Introduction
Success of invasive weeds can be attributed to several factors, including their adaptation for rapid dispersal, prolific seeding ability, rapid growth rates, and adaptability to diverse environmental conditions (Baker Reference Baker and Genetics1965). Adaptive trait diversity and phenotypic plasticity can facilitate survival and establishment in new areas, thereby aiding in rapid spread (Clements and Jones Reference Clements and Jones2021a). Reports of rapid evolution and spread of herbicide resistance in dominant weeds and invasive species further complicate the issue (Clements and Jones Reference Clements and Jones2021b; Norsworthy et al. Reference Norsworthy, Ward, Shaw, Llewellyn, Nichols, Webster, Bradley, Frisvold, Powles, Burgos, Witt and Barrett2012), due to the need of alternative herbicide chemistries or nonchemical weed control tools (Peterson et al. Reference Peterson, Collavo, Ovejero, Shivrain and Walsh2018; Swinton and Deynze Reference Swinton and Deynze2017). As invasive weeds are prolific seed producers, the selection pressure from herbicides will have greater chances of selecting for the resistant survivors among a large number of treated plants (Bagavathiannan and Davis Reference Bagavathiannan and Davis2018). This phenomenon is particularly evident in Palmer amaranth (Amaranthus palmeri S. Watson) and johnsongrass [Sorghum halepense (L.) Pers.], which have shown rapid adaptation to herbicide applications and diverse dispersal routes, resulting in widespread infestations that threaten both agricultural productivity and biodiversity (Adkins and Shabbir Reference Adkins and Shabbir2014; Nakka et al. Reference Nakka, Thompson, Peterson and Jugulam2017; Peterson Reference Peterson1999).
Weed invasion through rapid dispersal complicates management efforts and increases weed control costs (Tataridas et al. Reference Tataridas, Jabran, Kanatas, Oliveira, Freitas and Travlos2022). Weed dispersal occurs through movement of contaminated soil, water currents, wind dispersal, animals, human activities such as agriculture and landscaping, and the transportation of goods and vehicles (Benvenuti Reference Benvenuti2007; Thill and Mallory-Smith Reference Thill and Mallory-Smith1997). One major strategy is to prevent dispersal of resistant weed populations (Bagavathiannan and Davis Reference Bagavathiannan and Davis2018; Beckie and Harker Reference Beckie and Harker2017), as the spread of resistance genes is a more significant source of new infestations than the selection of new resistant cases on-site (Heap and Duke Reference Heap and Duke2018).
Endozoochory, dispersal of seeds via animal digestive systems (Figure 1), is a key seed dispersal method in integrated crop–livestock systems where ruminants act as dispersing agents that can facilitate the spread of herbicide-resistant weeds (Ciontu et al. Reference Ciontu, Jalobă, Șerban, Petcu and Grădilă2020; Schupp et al. Reference Schupp, Jordano and Gómez2010; Viero et al. Reference Viero, Schaedler, Azevedo, Santos, Scalcon, David and Rosa2018). Seeds can be transported long distances and deposited in viable form through animal fecal materials (Gardener et al. Reference Gardener, McIvor and Jansen1993; Guo et al. Reference Guo, Qiu, Ye, Jin, Mao, Zhang, Yang, Peng, Wang, Jia, Lin, Li, Fu, Liu and Chen2017). Pleasant and Schlather (Reference Pleasant and Schlather1994) reported that 1 kg of cattle (Bos spp.) manure could contain up to 42 viable seeds of field bindweed (Convolvulus arvensis L.), with about 20% of ingested seeds surviving digestion and manure processing. Endozoochory can provide seed scarification in the animal gut or allow microbial degradation of the seed coat that can stimulate germination (Lima et al. Reference Lima, Petter and Leandro2015; Traveset and Verdu Reference Traveset, Verdu, Levey, Silva and Galetti2002) or provide a nutrient-rich environment that supports weed seedling establishment (Milotić and Hoffmann Reference Milotić and Hoffmann2016; Traveset et al. Reference Traveset, Riera and Mas2001). Benvenuti (Reference Benvenuti2007) warned that although endozoochory accounts for 1.5% of seed dispersal, the economic losses can be potentially higher in case of herbicide-resistant weed biotypes because of greater difficulty in controlling them.
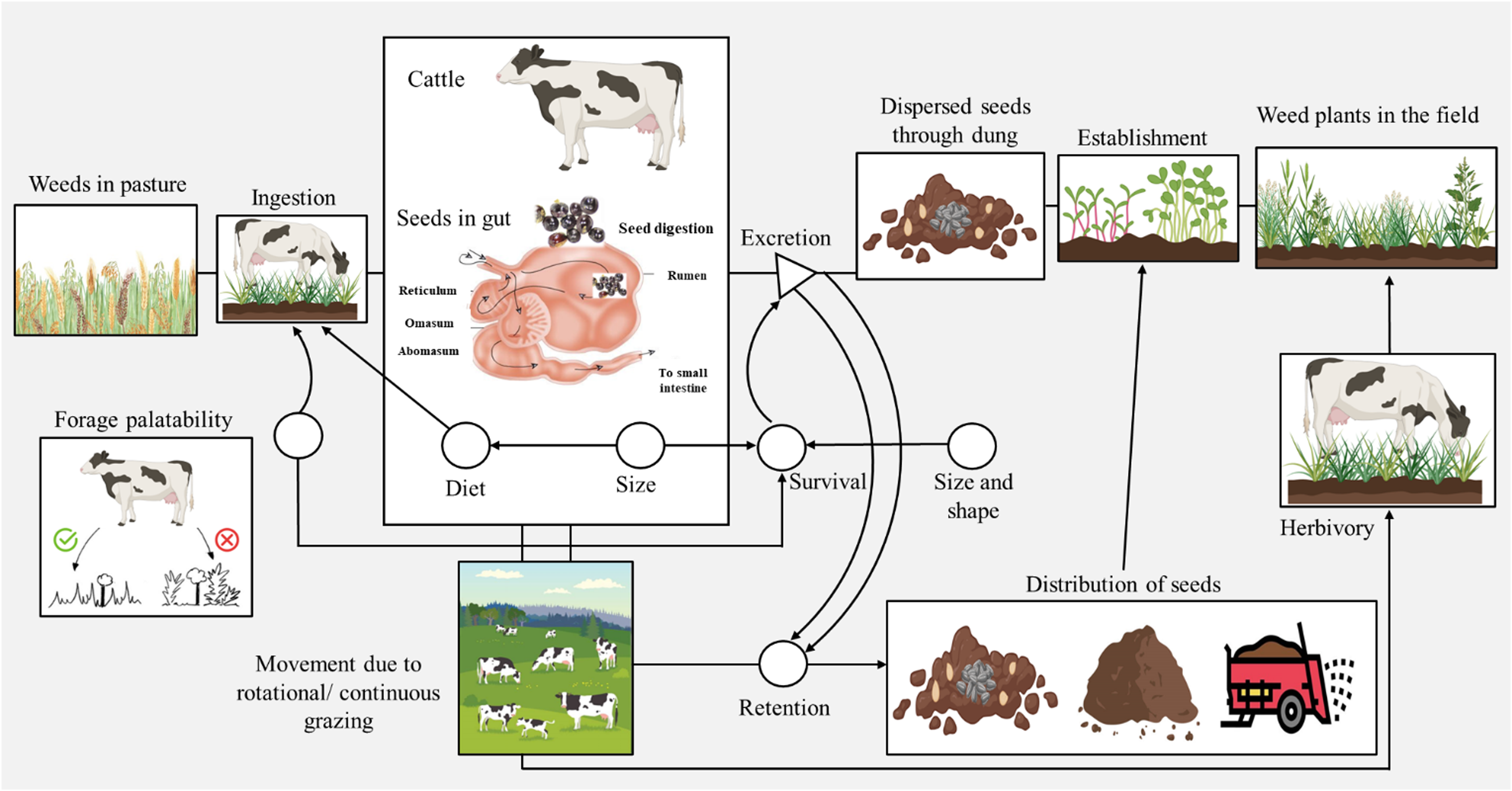
Figure 1. Flowchart illustrating the endozoochorous seed dispersal by cattle (modified from Mouissie et al. Reference Mouissie, Vos, Verhagen and Bakker2005). Boxes represent quantitative units, circles denote seed parameters, and triangles signify the spatial units essential for seed dispersal.
The effectiveness of endozoochorous seed dispersal can be influenced by the digestive physiology of the dispersing cattle, seed traits, and environmental conditions (Gilhaus et al. Reference Gilhaus, Freitag, Kunze and Hölzel2017; Karimi et al. Reference Karimi, Hemami, Tarkesh Esfahani and Baltzinger2020). Variations in seed retention time among herbivores impact seed viability and germination, while seed characteristics determine survival and establishment success (Xu et al. Reference Xu, Li, Li, Hu, Zhang, Iqbal and Du2022). The digestive process can variably affect seed viability, with some seeds experiencing reduced germination rates (Aper et al. Reference Aper, De Cauwer, De Roo, Lourenço, Fievez, Bulcke and Reheul2014; Gselman and Brus Reference Gselman and Brus2023). Studies show that seeds with hard coats maintain high viability after digestion, with survival rates of 28% to 31% for certain legumes (Deminicis et al. Reference Deminicis, Vieira, de Carvalho Almeida, Valente, do Carmo Araujo and Lima2020).
In the southern United States, several dominant weed species, including A. palmeri, S. halepense, and C. arvensis, have significant ecological and economic impacts on agricultural systems (Webster and Nichols Reference Webster and Nichols2012). Amaranthus palmeri has emerged as one of the most problematic weeds due to its rapid growth, high reproductive capacity, and ability to develop resistance to multiple herbicide classes (León and van der Laat Reference León and van der Laat2021). Similarly, S. halepense can reduce crop yields in cotton (Gossypium hirsutum L.), soybean [Glycine max (L.) Merr.], and corn (Zea mays L.) up to 90%, 88%, and 80%, respectively (Klein and Smith Reference Klein and Smith2021). Convolvulus arvensis is a perennial weed with an extensive root system that competes aggressively with crops, potentially reducing yields by up to 100% (Boldt et al. Reference Boldt, Rosenthal and Srinivasan1998). These weeds not only disrupt agricultural systems but also alter local ecosystems by outcompeting native flora, thereby reducing biodiversity (Webster and Nichols Reference Webster and Nichols2012). Seeds of these weeds can disperse spatially to long distances through waterways, animals, wind, and agricultural activities like tillage or animal ingestion, contributing to their widespread distribution and genetic diversity (Maity et al. Reference Maity, Young, Subramanian and Bagavathiannan2022; Martín et al. Reference Martín, Andújar, Fernández-Quintanilla and Dorado2015; Rudi et al. Reference Rudi, Bailly, Belaud and Vinatier2018; Sosnoskie et al. Reference Sosnoskie, Hanson and Steckel2020; Yu et al. Reference Yu, Blair, Hardel, Chandler, Thiede, Cortilet, Gunsolus and Becker2021).
While research has explored the role of cattle in seed dispersal of some forbs and woody plants (Brown and Archer Reference Brown and Archer1988; Chuong et al. Reference Chuong, Huxley, Spotswood, Nichols, Mariotte and Suding2016), the specific role they play in dispersing dominant U.S. southern weeds remains relatively understudied. As cattle grazing often overlaps with areas heavily infested with these invasive plants, understanding the endozoochorous dispersal potential of these weeds may have significant implications for weed management strategies in the region. We hypothesized that these weeds would survive digestion through cattle gut, thereby facilitating their dispersal and establishment in new areas. Therefore, this study aimed to analyze the effects of rumen fluid incubation on germination, viability, and seedling vigor of common weed species with contrasting seed traits found in pastures, thereby providing insights into the ecological consequences of endozoochorous seed dispersal.
Materials and Methods
Plant Material
Weed species that are known as common and troublesome in U.S. southern pastures were used in the experiment: A. palmeri, C. arvensis, pitted morningglory (Ipomoea lacunosa L.), yellow foxtail [Setaria pumila (Poir.) Roem. & Schult.], and S. halepense (Figure 2). These species are prevalent in various agricultural settings and exhibit aggressive growth patterns that can outcompete desirable forage species, leading to reduced pasture productivity and quality (Culpepper Reference Culpepper2006; Sarangi and Jhala Reference Sarangi and Jhala2018). The seeds of A. palmeri and S. halepense were collected in 2022 from research farms at College Station, TX, USA, and placed in permeable paper bags and stored under laboratory conditions at 20 ± 2 C in the dark until commencement of the experiment. The seeds of the other three species (C. arvensis, I. lacunosa, and S. pumila) were obtained from Azlin Seed Service, Leland, MS, USA. A preliminary viability test was conducted to ensure adequate seed viability before the experiment. This involved using 20 seeds per species with three replications, subjected to a tetrazolium test at a 0.1% concentration. An in vitro lab experiment was conducted in 2023 and 2024 at Auburn University’s Department of Animal Sciences, Upchurch Hall, Auburn, AL, USA. Each experiment was repeated three times.

Figure 2. Seeds of five weed species that underwent the rumen digestion experiment, arranged in the order of increasing seed size. From left to right: (A) Amaranthus palmeri, (B) Setaria pumila, (C) Sorghum halepense, (D) Convolvulus arvensis, and (E) Ipomoea lacunosa.
Rumen Fluid Extraction and Preparation
Simulated rumen digestion involved exposure of seeds to rumen fluid containing micro-organisms to undergo fermentation at a pH of about 6.5 for 0 to 96 h, followed by exposure to acidic conditions (pH 3.5) for 48 h, as found in the abomasum (true stomach) (Ocumpaugh and Swakon Reference Ocumpaugh and Swakon1993). The rumen fluid (solid and liquid fractions; approximately 1,000 ml) used in the experiment was collected from two heifers with rumen fistula fitted with a cannula and maintained on an all-forage diet. At sampling, the cannula was opened, the rumen contents were stirred, and rumen fluid was transferred into prewarmed (39 C) thermos containers to retain fluid temperature. The containers were completely filled with rumen fluid to exclude oxygen and transported to the laboratory. On arrival at the laboratory, rumen inoculum was strained through two layers of cheesecloth to separate liquid phase (rumen fluid) from solid phase.
Digestion Experiment/In Vitro Digestion
Fermentation was carried out in the laboratory using a Daisy II incubator (Ankom Technology, Macedon, NY, USA) according to the general procedure of Tilley and Terry (Reference Tilley and Terry1963). About 300 to 400 seeds per Erlenmeyer flask marked with the species, replication number, and duration of incubation (0, 4, 8, 12, 24, 48, 72, and 96 h) were added with 40 ml of McDougall’s buffer (McDougall Reference McDougall1948) under CO2 to maintain anaerobic conditions. Then, 10 ml of strained rumen inoculum was added to this solution and stirred. Samples were stoppered with fitted airlocks and incubated in a Thermo Scientific Precision Shaking Water Bath (Waltham, MA USA) at 39 C for their prescribed incubation period. For the 96-h samples (those used to simulate total tract digestion), 50 ml of pepsin solution was added to stop fermentation after 48 h, and the flasks were incubated for an additional 48 h. The flasks were taken out of the water bath at the decided time, and seeds were placed on a filter paper for drying overnight.
Evaluation of Seed Germination and Viability
To evaluate seed germination and viability, germination tests were performed using a completely randomized design (CRD) with three replications. For each replication, 50 seeds of each weed species were placed in petri dishes (United Scientific (Libertyville, Illinois, USA) Supplies K1004-J Petri Dish, 90-mm diameter, 15-mm height, polystyrene (Libertyville, Illinois, USA)) that had two disks of germination paper (Whatman 114, 90 mm (Sanford, ME, USA); and Zenpore ST001-90, 90 mm (Fotan, NT, Hong Kong)) and 10 ml of distilled water. Petri dishes were placed in a growth chamber (Incubator-I-36NL, Percival Scientific (Perry, Iowa, USA)) set to a diurnal light cycle of 16 h and a temperature of 25 C. Germination was counted daily based on radicle protrusion (2 mm) for 21 d, and total cumulative germination was recorded. The final germination percentage, the time taken for the first seed to germinate, and the mean time to germination (MTG) were calculated. MTG is an index of seed germination speed, determined using the formula: MTG = Σ (n × d)/N, where n represents the number of seeds that germinated on day d, and N is the total number of seeds that germinated in the treatment. The tetrazolium test was used to determine viable seeds. For this, nongerminated seeds were first pricked or scratched to break any hard seed coats and then sectioned to expose the embryo and soaked overnight in 2,3,5-triphenyl tetrazolium chloride solution (0.1%) in distilled water with pH 7 at 20 C in darkness. After 48 h, seeds whose embryos had stained red and had firm flesh were classified as viable. Nongerminated seeds with complete embryo coloring were considered to be dormant seeds (Maity et al. Reference Maity, Singh, Jessup and Bagavathiannan2021).
Membrane Integrity Test
For the membrane integrity test, 20 seeds of small-seeded weeds and 10 seeds of large-seeded weeds were selected and cleaned. These seeds were then soaked in 50 ml of deionized water at room temperature (approximately 25 C) for 24 h to allow for electrolyte leakage (Powell Reference Powell1986). The electrical conductivity of the soaking solution was measured using a Thermo-Scientific Orion Versastar Pro EC meter, calibrated with instrument-specific standard solutions before each measurement. Conductivity readings, recorded in microsiemens per centimeter (µS cm−1), indicated the extent of electrolyte leakage: high values suggested poor membrane integrity and lower seed vigor, while low values indicated good membrane integrity and higher seed vigor. Each sample was tested in triplicate to ensure reproducibility, and the average conductivity value was calculated for each seed sample.
Seedling Vigor
Seedling vigor was assessed by measuring seedling length and dry weight. At the end of the 21-d germination test, the length of five random seedlings were measured from the base to the tip of the longest leaf to evaluate growth performance. Subsequently, these seedlings were placed for drying in an oven at 65 C for 48 h. After drying, the five seedlings were weighed to determine their dry biomass. The seedling vigor was measured using two indices: vigor index I (percent germination by plant length) and vigor index II (percent germination by total dry biomass) (Abdul-Baki and Anderson Reference Abdul-Baki and Anderson1973).
Statistical Analysis
Seed germination and viability were expressed as percentages. All treatment combinations were treated as a CRD with four replicates and were repeated three times. Treatments comprised all combinations of five weed species with eight incubation times (digestion experiment; four replications). Data were analyzed in RStudio using Levene’s test for homogeneity of three runs. Data from the three experimental runs were pooled, as there was no significant difference (P > 0.05) among the runs. ANOVA was performed on seed germination, seed viability, seed membrane integrity, and seedling vigor data using JMP PRO v. 16 (SAS Institute, Cary, NC, USA). Means were separated using Tukey’s honest significant difference (HSD) test at a significance level of α = 0.05. Results are presented as estimated means ± SE.
The time to reduce seed germination by 50% (G50) was estimated using a three-parameter log-logistic regression model with the drc package in RStudio (Knezevic et al. Reference Knezevic, Streibig and Ritz2007; Ritz et al. Reference Ritz, Baty, Streibig and Gerhard2015) applied to cumulative seed germination data collected over 21 d. The following three-parameter regression model was fit (Equation 1):
In this equation, y is the response variable (i.e., seed germination [%]); x is the incubation period in rumen fluid; d is the upper limit; b is the relative slope around e; and e is G50, which is the incubation period in rumen fluid required for 50% reduction in germination.
Results and Discussion
Seed Germination Traits
In natural conditions, the majority of seeds are recovered from cattle within the first 24 to 48 h, with recovery continuing up to 72 h and beyond (Gardener et al. Reference Gardener, McIvor and Jansen1993; Xu et al. Reference Xu, Li, Li, Hu, Zhang, Iqbal and Du2022). We observed significant effects of rumen fluid digestion on weed seed germination among the weed species in this study, as seed germination rate progressively declined with increasing duration of incubation (Figure 3). Ipomoea lacunosa seeds were highly affected by rumen fluid incubation, starting with a high germination percentage of 85% at 0 h that fell dramatically to 0% by 24 h of incubation and beyond. This pattern is consistent with previous research, which suggests that prolonged exposure to rumen fluid can adversely affect needle grass (Stipa aliena Keng) seed germination, potentially due to microbial activity and changes in pH levels that inhibit germination (Chen et al. Reference Chen, Huang, Zhang, Wu and Liu2012). Blackshaw and Rode (Reference Blackshaw and Rode1991) reported that downy brome (Bromus tectorum L.) and barnyardgrass [Echinochloa crus-galli (L.) P. Beauv.] seeds had 0% germination after 24 h of rumen fluid incubation. Convolvulus arvensis and S. pumila showed a severe and continuous decline in germination rates as the incubation period increased. Germination percentages dropped from 27% at 0 h to 4% at 48 h for C. arvensis, and from 58% at 0 h to 5% at 48 h for S. pumila, with no germination observed beyond this period. Similar findings were reported by Blackshaw and Rode (Reference Blackshaw and Rode1991) for green foxtail [Setaria viridis (L.) P. Beauv.], with seed germination decreasing from 88% to 14% after 24 h of rumen fluid incubation. In all three species (C. arvensis, I. lacunosa, and S. pumila), there was no germination beyond 48 h of incubation. Reduced seed germination can be attributed to the degradation of the embryo due to digestive enzymes in animal gastrointestinal systems. Additionally, microbial attachment in the rumen can further inhibit germination (Obara et al. Reference Obara, Ogura, Shishido and Sugawara2009). The high microorganism content and acidity of rumen fluids can lead to a loss of seed germinability, particularly in seeds with higher seed coat permeability and thin coats (Horiguchi et al. Reference Horiguchi, Takahashi and Kayaba2008).
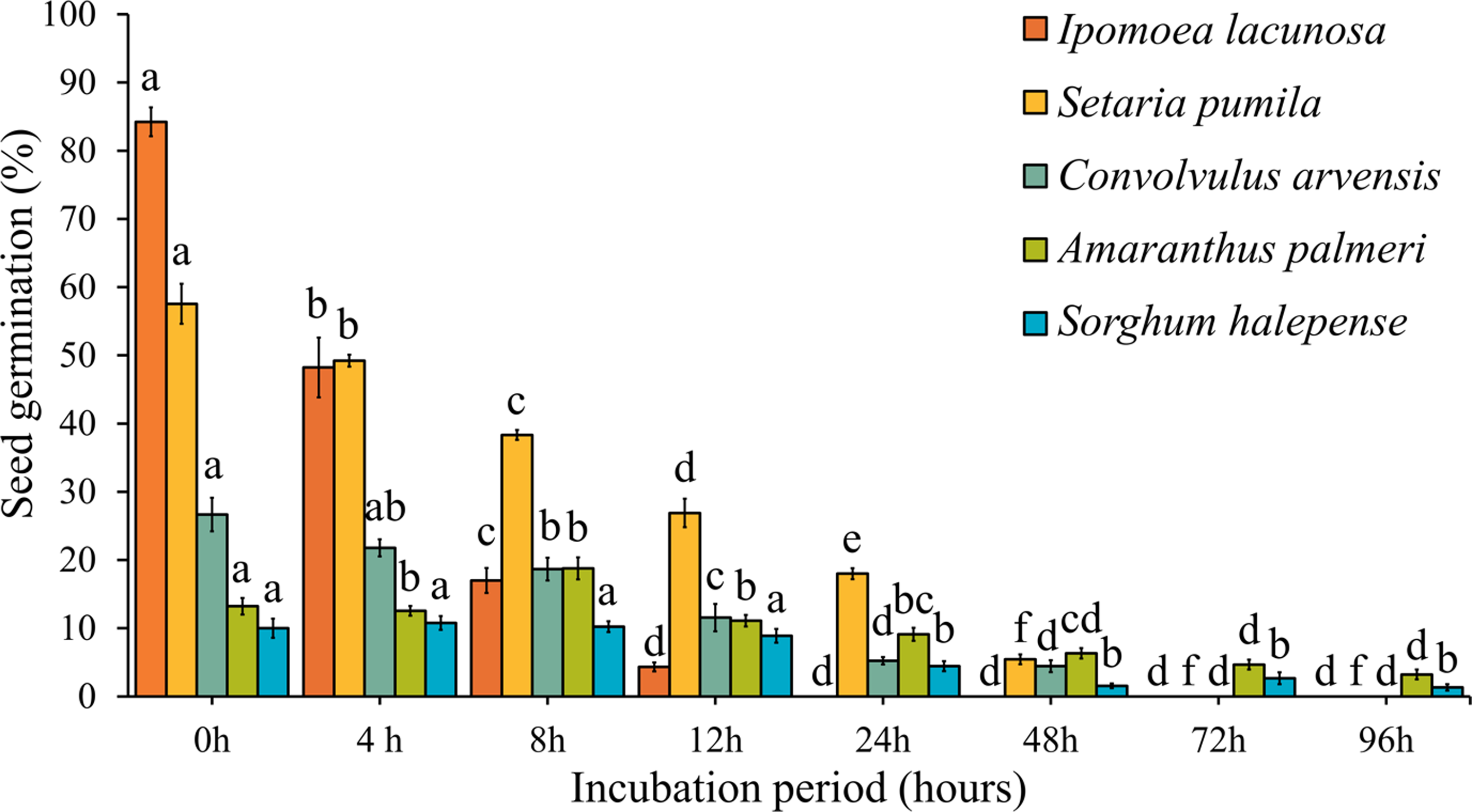
Figure 3. Effects of different duration of rumen fluid incubation on seed germination of five weed species. Bars with different letters indicate significant difference among the individual species across durations at P < 0.05. Errors bars indicate standard error of the mean (SEM).
Amaranthus palmeri and S. halepense exhibited a relatively stable germination pattern, although there was a decline in germination over the incubation periods (Figure 3). Notably, these two species were still germinating even after 96 h of incubation, with A. palmeri and S. halepense showing germination rates of 3% and 1%, respectively. Additionally, after 8 h of rumen fluid incubation, A. palmeri seed germination increased to 19% from 13% in control, likely due to the acidic environment affecting the seed coat’s integrity and permeability, similar to the effects of acid scarification, as observed in common lambsquarters (Chenopodium album L.) (Aper et al. Reference Aper, De Cauwer, De Roo, Lourenço, Fievez, Bulcke and Reheul2014; Buhler and Hoffman Reference Buhler and Hoffman1999).
Seeds of A. palmeri are reported to survive digestive tracts of various birds (De Vlaming and Proctor Reference De Vlaming and Proctor1968; Farmer et al. Reference Farmer, Webb, Pierce and Bradley2017) and ruminant animals (Blackshaw and Rode Reference Blackshaw and Rode1991; Yu et al. Reference Yu, Blair, Hardel, Chandler, Thiede, Cortilet, Gunsolus and Becker2021). Amaranthus palmeri is an aggressive invader by nature due to several advantageous adaptive traits, including its seed morphophysiology and diverse dispersal routes. Its high fecundity, tolerance to extreme temperatures and drought, and outcrossing nature due to dioecy leading to high genetic diversity, along with its ability to develop herbicide resistance, make it a formidable weed (Franssen et al. Reference Franssen, Skinner, Al-Khatib, Horak and Kulakow2001; Ward et al. Reference Ward, Webster and Steckel2013). These traits, combined with its prolonged survival in rumen fluid, suggest a high potential for the spread of herbicide-resistant populations. Similarly, S. halepense is a persistent invasive species with high seed dormancy and can thrive more aggressively under stress conditions (Acciaresi and Guiamet Reference Acciaresi and Guiamet2010; Ghersa et el. Reference Ghersa, Arnold and Martinez-Ghersa1992). Seeds of S. halepense need fluctuating diurnal temperatures for seed dormancy release (Taylorson and McWorther Reference Taylorson and McWorther1969). Upon animal grazing, seeds of S. halepense showed enhanced germination when retrieved from fecal materials in 24 to 48 h, primarily due to loosening of external bracts on seeds from scarification processes in the gut passage (Abbas et al. Reference Abbas, Al-Kahtani, Abdelazeem Mousa, Badry, Hassaneen, Ezzat-Ahmed, Mancilla-Leytón and Castillo2020; Grande et al. Reference Grande, Mancilla-Leytón, Vicente and Delgado-Pertíñez2016; Kaur and Soodan Reference Kaur and Soodan2017). Moreover, S. halepense can hybridize with other Sorghum species, creating resilient perennial and rhizomatous hybrids that thrive in harsh environments and establish in new habitats (Klein and Smith Reference Klein and Smith2021).
Days to First Germination, the MTG
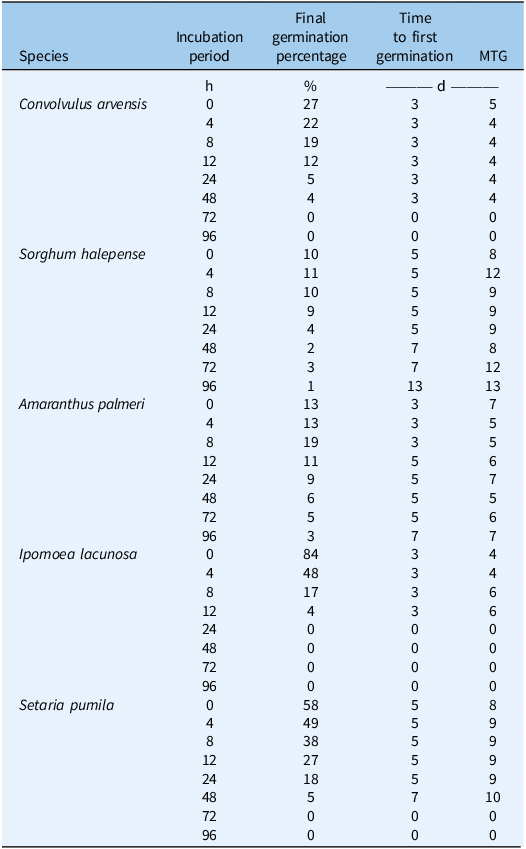
The time to first germination remained constant at 3 d for all incubation periods up to 48 h and 12 h, after which no germination was observed in C. arvensis and I. lacunosa, respectively (Table 1). In A. palmeri, the initial germination occurred rapidly within 3 d up to 8 h of incubation. This duration extended to 5 d up to 72 h and further increased to 7 d at 96 h of incubation. In S. halepense, the time to first germination was consistently 5 d for most periods of incubation, except for 48 and 72 h, where it increased to 7 d, and 96 h, where it significantly increased to 13 d. The decrease in speed of germination in A. palmeri and S. halepense may be due to genetic variability in seed coat impermeability, with more impermeable seeds surviving longer in rumen fluids and emerging more slowly in germination tests. This delay likely stems from solute leakage and membrane damage caused by exposure to rumen fluid disrupting osmotic balance and germination processes. Gama et al. (Reference Gama, Brum, Martins, Silva and Dias2021) reported that water deficits can significantly slow down germination rates, which may be a survival strategy in arid conditions. Early-germinated seeds may have a higher risk of dying from factors like desiccation, pathogens, or predators (Traveset et al. Reference Traveset, Rodríguez-Pérez and Pías2008) as observed in I. lacunosa and C. arvensis (Figure 3). Highly permeable seed coats in these species are likely digested by rumen fluids, suggesting that seed coat permeability plays a crucial role in seed survival.
Table 1. Percent final germination, number of days to first germination, and the mean time to germination (MTG) of the weed seeds after various durations of incubation in rumen fluid (hours).
Seed Viability
Overall, seed viability was significantly affected by rumen fluid incubation across the species (Figures 4 and 5). Although the initial germination percentage varied across the weed species, the initial viability was more than 95%, except for C. arvensis (89%). Similar to germination reduction, there was a gradual decrease in seed viability with increased duration of rumen fluid incubation. Simao Neto and Jones (Reference Simao Neto and Jones1987) observed an inverse relationship between incubation time and seed viability in six pasture species (signal grass [Urochloa decumbens (Stapf) R. Webster; syn.: Brachiaria decumbens Stapf], common carpetgrass [Axonopus fissifolius (Raddi) Kuhlm.; syn. Axonopus affinis Chase], perennial soybean [Neonotonia wightii (Wight & Arn.) Lackey], Kenya clover [Trifolium semipilosum Fresen.], cheesy-toes [Stylosanthes hamata (L.) Taubert] and shrubby stylo [Stylosanthes scabra Vog.]). However, this decline was not gradual for all species in our data. The viability of I. lacunosa seeds decreased sharply from 100% at 0 h to 34% by 24 h, and ultimately to 0% after 48 h. Similarly, the viability of C. arvensis seeds dropped from 96% at 0 h to 54% by 24 h, and finally to 0% after 96 h; and S. pumila began with a viability of 99% and maintained viability above 80% up to 24 h, declining sharply to 5% by 96 h. Haidar et al. (Reference Haidar, Gharib and Sleiman2010) observed a similar trend, with a steep decrease in viability of C. arvensis seeds in the first 24 h of rumen fluid incubation from 80% at 0 h to 30% by 24 h. Similar observations were made in case of kochia [Bassia scoparia (L.) A.J. Scott], for which viability sharply declined from 94% at 0 h to 15% after 24 h of incubation (Blackshaw and Rode Reference Blackshaw and Rode1991). This rapid loss of viability suggests that this species is highly sensitive to the conditions created by rumen fluid. Ipomoea spp. are highly sensitive to flood-induced anaerobic conditions, as high temperatures and humidity in rumen fluid triggered the water gap (a specialized area in the seed coat that facilitates the entry of water into the seed) to open, as seen in the case of rumen fluid (Jayasuriya et al. Reference Jayasuriya, Baskin, Geneve and Baskin2007; Siahmarguee et al. Reference Siahmarguee, Gorgani, Ghaderi–Far and Asgarpour2020) which inhibited their germination.
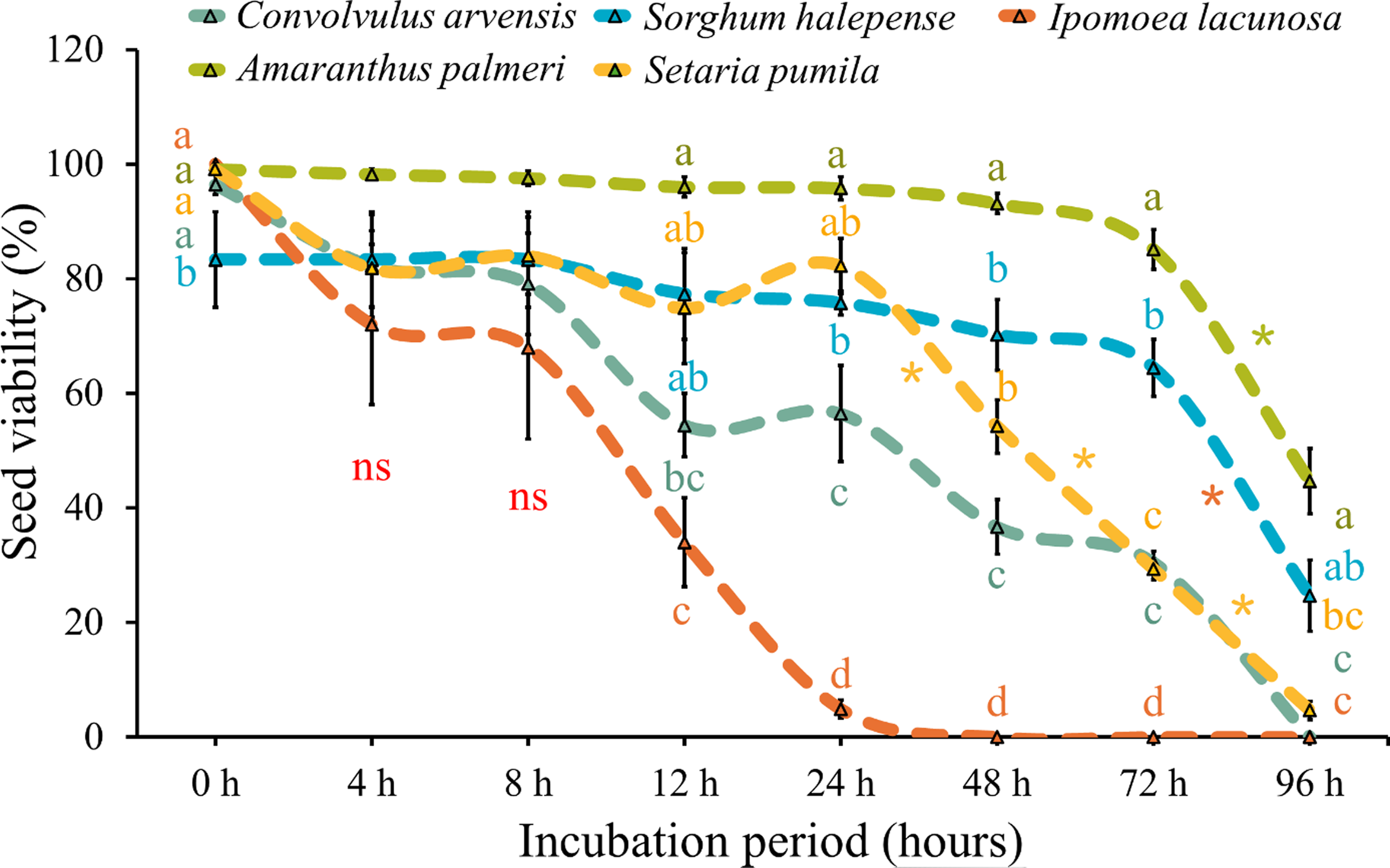
Figure 4. Effect of different duration of rumen fluid incubation on seed viability (germinated + dormant but viable seeds) of five weed species. Letters on the data points indicate significant difference (P < 0.05) among the species within each observation timing. Asterisks (*) indicate significant difference (P < 0.05) between two consecutive observation timings for an individual species. Errors bars indicate standard error of the mean (SEM).
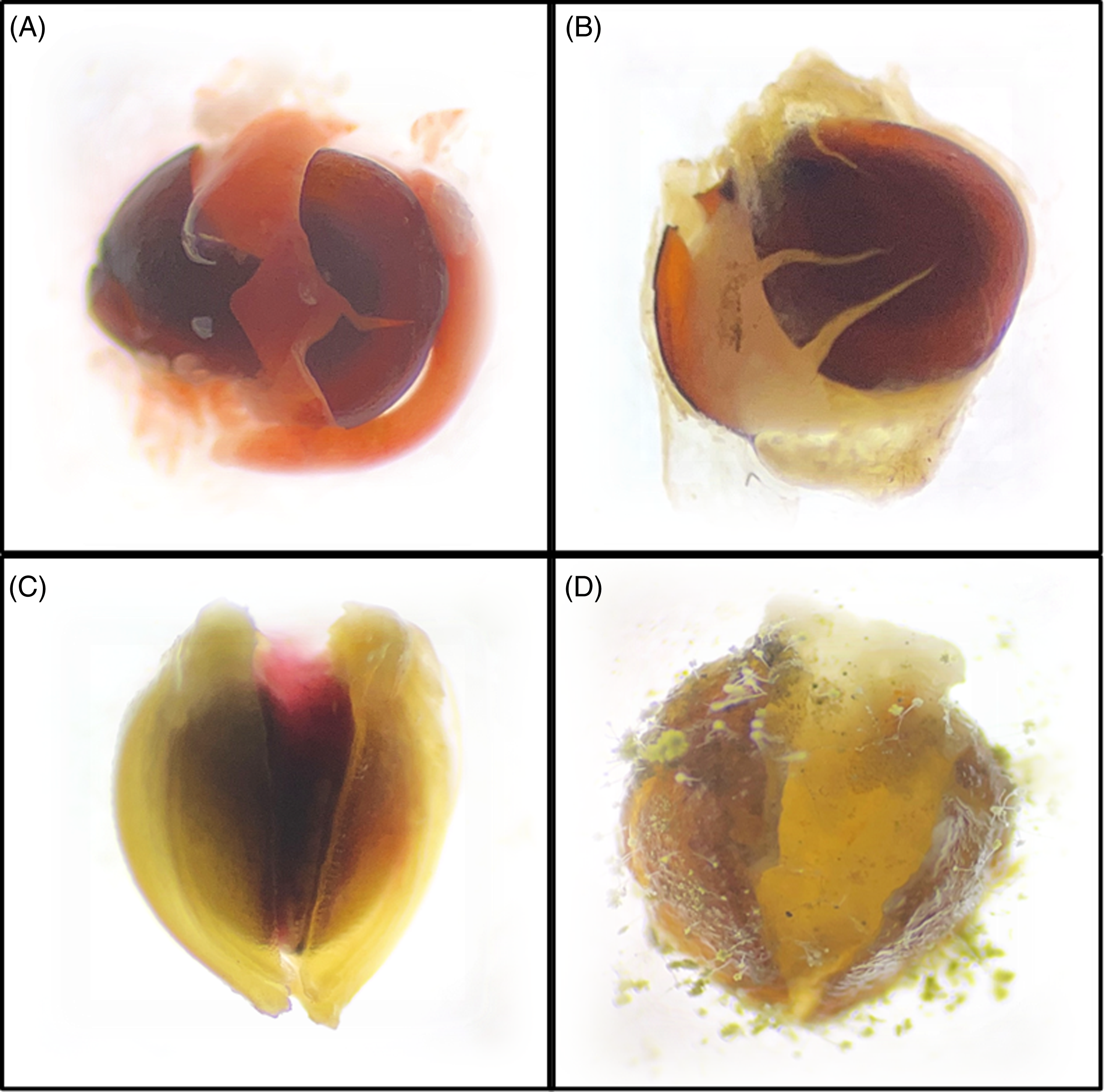
Figure 5. (A) Viable and (B) dead seed of Amaranthus palmeri; (C) viable and (D) dead seed of Setaria pumila, after a tetrazolium test under a Leica EZ4 Stereo Microscope at 35× magnification.
On the other hand, A. palmeri maintained relatively high viability throughout the incubation period, starting at 99% and decreasing to 47% by 96 h. Similarly, S. halepense started at 83% and decreased to 27% by 96 h. The slower decline in viability suggests that A. palmeri and S. halepense may have mechanisms like hard seed coats and high seed tannin content (Adjesiwor et al. Reference Adjesiwor, Liu, Felix and Alder2024; Bagavathiannan and Norsworthy Reference Bagavathiannan and Norsworthy2013; Burt Reference Burt1954; Egley and Chandler Reference Egley and Chandler1978; Schutte et al. Reference Schutte, Klypin and Shukla2016) that allow them to withstand the inhibitory effects of rumen fluid longer than other species. Blackshaw and Rode (Reference Blackshaw and Rode1991) found that seeds of redroot pigweed (Amaranthus retroflexus L.) and C. album retained viability rates of 45% and 52%, respectively, even after 24 h of rumen fluid incubation. This remnant viability suggests that even after being subjected to harsh conditions, such as digestion in the rumen, these seeds retain the post-excretion potential for germination upon onset of favorable environmental conditions. This adaptive strategy is critical for their survival and proliferation in competitive agricultural ecosystems.
Time to 50% Germination Reduction (G50)
All species exhibited a significant reduction in seed germination compared with the control (Figure 6). Amaranthus palmeri and S. halepense demonstrated a gradual decrease in germination over time, with the greatest reduction occurring within the first 24 h. In contrast, C. arvensis, I. lacunosa, and S. pumila showed a more rapid decline in germination, reaching 50% within the first 12 h. The time required to reduce germination by 50% (G50) also differed significantly (P < 0.001) among the species (Figure 6). The G50 values for individual weed species reflect their unique physiological and ecological adaptations to environmental conditions, particularly in response to rumen fluid treatment. The rapid G50 value for I. lacunosa of only 5 h indicates a high sensitivity to the inhibitory effects of rumen fluid. Convolvulus arvensis and S. pumila G50 values (11 and 12 h, respectively) suggest a moderate sensitivity to rumen fluid. The longer G50 for A. palmeri (42 h) and S. halepense (24 h) suggests a high level of resilience to the inhibitory effects of rumen fluid. The differences in G50 values indicate that different weed species exhibit varying degrees of sensitivity and resilience to rumen fluid treatment that ultimately dictate their establishment speed and success under natural conditions. The decline in germination and viability might be attributed to alterations in membrane and protein structures, as well as a rise in embryo mortality (Hendricks and Taylorson Reference Hendricks and Taylorson1976). Haidar et al. (Reference Haidar, Gharib and Sleiman2010) found that seed germination and viability of various weed species decreases with longer incubation in the rumen due to microbial activity. Additionally, volatile fatty acids from fermentation can inhibit germination by altering osmotic potential, as noted by Cordeiro et al. (Reference Cordeiro, Cabrita, Oliveira, Maia, Rodrigues, Fonseca and Valente2022).
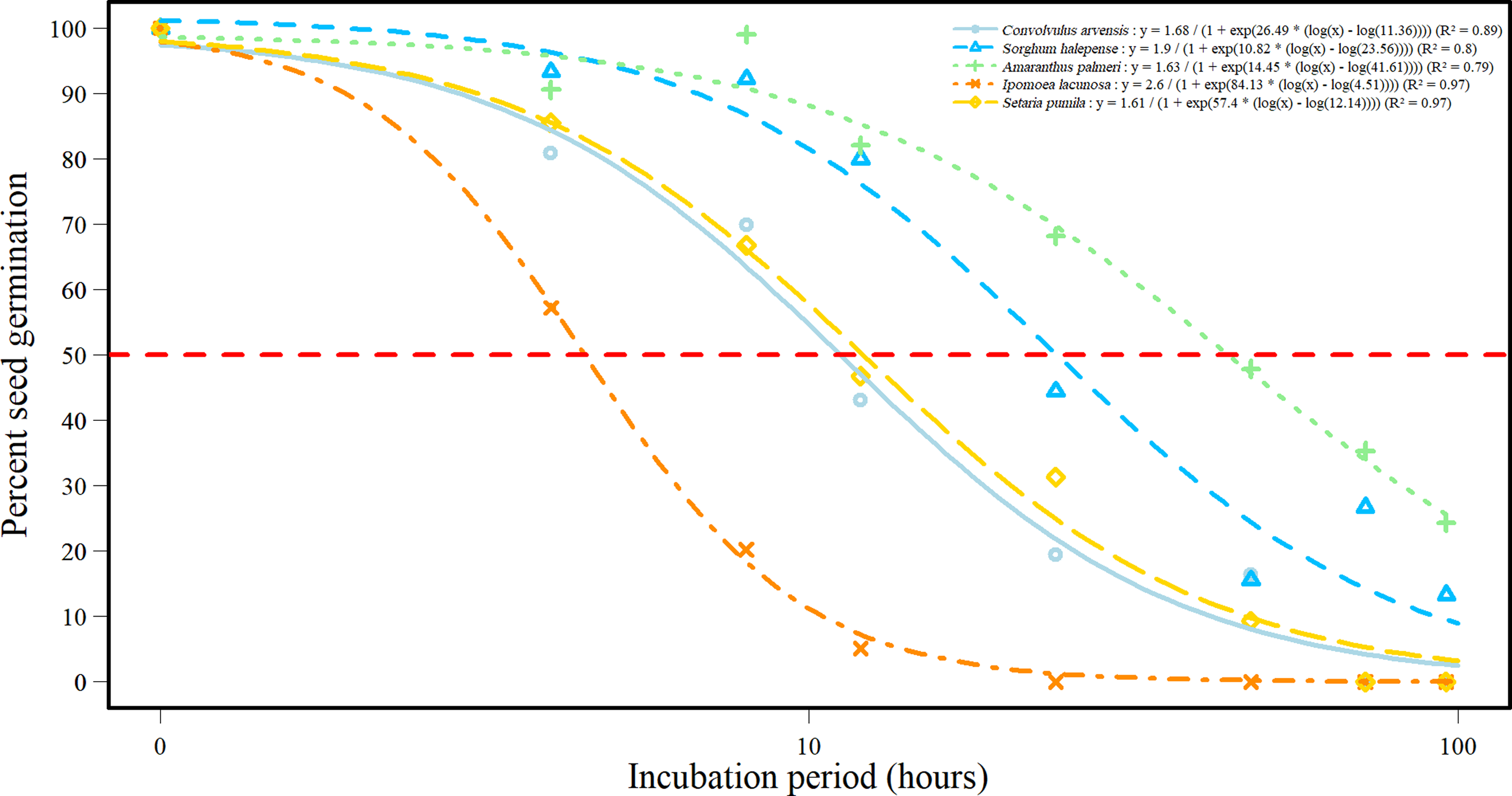
Figure 6. Percent germination response curve for five weed species over the incubation period at 3 wk after treatment with rumen fluid. The horizontal red dashed line in the plot represents 50% of the relative germination percentage. Data normalized to 100% according to control.
The R2 values for the fitted regression models indicate a strong relationship between incubation period and germination for all species, suggesting that rumen fluid treatment effectively inhibits seed germination in these weed species (Figure 6). The nonlinear model (Equation 1) provided a good fit to the germination data for all five weed species, as indicated by the high R2 values (0.79 to 0.97). However, the model’s predictive accuracy varied among species, as reflected in the root mean-square error (RMSE) values (Figure 6). RMSE values were higher for C. arvensis (12.33), S. halepense (17.00), and A. palmeri (14.05), suggesting that the model’s predictions for these species were less precise. In contrast, I. lacunosa and S. pumila had the lowest RMSE values (5.77 and 5.76, respectively), indicating a higher degree of model accuracy for these two species.
Seed Coat (Membrane) Integrity
Membrane integrity of seed coat was significantly affected by different periods of rumen fluid incubation among the weed species (Figures 7 and 8). The seed coats of I. lacunosa and C. arvensis were highly damaged after 48 h of incubation and were completely disintegrated at 96 h. The conductivity values for C. arvensis seeds increased from 31.09 µS cm−1 at 0 h to a peak of 70.71 µS cm−1 at 72 h, before slightly decreasing to 69.93 µS cm−1 at 96 h. This gradual increase in conductivity suggests a decline in membrane integrity over time, indicating leakage of solutes from the seed, which ultimately diminishes seedling vigor. Ipomoea lacunosa displayed the highest conductivity values, starting at 111.50 µS cm−1 and peaking at 408.73 µS cm−1 at 48 h, before declining to 133.95 µS cm−1 at 96 h. Declining value suggests that seeds were fully exhausted of ions and completely dead following 72 h of incubation. Sorghum halepense seeds had the least damage, even after 96 h of rumen fluid incubation. Amaranthus palmeri seeds were significantly damaged after 96 h of incubation, but plenty of seeds survived the rumen fluid. Amaranthus palmeri showed relatively low conductivity values, ranging from 4.62 µS cm−1 at 0 h to 11.19 µS cm−1 at 96 h. The low values indicate good membrane integrity and higher seed vigor, suggesting that this species is more resilient to stress conditions.
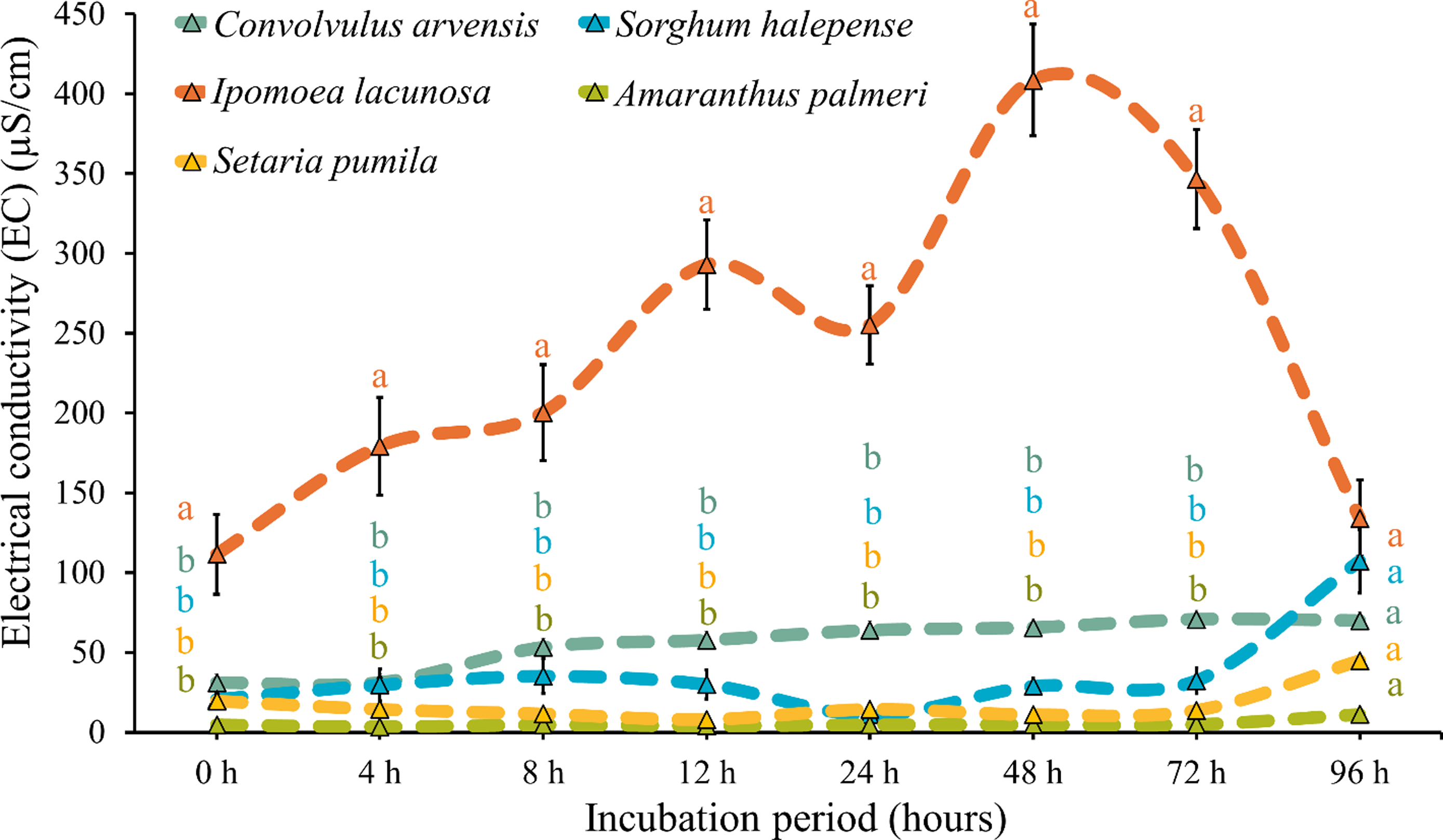
Figure 7. Membrane integrity test to examine damage to seed coat due to incubation in rumen fluid. Letters on the data points indicate significant difference (P < 0.05) among the species within each observation timing. There was no significant difference between two consecutive observation timings for an individual species. Errors bars indicate standard error of the mean (SEM).
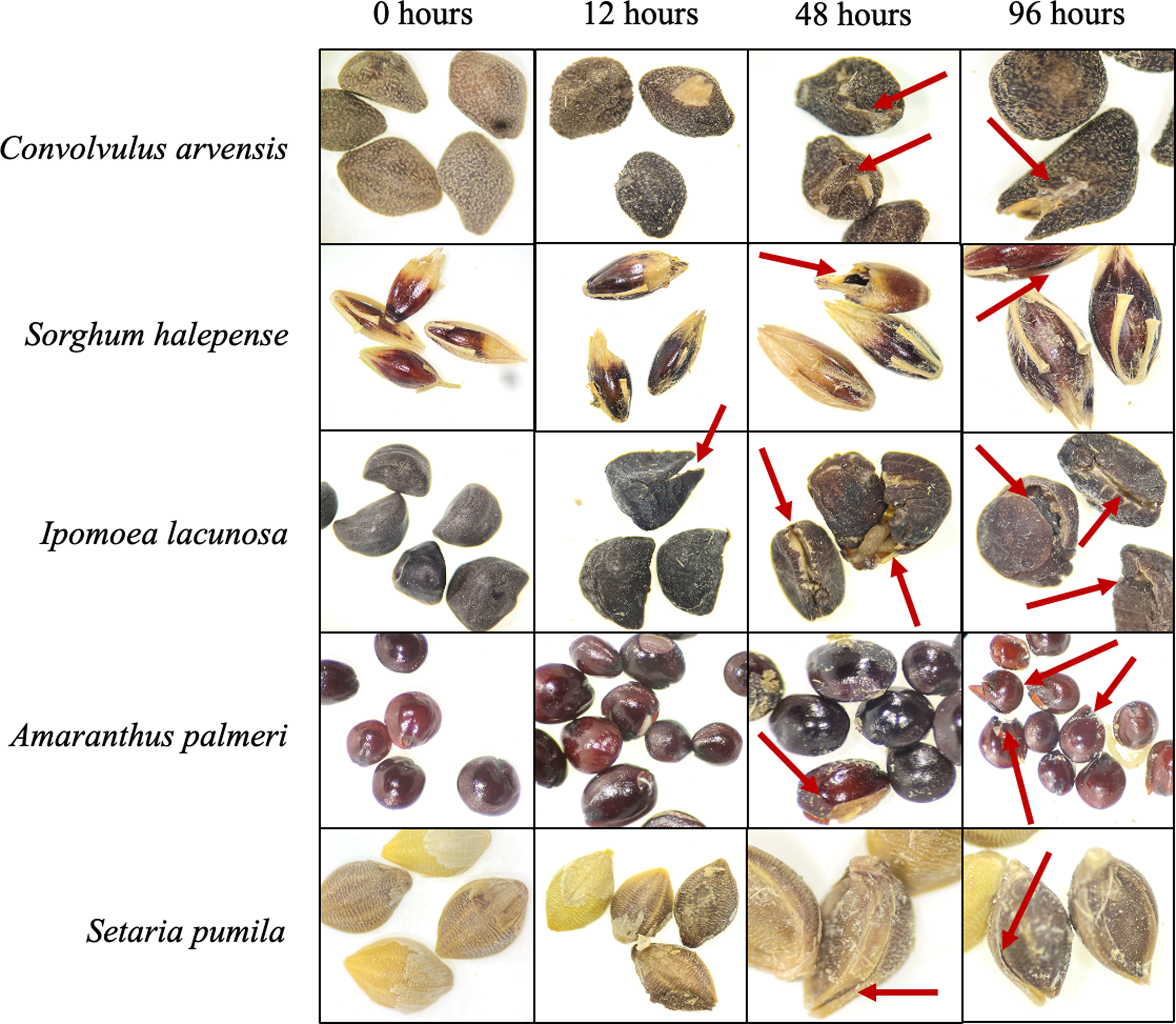
Figure 8. Seed morphology after incubation in rumen fluid at different time intervals under a Leica EZ4 Stereo Microscope at 35× magnification. Red arrows show the damage and cracks on seed coats with increasing duration of rumen fluid incubation.
Amaranthus palmeri’s hard seed coat may have contributed to its survival in rumen fluid, unlike I. lacunosa’s larger seed size and quickly imbibed seed coat due to the opening of the water gap in rumen fluid. Amaranthus spp. seeds have a high lignin content (Calabrò et al. Reference Calabrò, Oteri, Vastolo, Cutrignelli, Todaro, Chiofalo and Gresta2022) and peptides with antimicrobial activity (Lipkin et al. Reference Lipkin, Anisimova, Nikonorova, Babakov, Krause, Bienert, Grishin and Egorov2005), which primarily provides structural integrity and impermeability to seed coats. Additionally, lignin may protect seeds from microbial pathogens and various biotic and abiotic stresses (Tobimatsu et al. Reference Tobimatsu, Chen, Nakashima, Escamilla-Treviño, Jackson, Dixon and Ralph2013). On the other hand, S. halepense relies on its substantial hemicellulose and cellulose content for protective functions. The hard, impermeable seed coat blocks rumen fluid from entering the seed and acts as a barrier against microbial enzymes, ensuring seed survival (Michael et al. Reference Michael, Steadman, Plummer and Vercoe2006). The hard and impermeable seed coat of small-flowered mallow (Malva parviflora L.) helped it to survive rumen digestion (Michael et al. Reference Michael, Steadman, Plummer and Vercoe2006). On the other hand, Olson and Wallander (Reference Olson and Wallander2002) found that in some species, the seed coat softens over time in rumen fluid and becomes susceptible to microbial digestion.
Seedling Vigor
Seedling length and weight, which control the seedling vigor indices, decreased significantly over incubation time in most of the weed species but were not much affected in the case of A. palmeri (Figure 9). Seedling vigor, which indicates the strength of a seed or seedling to thrive and establish a healthy plant, was significantly affected by rumen fluid, and there was a gradual decrease in seedling vigor with increased duration of incubation for all the species (Figures 10 and 11). The initial vigor index of I. lacunosa was high, but it decreased to 0 after 24 h of exposure to rumen fluid. Similarly, S. pumila began with the highest seedling vigor index among all the species, which decreased to 0 by 72 h. This rapid decline suggests that, despite their higher initial vigor, both species are highly susceptible to the conditions imposed by rumen fluid. Although both species exhibited similar trends, S. pumila maintained its vigor for a longer duration. Similarly, the other three species showed lower initial values but sustained their vigor for a longer period. Although A. palmeri and S. halepense exhibited low initial vigor, they were able to maintain their vigor for the longest duration among all the species. Notably, A. palmeri and S. halepense retained substantial vigor even after 96 h of rumen fluid incubation, demonstrating their high resistance and adaptability to stress conditions. While A. palmeri and S. halepense are resilient, prolonged exposure to rumen fluid significantly impairs their growth potential. Early seedling vigor, a key seed-quality attribute, ensures rapid, uniform germination and strong seedling establishment under various environmental conditions, with high-vigor seeds providing better resistance to stresses compared with low-vigor seeds (Bourgeois et al. Reference Bourgeois, Munoz, Fried, Mahaut, Armengot, Denelle, Storkey, Gaba and Violle2019; Foolad et al. Reference Foolad, Subbiah and Zhang2007). Rapid seedling growth, positioning S. halepense as the leading species in terms of seedling size among dominant warm-season grasses, contributes significantly to its invasive success (Reichmann et al. Reference Reichmann, Schwinning, Polley and Fay2016).
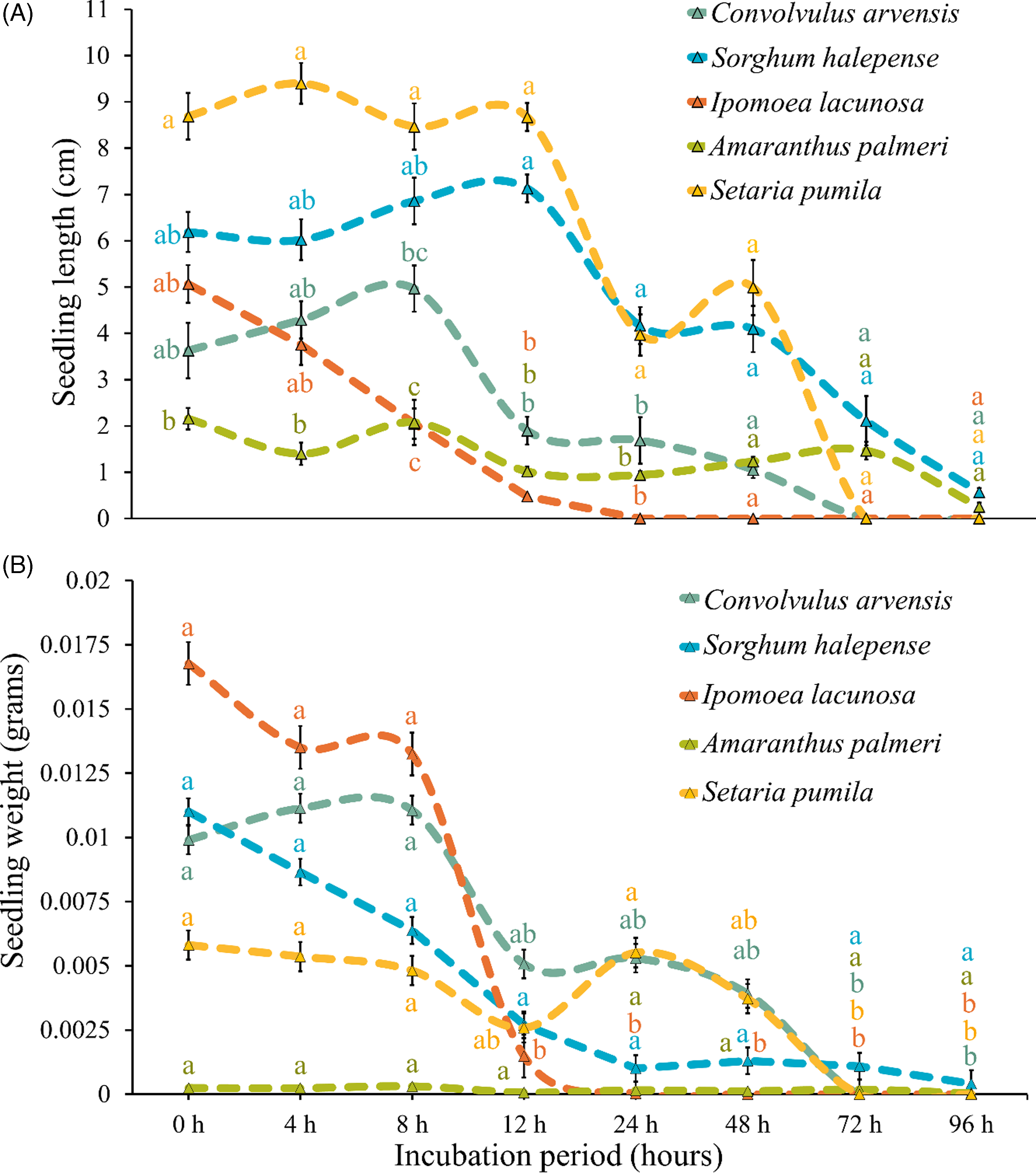
Figure 9. Effect of different durations of rumen fluid incubation on (A) seedling length and (B) seedling weight of five weed species. (A) Letters on the data points indicate significant difference (P < 0.05) among the species within each observation timing. There was no significant difference between two consecutive observation timings for an individual species. (B) Letters on the data points indicate significant difference (P < 0.05) between two consecutive observation timings for an individual species. There was no significant difference (P < 0.05) among the species within each observation timing. Errors bars indicate standard error of the mean (SEM).
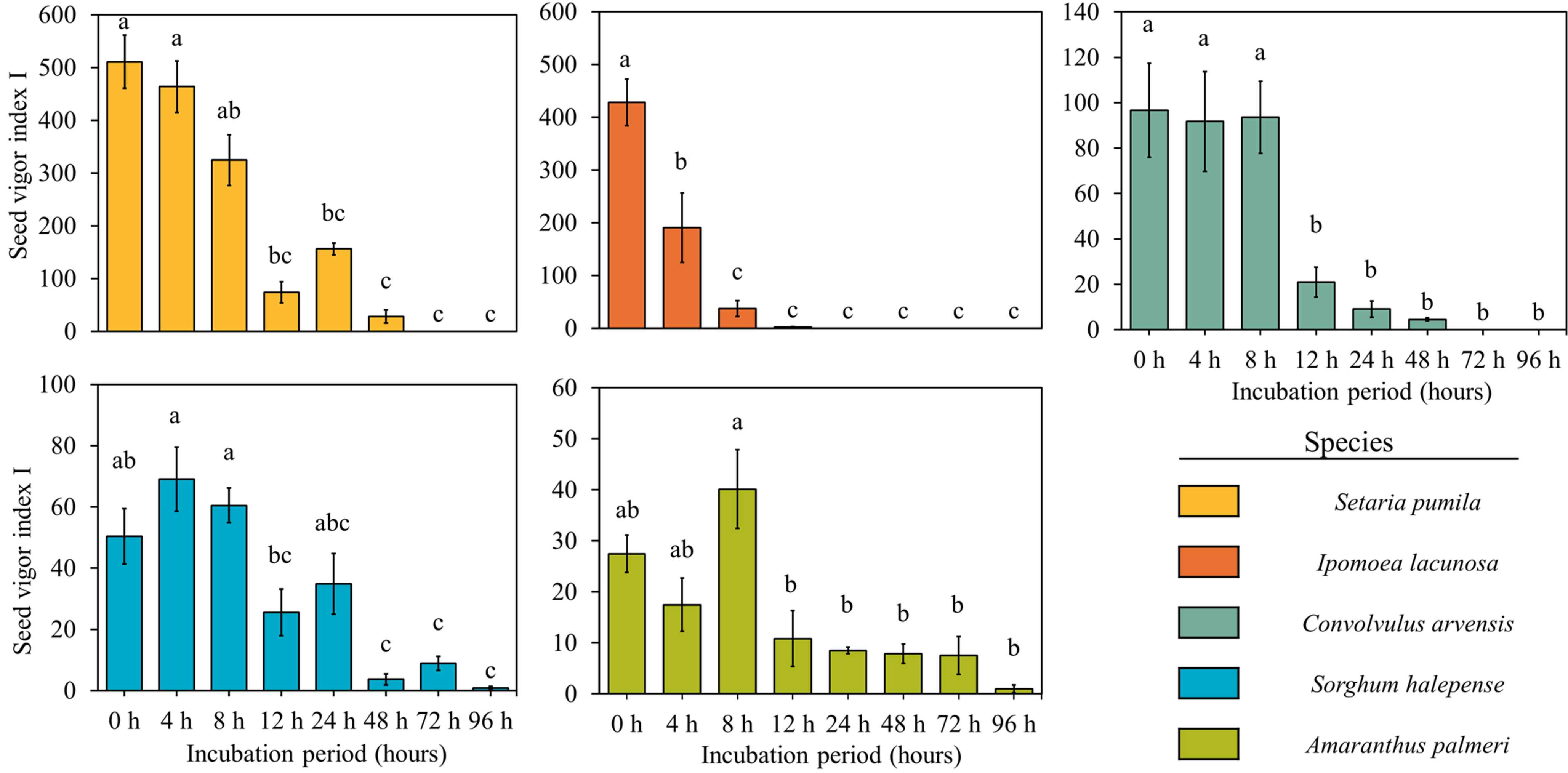
Figure 10. Effect of different duration of rumen fluid incubation on seedling vigor index I of five weed species. Bars with different letters indicate significant difference (P < 0.05) between two consecutive observation timings for an individual species. Errors bars indicate standard error of the mean (SEM).
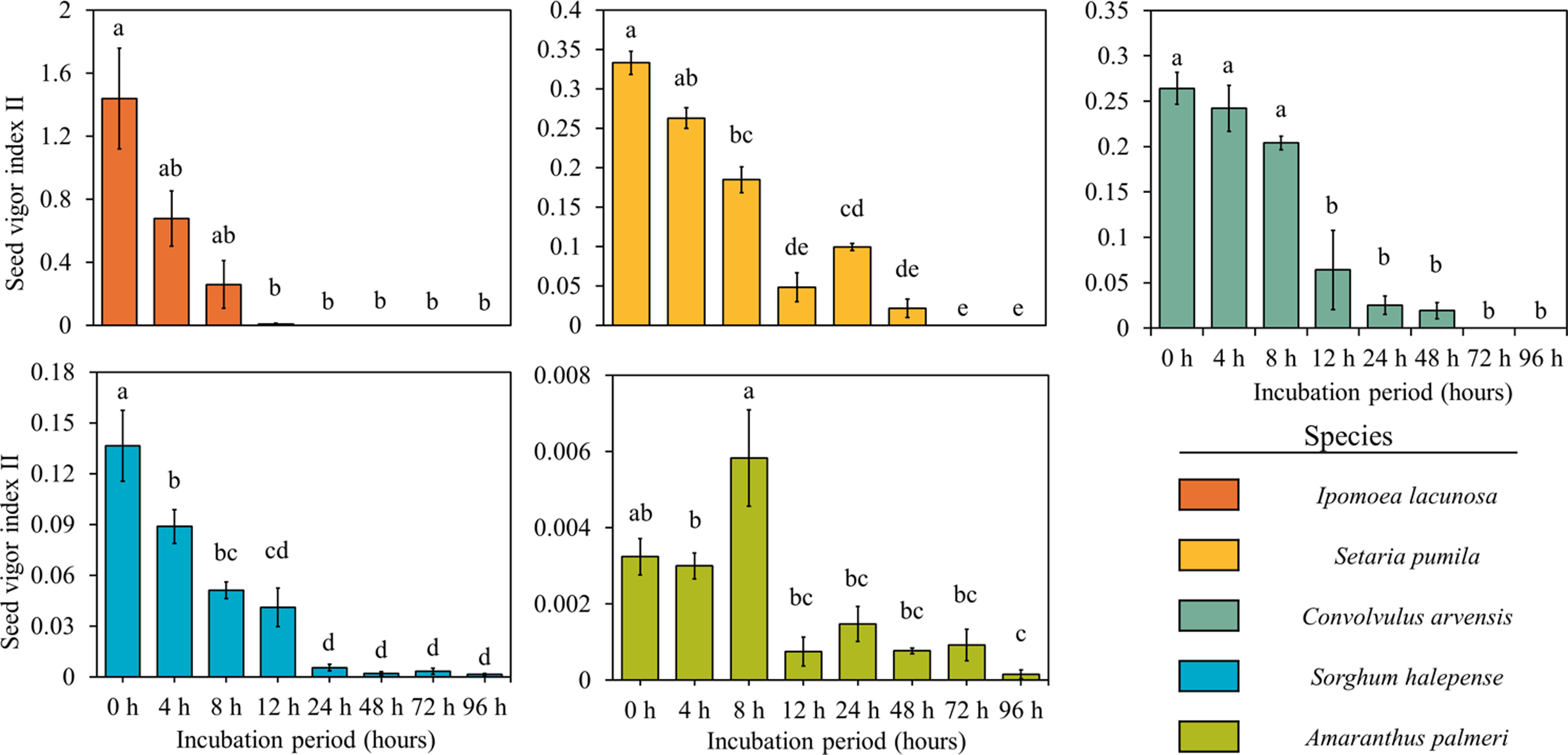
Figure 11. Effect of different duration of rumen fluid incubation on seedling vigor index II of five weed species. Bars with different letters indicate significant difference (P < 0.05) between two consecutive observation timings for an individual species. Errors bars indicate standard error of the mean (SEM).
Our study showed that passage of weed seeds through the digestive tract of cattle reduces the viability of weed seeds. Consequently, cattle grazing on weeds can help in decreasing the number of seeds entering the weed seedbank, further aided by the mechanical damage caused by their teeth (Stanton et al. Reference Stanton, Piltz, Pratley, Kaiser, Hudson and Dill2002). However, some seeds remain viable after digestion, depending on the weed species (Asaduzzaman et al. Reference Asaduzzaman, Piltz, Koetz, Hopwood, Shephard and Wu2022; Wang et al. Reference Wang, Lu, Waly, Ma, Zhang and Wang2017). Weed seeds are generally less affected by ingestion by cattle compared with sheep (Ovis spp.) or birds (Farmer et al. Reference Farmer, Webb, Pierce and Bradley2017; Gardener et al. Reference Gardener, McIvor and Jansen1993; Haidar et al. Reference Haidar, Gharib and Sleiman2010; Michael et al. Reference Michael, Steadman, Plummer and Vercoe2006). Thus, viable weed seeds in cattle excreta can potentially lead to weed infestations in fields (Wilson et al. Reference Wilson, Brusa, Christensen, Strack, Alto, Allen, Cortus, Modderman and Becker2022).
In our study, A. palmeri and S. halepense seeds showed a high tolerance to rumen fluid compared with I. lacunosa, C. arvensis, and S. pumila. Specifically, I. lacunosa seeds were dead after 48 h of incubation, with no germination observed after 24 h. Seeds of C. arvensis and S. pumila were almost dead after 96 h of incubation, with no germination occurring after 72 h. Amaranthus palmeri is particularly concerning due to its prolific seed production, with a single female plant capable of producing up to 600,000 seeds (Keeley et al. Reference Keeley, Carter and Thullen1987). Additionally, looking at the herbicide-resistance scenario in these two weeds (Heap Reference Heap2024), there is a risk of these seeds being spread by cattle and a potential risk of herbicide resistance spread. Results indicated that, unlike soft seeds, some hard-coated seeds remained viable in the digestive system of cattle for more than 4 d. Use of manure compost piles by farmers in the U.S. Southeast might contribute unknowingly to the spread of weeds and, consequently, herbicide resistance. Consequently, farmers should avoid grazing cattle in areas infested with weeds like A. palmeri and S. halepense, especially after the formation of viable seeds. Cattle that have grazed in such infested fields should not be moved to new, uninfested areas, and their fresh manure should not be used on arable lands. This is crucial, because cattle can inadvertently transport viable weed seeds through their manure, which can lead to the establishment of these weeds in previously noninfested fields.
Effective grazing management is crucial for herders aiming to mitigate weed invasion while optimizing livestock productivity. Cattle can consume various weeds like C. album, Canada thistle [Cirsium arvense (L.) Scop.], Setaria spp., and S. halepense. Marten and Anderson (Reference Marten and Andersen1975) observed that A. retroflexus, a species similar to A. palmeri, has forage nutritive value similar to high-quality alfalfa (Medicago sativa L.) when harvested at the vegetative stage. Strategic grazing timing can significantly affect weed phenology, particularly preventing flowering stages of certain weed species. Grazing before the flowering of summer annuals such as common ragweed can reduce local weed occurrence effectively; however, this has been shown to be context dependent and influenced by various factors throughout the growing season. Rotational grazing practices tailored to specific weed phenologies further enhance this effect by allowing herders to manage the grazing of different areas over time and avoid periods that favor weed reproduction.
Furthermore, the treatment of manure before field application can be an effective strategy to mitigate the risk of weed seed dispersal. Composting manure at high temperatures (≥60 C) can kill most of the viable weed seeds, thereby reducing the potential for these seeds to germinate and establish in agricultural fields (Larney and Blackshaw Reference Larney and Blackshaw2003). High temperature in combination with organic acids reduces seed viability during composting (Liu et al. Reference Liu, Wang, Awasthi, Chen, Awasthi, Duan and Zhang2020). This practice is particularly relevant for farmers who rely on manure as a fertilizer, as it allows them to utilize this resource without inadvertently introducing weed seeds into their cropping systems.
Acknowledgments
We are grateful to Diva Rigney, Madison Boone, and other team members of Brandon Smith’s group for their assistance with rumen fluid collection and digestion experiment.
Funding statement
We are grateful to the Alabama Farmers Federation (ALFA) for funding to DR and startup funds provided by Auburn University to AM for this research.
Competing interests
The authors declare that they have no conflicts of interest.














