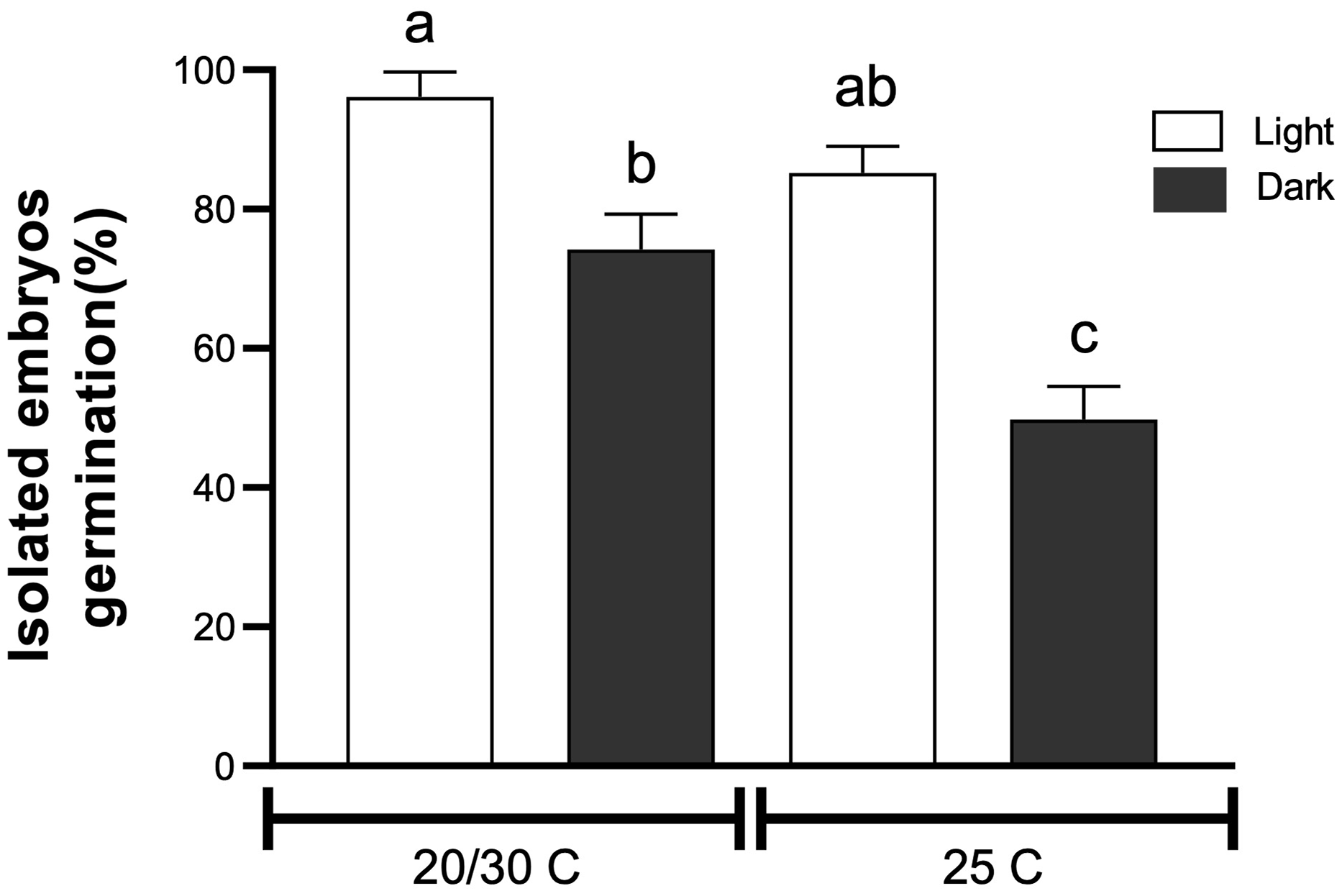
Figure 3. Tithonia tubaeformis final germination percentage of isolated embryos incubated at constant (25 C) or alternating temperatures (20/30 C) in light or in darkness. Data are means of three replicates ± SE. Different letters at the top of each bar indicate significant differences according to Tukey’s test (α = 0.05).
Effect of the Pericarp on Germination
The highest water uptake (%) was scored 16 h after the start of imbibition (Figure 2A), indicating that there is no coat impermeability. Mechanical scarification for 16 s increased germination (P < 0.0001) up to 84.5% ± 2.00% (t 50 of 3.18 d), increasing germination by 53% in relation to the control (55.5% ± 4.05%) (Figure 2B). Likewise, chemical scarification by means of sulfuric acid for 0.5 min promoted germination up to 90% ± 1.92%, with a T50 of 3.17 d (P < 0.0001) (Figure 2C). Germination of isolated embryos showed an interaction between temperature and light (P = 0.027) (Figure 3), while excised embryos incubated at 20/30 and 25 C in light had the highest final germination percentages, with 96.11% ± 2.06% and 85.5% ± 2.2%, respectively. These results highlighted that pericarp removal, by chemical or mechanical scarification, increased germination. Interestingly, exposure to light and alternating temperatures was necessary even for excised embryos to achieve almost full germination percentage, suggesting a significant stimulative effect and the maintenance of some degree of embryo dormancy in the absence of light. Water uptake measurement performed on fresh achenes rules out the possibility that impermeability of the pericarp reduces the water entry to the embryo. Thus, this finding evidences that the block to germination is mostly exerted by a mechanical constraint impairing the embryo growth required for the completion of germination. This feature and the fast seedling emergence of this weed connect well with the effects of tillage management that can be carried out in the field, which could weaken the pericarp tissue and make the achene ready for germination once environmental conditions are met. Lesser swinecress [Coronopus didymus (L.) Sm.]; syn.: Lepidium didymium L.) showed a similar process based on mechanical seed coat weakening by certain ascomycetes causing fruit fractures (Sperber et al. Reference Sperber, Steinbrecher, Graeber, Scherer, Clausing, Wiegand, Hourston, Kurre, Leubner-Metzger and Mummenhoff2017). Overall, these findings demonstrate that a combination of pericarp constraint and embryo dormancy governs germination in T. tubaeformis. This condition was observed in the Asteraceae family, as was already documented for sunflower (Dominguez et al. Reference Dominguez, Batlla, Rodríguez, Windauer, Gerbaldo and Benech-Arnold2016) or G. coronaria (Puglia et al. Reference Puglia, Grimaldi, Carta, Pavone and Toorop2015).
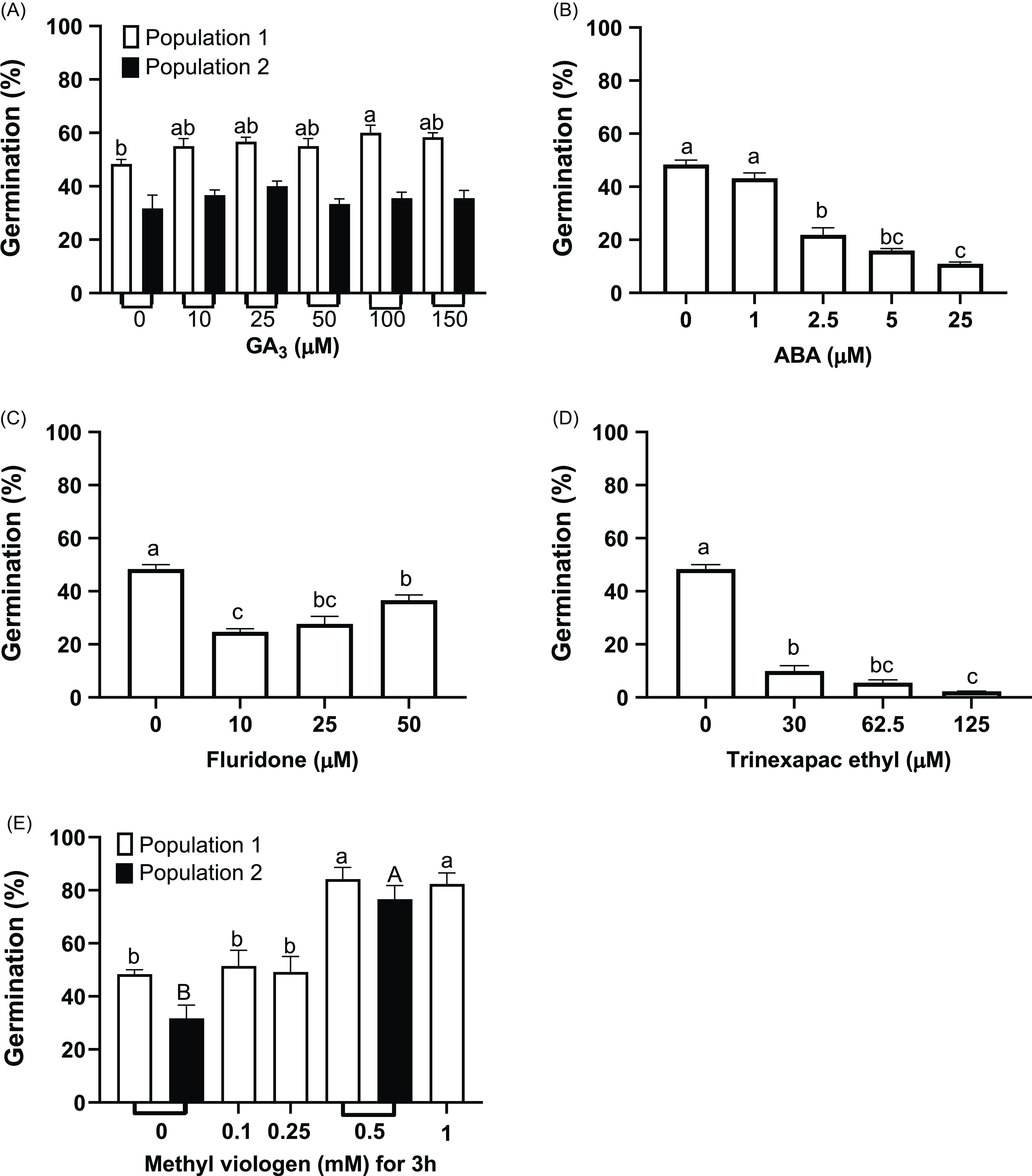
Figure 4. Tithonia tubaeformis germination percentage of fresh achenes in the presence of (A) gibberellic acid (GA3), (B) abscisic acid (ABA), (C) fluridone, (D) trinexapac-ethyl (TE), or (E) methyl viologen (MV). For GA3 and MV treatments (0 and 0.5 mM), population 2 was also tested. Data are means of three replicates ± SE. Different letters at the top of each bar indicate significant differences according to Tukey’s test (α = 0.05).
Effect of GAs, ABA, Fluridone, TE, and ROS Compounds on Achene Dormancy Breakage
The presence of GA3 did not affect T. tubaeformis germination (Figure 4A) (P = 0.058 and 0.37 for populations 1 and 2, respectively). Maximum final germination percentage was scored with the addition of 100 µM GA3 (60% ± 2.36%). ABA at doses of 2.5, 5, and 25 µM reduced germination up to 10.89% ± 0.73% for ABA 25 µM (P < 0.0001), while the presence of fluridone did not increase germination (P < 0.0001) (Figure 4B and C, respectively). TE reduced germination in comparison with the control treatment (P < 0.0001) (Figure 4D). Imbibition of achenes in MV (0.5 and 1 mM) increased germination (P < 0.05) (Figure 4E), with 84% ± 4.04% and 76.33% ± 5.09% for 0.5 mM in populations 1 and 2, respectively. This set of results suggested that only a shift in the ABA content affected the germination percentage. Indeed, the addition of ABA at doses from 2.5 µM onward resulted in a significant reduction in germination.
In contrast, GA and fluridone (i.e., an inhibitor of the ABA synthesis pathway) (Nonogaki et al. Reference Nonogaki, Sall, Nambara and Nonogaki2014; Yoshioka et al. Reference Yoshioka, Endo and Satoh1998) did not promote germination. Therefore, low germination scores for fresh achenes were likely based on stored ABA produced during seed development and not by ABA de novo synthesis from imbibition onward. The addition of TE (i.e., an inhibitor of GA biosynthesis) reduced germination. This compound interferes with the action site of the GA3 oxidase enzyme, which is the pivotal final step of GA biosynthesis (Rademacher Reference Rademacher2000). Germination scores in the present study using fluridone and TE, respectively, agree with results presented by Sano and Marion-Poll (Reference Sano and Marion-Poll2021) and Huarte et al. (Reference Huarte, Luna, Pagano, Zavala and Benech-Arnold2014a, Reference Huarte, Ruiz-Carmona and Zapiola2014b).
On the other hand, the addition of MV, an ROS donor compound, was the compound most effective for improving germination. As extensively documented, ROS play a role as signaling molecules in the regulation of responses to environmental stimuli that drive the germination processes (Bailly et al. Reference Bailly, El-Maarouf-Bouteau and Corbineau2008; Puglia Reference Puglia2024). The lack of germination response to GA3 addition and the substantial increase in final germination percentage in the presence of MV suggest a direct role of the ROS compounds for T. tubaeformis germination, for instance, contributing to pericarp weakening, thus loosening pericarp restriction.
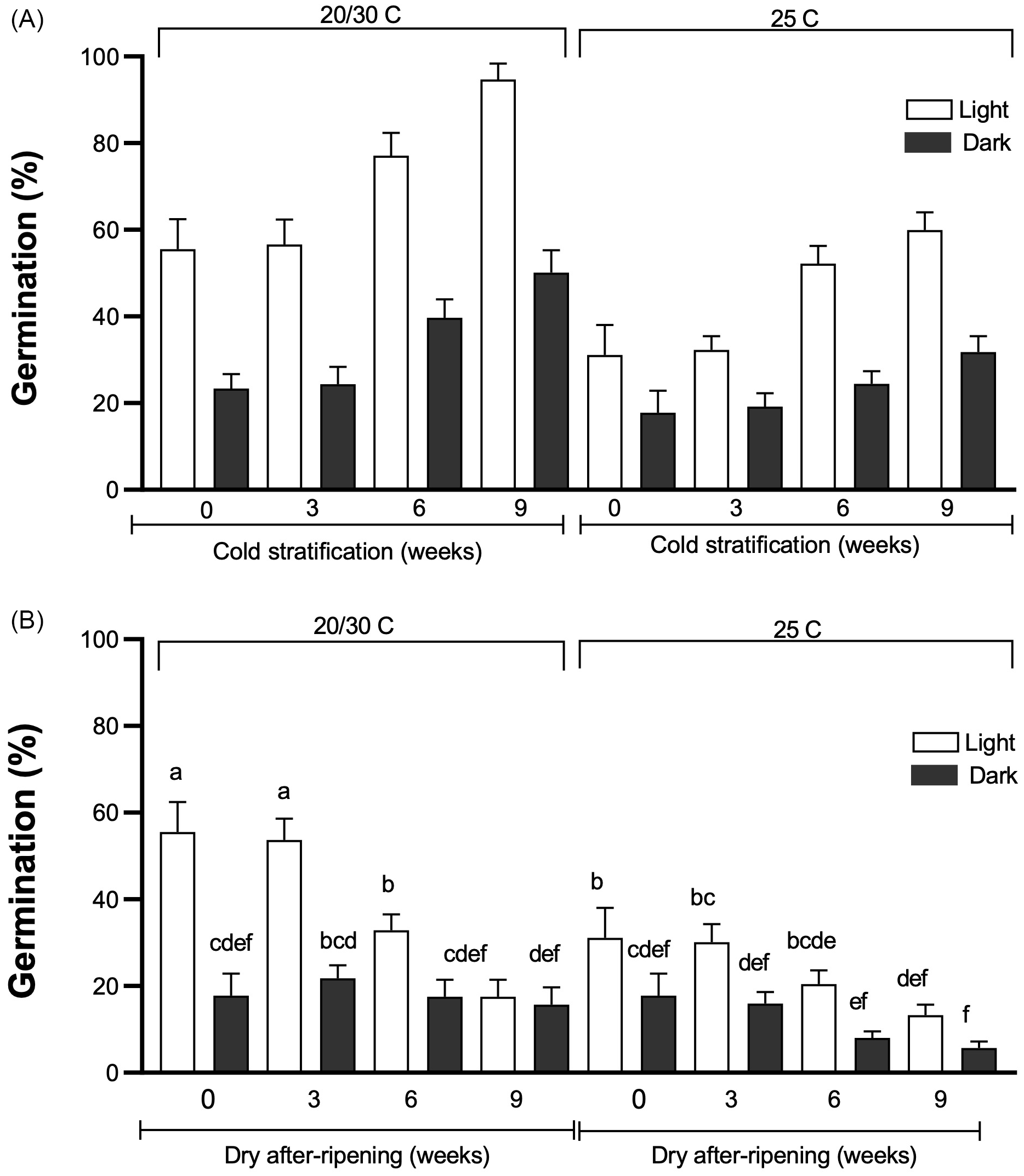
Figure 5. Tithonia tubaeformis germination average percentage of achenes exposed to 0, 3, 6, or 9 wk of cold stratification (A) or dry afterripening (DAR) (B) treatments. After each treatment, achenes were imbibed at constant (25 C) or alternating temperatures (20/30 C) in light or in darkness. Data are means of three replicates ± SE. Different letters at the top of each bar indicate significant differences according to Tukey’s test (α = 0.05).
Effect of Cold Stratification and DAR on Dormancy Release
Simple main effects of cold stratification, light exposure, and temperature were significant (P < 0.0001) (Figure 5A), with no interaction among these effects. Longer cold stratification treatments more effectively released achenes from dormancy. This result was evidenced by a gradual increase in sensitivity to alternating temperatures, under both light or dark conditions, and constant temperature + light for germination in agreement with data reported by Batlla and Benech-Arnold (Reference Batlla and Benech-Arnold2010). Also, but to a much lesser extent, the effects of longer cold stratification were made evident by an enhancement of germination in constant dark conditions in comparison with nontreated achenes (i.e., 17.78% ± 2.6% vs. 31.77% ± 2.6% for 0 and 9 wk, respectively). Germination of cold-stratified achenes for 6 and 9 wk incubated at 20/30 C + light reached 77.11% ± 3.02% and 94% ± 2.15%, respectively, improving the 55.55% ± 4.01% scored at the same condition in fresh achenes.
In contrast, for the DAR condition, a significant three-way interaction among treatment duration, temperature, and light influenced seed dormancy (P = 0.0004). Results scored showed DAR was not effective in releasing T. tubaeformis dormancy (Figure 5B). This was proved by the progressive reduction in germination at alternating and constant temperatures, both in light and darkness. These results agree with those found in other Asteraceae and summer annual species (Batlla et al. Reference Batlla, Ghersa and Benech-Arnold2020; Brown and Allen Reference Brown and Allen2023; Zhang et al. Reference Zhang, Huang, Liu, Hu, Panero, Luebert, Gao and Ma2021). They indicate that T. tubaeformis behaves as a typical summer annual weed, with achenes exhibiting increased sensitivity to alternating temperatures and light and, to a lesser extent, to alternating temperatures in darkness as cold stratification progressed. In contrast, at constant temperatures, even for longer cold stratification progressed, germination did not reach the level observed at alternating temperatures, mainly in the dark. This is consistent with reports by Benech-Arnold et al. (Reference Benech-Arnold, Sánchez, Forcella, Kruk and Ghersa2000) and Batlla and Benech-Arnold (Reference Batlla and Benech-Arnold2015) that seed dormancy release, in this case by cold stratification, involves a higher sensitivity to environmental factors that trigger germination whose perception may indicate an open area to colonize before other seeds can germinate.
The reported behavior led us to categorize its germination as typical of weeds and invasive plants living in disturbed habitats that are sensitive to spatial information, such as light and alternating temperatures (Catara et al. Reference Catara, Cristaudo, Gualtieri, Galesi, Impelluso and Onofri2016; Martinková et al. Reference Martinková, Honěk and Brabec2021).
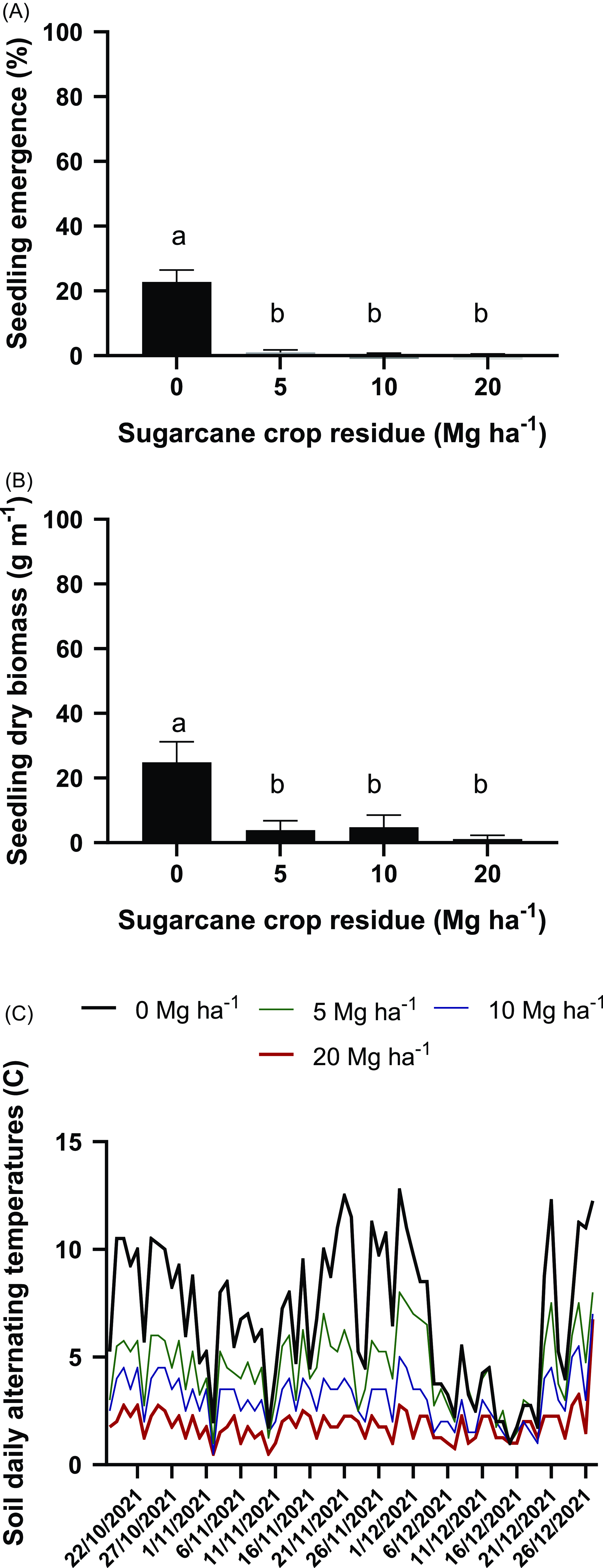
Figure 6. Effect of the presence of 0, 5, 10, or 20 Mg ha−1 of sugarcane residue on Tithonia tubaeformis seedling emergence percentage (A); seedling biomass grams of dry matter (B); and soil thermal amplitude (C). Data shown in A and B represent the means ± SE. Due to the lack of interaction between 2019 and 2021, results scored in each test (four replicates each) were pooled. Different letters at the top of each bar indicate significant differences according to Tukey’s test (α = 0.05).
Effect of the Sugarcane Residue on Seedling Emergence and Seedling Biomass and Soil Thermal Parameters
The percentage of seedlings that emerged on bare soil was 22.75% ± 3.7%. The presence of an amount of residue equivalent to 5 Mg onward drastically reduced the percentage of emerged seedlings, and this was not significantly different in the 2 yr considered in this study (Figure 6A; Supplementary Tables S1 and S2). Also, the weed seedling biomass in the 2 yr considered was reduced by the presence of crop residues (P < 0.0001) from 35.43 ± 4.89 g m−1 under bare soil conditions to 3.87 ± 2.81 g m−1 with the presence of a residue amount equivalent to 5 Mg ha−1 (Figure 6B; Supplementary Tables S3 and S4). The soil thermal amplitude scored during the seedling emergence window of 2021 was consistent with the assumption that as the amount of residue increases, the soil daily thermal amplitude is progressively reduced. Throughout the seedling emergence period, mean soil thermal amplitude was 6.93 ± 0.28 C, 4.51 ± 0.15 C, 3.06 ± 0.1 C, and 1.86 ± 0.07 C for residue amounts of 0, 5, 10, and 20 Mg ha−1, respectively (P < 0.0001) (Figure 6C). These results are consistent with those of Ranaivoson et al. (Reference Ranaivoson, Naudin, Ripoche, Affholder, Rabeharisoa and Corbeels2017) and Okeyo et al. (Reference Okeyo, Norton, Koala, Waswa, Kihara and Bationo2016), who revisited the effects of crop residues on soil thermal regimes. On the other hand, the soil mean temperatures during the experiment were 26.02, 25.18, 25.13, and 24.44 C for residue amounts of 0, 5, 10, and 20 Mg ha−1, respectively.
Together with the scored reduction in the soil thermal amplitude, crop canopies modify the light quantity and quality reaching the soil surface. Previous research has already documented this fact, with a large body of evidence pointing to this effect and the importance of light. At the same time, crop residues also reduced the light quantity (Mahajan et al. Reference Mahajan, Prasad and Chauhan2021) and quality (i.e., the low R/FR ratio) (Roso et al. Reference Roso, Nunes, Müller, Paranhos, Lopes, Dornelles, Bertagnolli, Huth, Forte and Menegaes2021), which were also essential cues for dormancy breakage in many weed species.
The current study provides important information on the germination ecophysiology of T. tubaeformis. Based on our observations, in dispersed achenes, physiological dormancy may be released by means of cold stratification, while exposure to light and/or alternating temperatures provides necessary spatial information for the achenes to germinate in the right place. The dependence of these environmental cues to break their dormancy can be used to control T. tubaeformis emergence. For instance, privation of these stimuli by leaving crop residues on the soil surface may reduce T. tubaeformis emergence. However, further research is required to effectively implement this approach on an industrial scale in the field. In addition, because T. tubaeformis emergence is linked to a specific time in the year, that is, the end of the winter, the anticipation of the sugarcane planting date could reduce T. tubaeformis emergence by means of an early canopy closure of the interrow spaces.
Both of the proposed management approaches could be useful alternatives to reduce the amount of herbicide needed to control this weed. Complementary research is needed to investigate T. tubaeformis seedbank resilience and longevity in the soil and determine the quality and quantity of light beneath various crop residue amounts to gain a more comprehensive understanding of the crop residue effect.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/wsc.2025.19
Funding statement
This research was partly founded by the LOMASCyT Universidad Nacional de Lomas de Zamora.
Competing interests
The authors declare no conflicts of interest.









