Obsessive–compulsive disorder (OCD) is an impairing illness that, if left untreated, is characterised by a chronic, waxing and waning disease course. Reference Ruscio, Stein, Chiu and Kessler1 Selective serotonin reuptake inhibitors (SSRIs) are considered as first-line pharmacotherapy. Reference Bloch, Green, Kichuk, Dombrowski, Wasylink and Billingslea2–Reference Herzog, Osen, Stierle, Middendorf, Voderholzer and Koch4 Despite subtle differences in chemical structure, no differences in efficacy have been found between different SSRIs. Reference Miller, Shoup and Recktenwald5 Meta-analyses of short-term efficacy OCD studies have consistently found a small effect of SSRIs compared to placebo. Reference Skapinakis, Caldwell, Hollingworth, Bryden, Fineberg and Salkovskis6,Reference Soomro, Altman, Rajagopal and Oakley-Browne7 Non-response after adequate treatment with a SSRI, defined as a symptom decrease of 35% or less after treatment, occurs in 40–60% of patients. Reference Pallanti and Quercioli8 It is unknown whether the response to SSRIs is primarily driven by the reduction of obsessions or compulsions, or by an equal contribution of both. Attempts to identify baseline patient characteristics that modify treatment effects through meta-analyses have been unsuccessful. Reference Ackerman and Greenland9,Reference Piccinelli, Pini, Bellantuono and Wilkinson10 However, salient patient-level modifiers could remain undetected in regular meta-analyses with aggregate data, or findings could be positive by spurious correlations, caused by the confounding influence of an unidentified third variable. Reference Fisher, Copas, Tierney and Parmar11 In contrast, an individual patient data meta-analysis (IPDMA) can mitigate these limitations by using crude data to detect within-study associations before pooling between-study results. Furthermore, IPDMA enables data standardisation and applying consistent analysis methods for each trial, thereby providing more reliable results. Reference Tierney, Vale, Riley, Smith, Stewart and Clarke12
Using crude patient data of short-term, double-blind placebo-controlled trials of SSRIs in adult OCD, we aimed to analyse overall efficacy and acceptability of SSRIs for the treatment of OCD, and to explore patient-level modifiers of efficacy.
Method
We pre-specified analyses and we preregistered the study protocol before commencement of the analysis (registration identifier https://doi.org/10.17605/OSF.IO/KVMN6). We followed Preferred Reporting Items for Systematic Review and Meta-Analyses of individual participant data (PRISMA-IPD) guidelines for data analysis and presentation. As our study is not a systematic review of the literature, we clearly noted in the PRISMA-IPD checklist which points did not apply (see the supplementary material available in https://doi.org/10.1192/bjp.2025.87).
Data selection
We obtained crude trial data of studies submitted to the Dutch regulatory authority (Medicines Evaluation Board, MEB) for registration and market authorisation. We included double-blind, randomised, placebo-controlled short-term efficacy trials with SSRIs for the treatment of OCD, performed between 1988 and 2004 in adult (18 years and over) patients with a DSM-III, 13 III-R, 14 -IV 15 classification of OCD. Collection of trial sets from the records of the MEB was carried out between 2022 and 2024, with the first data extracted on 26 August 2022. Final preparation of the dataframe, including potential patient-level modifiers, was done after finalisation of the study protocol.
The MEB database encompasses studies on new drug applications, including both positive and negative trial outcomes. It includes all SSRIs that are approved for the treatment of OCD in Europe. Reference Koran, Hanna, Hollander, Nestadt and Simpson16 We included short-term efficacy trials with a study duration of between 8 and 16 weeks, as is recommended by the guideline for clinical investigation of medical products for the treatment of OCD, by the European Medicines Agency Committee for Medical Products for Human use (CHMP). 17 All trials used the Yale–Brown Obsessive–Compulsive Scale (YBOCS) for measuring symptom severity. In trials with a third clomipramine study arm, we excluded patients randomly assigned to clomipramine.
Outcomes and data extraction
Our primary outcome was the mean difference in YBOCS change scores between SSRIs and placebo at week 12. If week 12 was not available, we selected the outcome closest to week 12. Secondary outcomes were the difference in response rate between active treatment and placebo at the study end-point, with response defined as a ≥35% reduction in YBOCS score from baseline, and the difference in acceptability. We operationally defined acceptability as the percentage of patients completing the full trial period, and not dropping out because of harm, non-response or any other reason. Furthermore, we analysed the mean difference between active treatment and placebo in obsessions and compulsions. Obsessions were defined as YBOCS questions 1−5 and compulsions as YBOCS questions 5−10.
We used the following patient-level characteristics as potential effect modifiers: age, gender, weight, duration of illness, use of an antidepressant (clomipramine or SSRI) in medical history, smoking status, symptom severity at baseline (as measured by the YBOCS) and depression symptom score at baseline (using the Hamilton Rating Scale for Depression (HDRS), after converting all scores to equivalents of the HDRS-17). We further extracted study region and ethnicity of patients; however, because of unequal distributions of groups, we were not able to include these in our quantitative analysis.
After deliberation of the study team, clinical variables were chosen on the basis of likelihood of availability and clinical relevance. Selection of treatment modifiers was further guided by a previously conducted aggregated data meta-analysis, IPDMA of SSRIs for paediatric OCD and multiple studies on prognostic factors for pharmacotherapy among which there was an exploratory IPDMA in SSRI trials for depression. Reference Cohen, Zantvoord, Storosum, Mattila, Daams and Wezenberg18–Reference Denys, Burger, van Megen, de Geus and Westenberg22
Study quality
Two investigators (S.E.C. and B.W.S.) independently used the Risk of Bias 2.0 tool (Cochrane; see https://methods.cochrane.org/risk-bias-2) to assess risk of bias for our primary outcome, using our analysis plan and individual patient data analysis as reference for outcome measures. Reference Sterne, Savović, Page, Elbers, Blencowe and Boutron23 Any discrepancies were resolved through consensus or discussion with a third reviewer (J.B.Z., T.K.M. and/or A.d.B.). We used the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) assessment to rate the certainty of evidence of our primary outcome. Reference Prasad24
Data analysis
Data was checked for completeness and consistency. We used an adjusted intent-to-treat analysis including all randomised patients for which at least one follow-up measure was recorded after randomisation. We handled missing data by linear interpolation using a last-observation carried forward (LOCF) approach. As a sensitivity analysis, we used multiple imputation by chained equations (MICE), as well as a completer analysis, that is, only including those patients who reach the week of the study end-point. For the MICE analysis we leveraged baseline and end-point data, as well as intermediate (auxiliary) outcome measurements collected between the baseline and final end-point.
We conducted a two-stage IPDMA; in the first stage, we estimated efficacy and treatment–modifier interactions for each study separately with treatment arm (active versus placebo) as the independent variable, correcting for baseline symptom severity, gender and age. For calculating the standardised mean difference (SMD), we used Hedges’ g. We used odds ratios for meta-analysis of response and acceptability.
In the second stage, we pooled individual study findings using a random effects meta-analysis with Hartung–Knapp correction and a restricted maximum likelihood estimator, assuming between-study variability. We quantified heterogeneity with tau-squared and used I-squared to estimate the proportion of variability attributable to heterogeneity. In addition to a 95% confidence interval, we computed a 95% prediction interval to estimate the range within which future patient outcomes are expected to fall. We calculated the number needed to treat (NNT) using the odds ratio for response and weighted control event rate (CER).
For analysis of potential effect modifiers, we used a similar two-stage approach. In the first stage we included an interaction term (treatment*covariate) for the patient characteristics described earlier, also correcting for baseline severity, gender and age. In the second stage, we calculated the pooled effect of the regression coefficient (beta), with a 95% confidence interval indicating the uncertainty of the coefficient.
In a post hoc analysis we combined the results for obsessions and compulsions, and then performed subscale as a moderator, accounting for study-level random effects. Furthermore, we repeated our efficacy analyses including clomipramine in the active arm (which was excluded in the preplanned analysis). In a head-to-head comparison of clomipramine versus SSRIs in three trials, we calculated differences in efficacy and acceptability. Finally, in the seven trials where Clinical Global Impression Scale – Improvement (CGI-I) outcomes were available, we included a post hoc analysis in which we used a more narrow definition of ‘response’, that is, a YBOCS score of 35% or more, and a CGI-I of 1 or 2, meaning much improved or very much improved. Reference Mataix-Cols, Fernández de la Cruz, Nordsletten, Lenhard, Isomura and Simpson25
We used R version 4.1 for Windows (R Foundation for Statistical Computing, Vienna, Austria; see https://cran.r-project.org/), with packages lme4 and dmetar. 26–Reference Harrer, Cuijpers, Furukawa and Ebert28
Results
Included trials and patient characteristics
From the 14 industry-sponsored trials in our database, we included 11 in our analysis (see Table 1 for an overview of trial characteristics and Supplementary Fig. 1 for the flowchart). We excluded two trials (n = 541) because the sponsors had not provided outcome data, and one trial (n = 466) because the sponsor had not provided information on patient randomisation. We contacted the sponsors but did not receive further details of their proprietary data, meaning we were able to report on 79% of trials in our data-set, and 73% of crude patient data. Three trials used a third clomipramine arm (total n = 267), which we excluded for our main analysis, resulting in a total of 2372 patients (885 placebo, 1487 SSRI treatment). Study sites were mostly located in the USA and the majority of patients were White. From the included sample, results of seven trials were published in peer-reviewed journals, while results of four were not.
Table 1 Trial characteristics
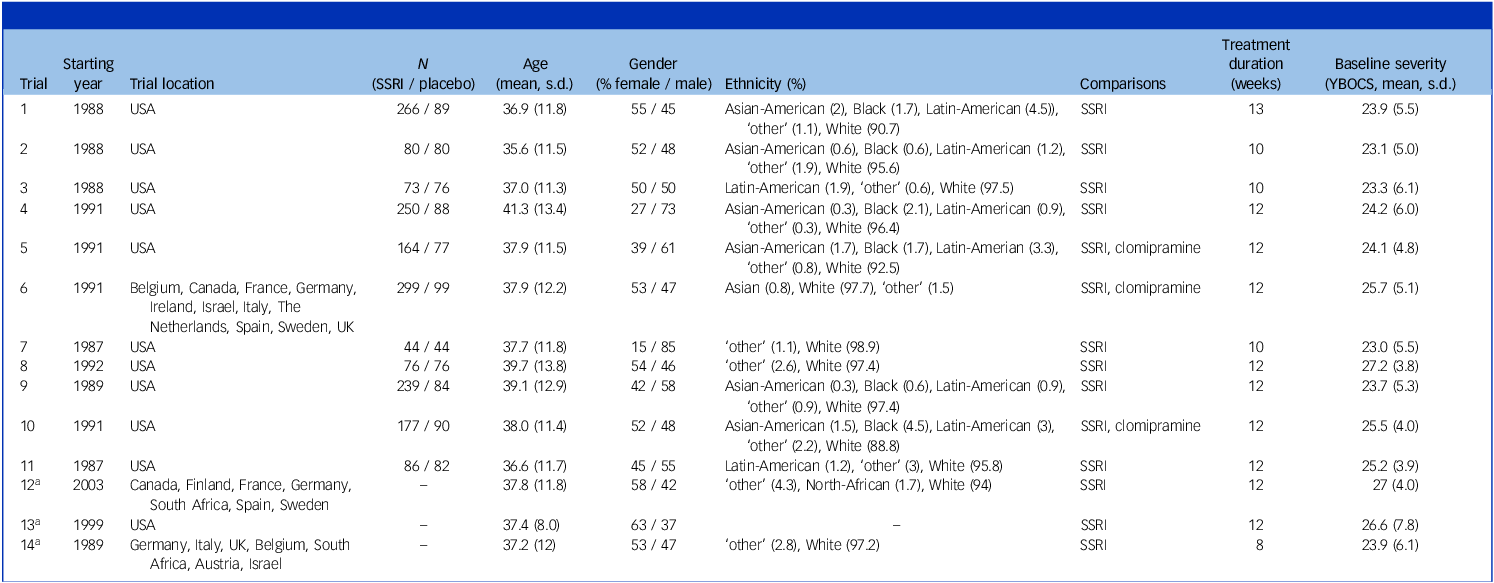
SSRI, selective serotonin reuptake inhibitor; YBOCS, Yale–Brown Obsessive–Compulsive Scale.
Ethnicity was labelled as ‘other’ only if the ethnicity was not specified in the original data.
a. Trials 12–14 are not included in our analyses as information regarding randomisation and follow-up is missing. Here, we do give an overview of the baseline characteristics of the study.
In the full group, 45% of patients were female and mean age was 38 years (12.3 s.d.). Mean YBOCS score at baseline was 24.5 (4.5 s.d.); 28% had mild, 56% moderate and 16% severe symptoms. Reference Cervin, Consortium and Mataix-Cols29 Mean duration of illness was 17.4 years (12.3 s.d.). For an overview of patient characteristics at baseline separately for the active and placebo treatment arm, see Table 2.
Table 2 Patient characteristics at baseline
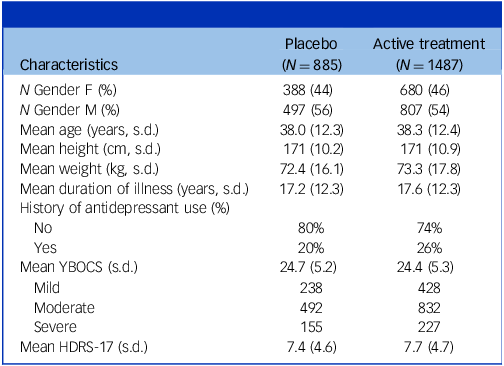
HDRS-17, Hamilton Depression Rating Scale – 17 items; YBOCS, Yale–Brown Obsessive–Compulsive Scale. Severity categories ‘mild’ (YBOCS ≤21), ‘moderate’ (YBOCS 22–29 and ‘severe’ (YBOCS ≥ 30) are according to empirically validated benchmarks. Reference Cervin, Consortium and Mataix-Cols29
Risk of bias analysis
Risk of bias was low for seven trials (see Fig. 1). In three trials, there were some concerns regarding the randomisation process, as the trial protocols did not explicitly state the allocation sequence generation and the allocation sequence concealment. One trial, which did not specify allocation sequence generation, showed significant differences in baseline groups. Even though this might suggest chance baseline imbalances rather than issues with randomisation, this could not be ascertained as the allocation sequence was unclear. Thus, according to current recommendations, we judged this trial to be at high risk of bias. Reference Corbett, Higgins and Woolacott30
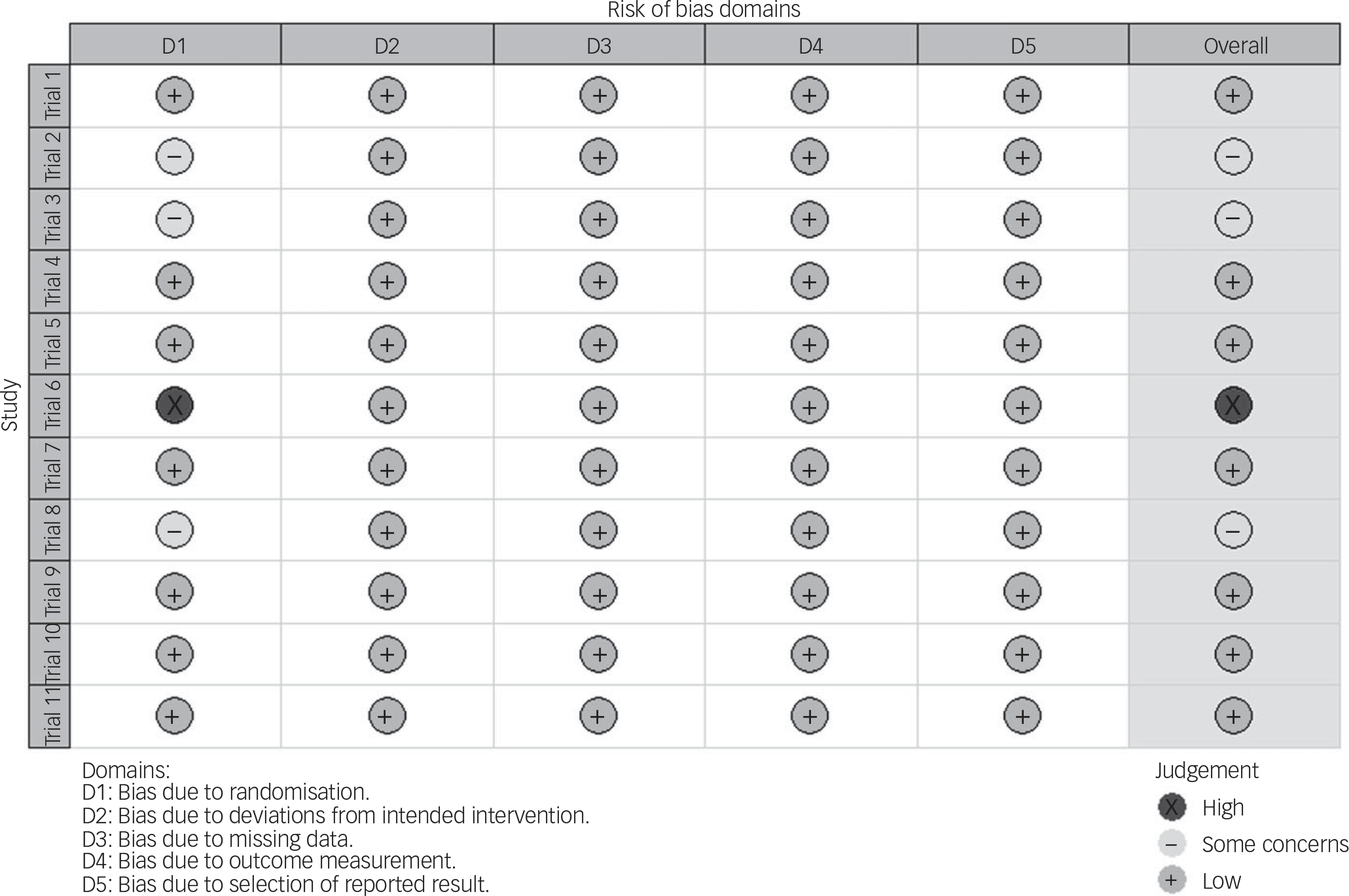
Fig. 1 Analysis of the risk of bias according to the Cochrane Risk of Bias 2.0 tool. Reference Noma, Furukawa, Maruo, Imai, Shinohara and Tanaka20
Efficacy of SSRIs compared to placebo
Our two-stage random effects meta-analysis of the 11 included randomised controlled trials (RCTs) resulted in a mean difference of 2.65 YBOCS points of active treatment compared to placebo (95% CI 1.85–3.46, p < 0.0001; see Fig. 2) and a SMD of 0.33 Hedges’ g (95% CI 0.23–0.44), equalling a small effect size. The 95% prediction interval of the mean difference was 0.89–4.4. Measures for heterogeneity showed non-significant variability (Q 15.1, p = 0.13), an I-squared of 34% and tau-squared of 0.48. Analysis of response showed an odds ratio of 2.21 in favour of active treatment versus placebo (95% CI 1.72–2.83, p < 0.0001, see Fig. 3), with a 95% prediction interval from 1.71 to 2.84, with a CER of 0.18 and a NNT of seven. Acceptability was equal in the active and placebo groups (odds ratio 0.96 in active treatment compared to placebo, 95% CI 0.67–1.37, p = 0.81). For an overview of attrition rates per trial, see Supplementary Table 1.
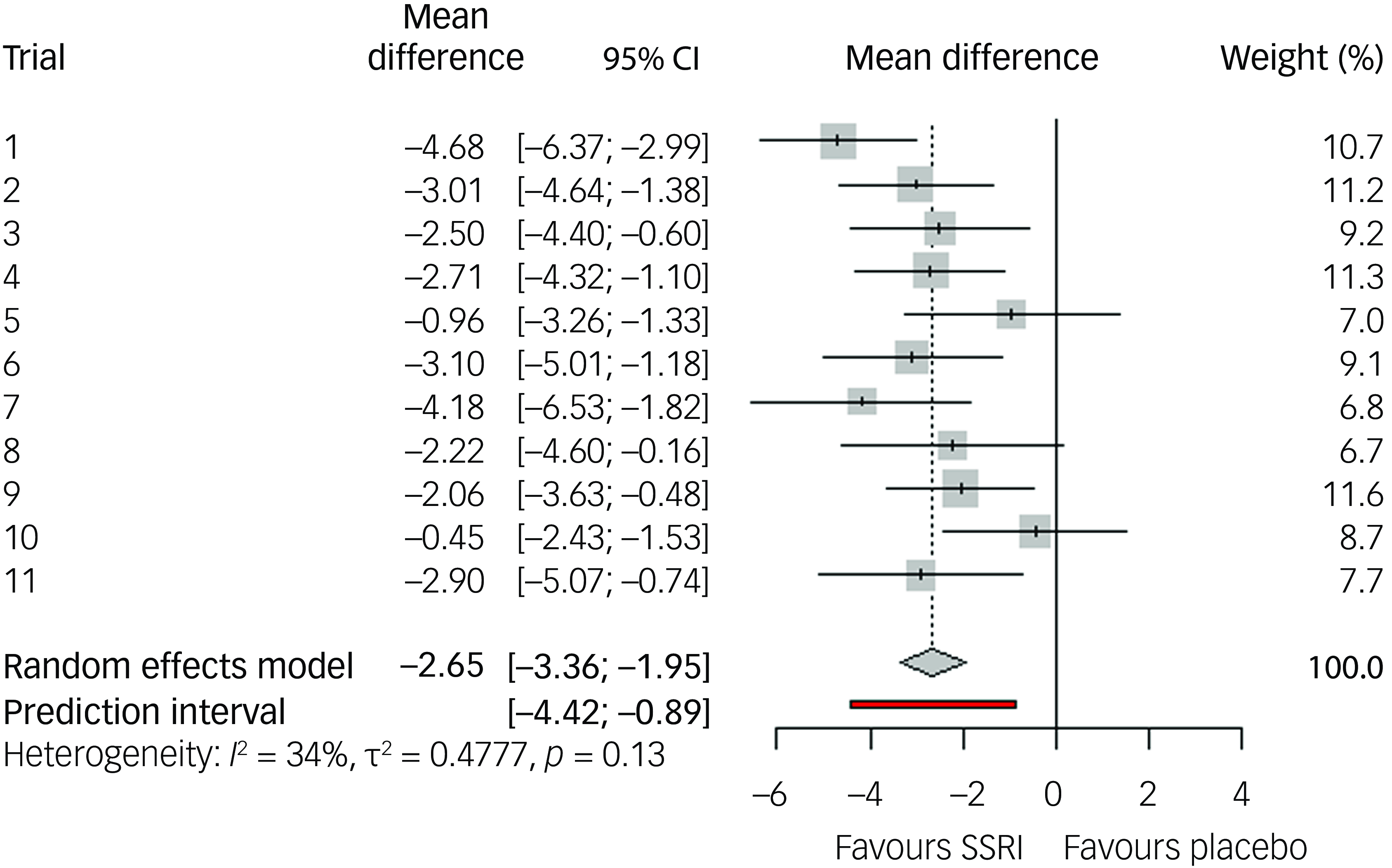
Fig. 2 Effect size regarding change in Yale–Brown Obsessive–Compulsive Scale symptoms compared to baseline, for selective serotonin reuptake inhibitors (SSRIs) compared to placebo, random effects model.
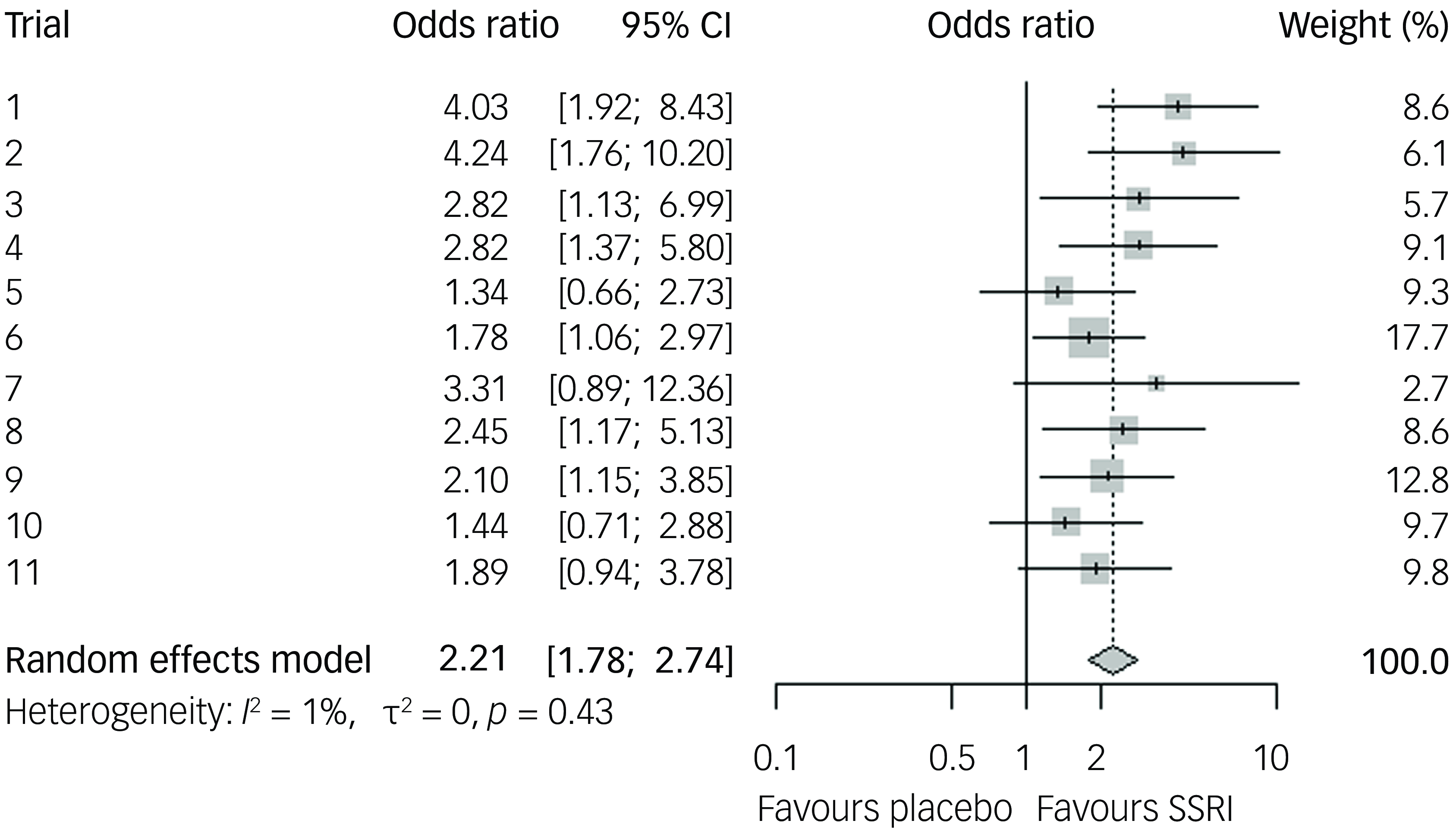
Fig. 3 Odds ratio for response of 35% or more compared to baseline, for selective serotonin reuptake inhibitors (SSRIs) compared to placebo, random effects model.
Sensitivity analyses with a complete-case analysis yielded a slightly increased mean difference and odds ratio for response, while multiple imputation yielded comparable point estimates with increased heterogeneity (see Supplementary Figs 2 and 3).
Efficacy for obsessions and compulsions separately
We used ten out of 11 trials for calculating the mean difference in obsessions and compulsions, as for one trial the YBOCS subscales were not available. For obsessions, mean difference was 1.53 in favour of active treatment compared to placebo (95% CI 1.03–2.03, p < 0.0001), against 1.05 for compulsions (95% 0.58–1.52, p < 0.0001), meaning 59% of symptom change was caused by reduction of obsessions, and 41% was caused by reduction of compulsions. A post hoc meta-regression comparing outcomes between obsessions and compulsions shows an estimate of –0.46 YBOCS points (95% CI −0.92 to 0.0042, p = 0.052), indicating a trend towards greater improvement in obsessions, although it did not reach statistical significance.
Effect modification
Age, gender, baseline illness severity, baseline depressive symptoms, weight at baseline, duration of illness and history of SSRI use did not show effect modification for mean difference and response (for a full table of regression coefficients, see Supplementary Table 2).
Post hoc analyses
Including clomipramine in the analysis had little impact on the overall efficacy. In the three trials where a head-to-head comparison of clomipramine versus a SSRI was possible, mean difference was in favour of clomipramine but did not reach statistical significance. A post hoc analysis using a more narrow definition of response did not change the odds ratio for response, with a CER of 0.15 and a NNT of six. A post hoc sensitivity analysis removing non-published trials from the meta-analysis resulted in a mean difference of 3.1 YBOCS points (95% CI 2.2–4.0). For details on the results from the post hoc meta-analyses, see Supplementary Table 3.
GRADE assessment
We graded the quality of evidence as high for the primary outcome (YBOCS point change) and the secondary outcome (≥35% response on the YBOCS), because of high precision with a substantial sample size, consistency of results, directness of the effect and a predominantly low risk of bias with some concerns in the allocation sequence domain that most likely did not influence effect estimates.
Discussion
This is the first IPDMA of placebo-controlled SSRI trials in adult OCD. We included data from 11 short-term efficacy trials, with a duration between 10 and 13 weeks, which were submitted to the Dutch MEB for market authorisation. We showed that SSRIs were well accepted and significantly more effective than placebo in primary and secondary outcome measures.
The mean difference in symptom score reduction of 2.65 YBOCS points equals a small effect size and is 0.5–1 YBOCS points lower than current estimates in aggregate data meta-analyses. Reference Skapinakis, Caldwell, Hollingworth, Bryden, Fineberg and Salkovskis6,Reference Soomro, Altman, Rajagopal and Oakley-Browne7 Furthermore, we found a NNT of seven, which is two more than was found by a Cochrane meta-analysis. Reference Soomro, Altman, Rajagopal and Oakley-Browne7 The difference in efficacy outcomes might be explained by the advantage that we used crude patient data, which allowed us to process the data and employ analysis methods consistently across trials, where aggregate data meta-analyses depend on published results. Reference de Boer, Waterlander, Kuijper, Steenhuis and Twisk31,Reference Holmberg and Andersen32 Another advantage is that we included four non-published trials that were not included in earlier systematic reviews, three of which were negative studies. Reference Skapinakis, Caldwell, Hollingworth, Bryden, Fineberg and Salkovskis6,Reference Soomro, Altman, Rajagopal and Oakley-Browne7,Reference Cohen, Zantvoord, Storosum, Mattila, Daams and Wezenberg18 Removing these negative trials resulted in an inflated effect size that resembles results from aggregate data meta-analyses, which may point to publication bias in OCD SSRI trials.
At the group level, the symptom reduction achieved with SSRIs compared to placebo is half as large as the minimal important difference (MID) identified in recent studies of OCD trials. Reference Cohen, Zantvoord, Mattila, Storosum, de Boer and Denys33,Reference Cervin, Storch, Farrell, Andersson, Rück and Geller34 The MID, the point at which a certain amount of symptom reduction becomes clinically meaningful, is dependent on baseline severity but was found to be about 5–6 YBOCS points on average. It is important to acknowledge, however, that individual patient responses within trials vary considerably, and a group-level effect smaller than the MID does not preclude the possibility of substantial treatment benefit for some patients. The MID should therefore be interpreted as an indicator of clinically meaningful change for individual patients, rather than a definitive threshold for clinical significance at the group level. Nevertheless, comparing the observed group-level effects of SSRIs to the MID highlights the modest magnitude of these effects and underscores the importance of carefully weighing the potential benefits against the risks of side-effects when considering treatment.
We included only studies submitted for registration and not published investigator-initiated trials. Therefore, we cannot exclude the possibility of bias in our effect estimates. However, results from sponsored studies still seem to reflect general information regarding evidence, as a recent systematic review of all published placebo-controlled OCD pharmacotherapy trials from this research group found that from a total of about 4000 included patients in serotonin reuptake inhibitor trials, including clomipramine, only 5.6% were from non-industry-sponsored trials. Reference Cohen, Zantvoord, Storosum, Mattila, Daams and Wezenberg18 Furthermore, in this systematic review and meta-analysis a trend towards a small negative influence on efficacy of sponsorship was found, in contrast to earlier evidence for investigator bias inflating effect size in medical trials in general. Reference Lundh, Lexchin, Mintzes, Schroll and Bero35
SSRIs did not differ relative to placebo regarding acceptability, defined as the percentage of patients completing the full trial period, and not dropping out because of harm, non-response or any other reason. In both the placebo group and the SSRI group, 22% of patients dropped out before the week of the primary end-point. This is similar to earlier results found in a meta-analysis of drop-out in placebo groups for OCD. Reference Li, Nasir, Olten and Bloch36 In major depressive disorder (MDD), SSRIs were also found to be as acceptable as placebo. Reference Cipriani, Furukawa, Salanti, Chaimani, Atkinson and Ogawa37 These results indicate that, even though adverse effects of SSRIs are common and may be a reason for discontinuation, SSRIs are as well accepted as a placebo. Reference Carvalho, Sharma, Brunoni, Vieta and Fava38,Reference Kelly, Posternak and Alpert39 This might be because of the higher efficacy of SSRIs compared to placebo (and therefore less attrition because of non-response). Acceptability, as an outcome measure, does not offer a distinction between drop-out caused by adverse events, lack of efficacy or other reasons. It is therefore important for trialists to investigate the reasons for attrition in both placebo and active treatment arms to gain a more nuanced understanding of tolerability. A key advantage of acceptability as an outcome is its applicability to both trial and real-world data, where drop-out rates are often available, but reasons for discontinuation may not be recorded. It also facilitates comparisons with psychological interventions, where adverse events are less commonly reported. Reference Duggan, Parry, McMurran, Davidson and Dennis40 However, acceptability in well-controlled trials may be higher than in clinical practice, as trial participants are often more motivated to complete the study and benefit from more intensive guidance and monitoring.
To our knowledge, this study is the first meta-analysis of effects of SSRIs on obsessions and compulsions separately. Both were significantly reduced by SSRIs compared to placebo. Differences between obsessions and compulsions did not reach statistical significance, although there was a trend in favour of obsessions, and 59% of total mean difference was caused by reduction of obsessions. Earlier literature reviews suggested that SSRIs affect obsessions more than compulsions, but this has not been shown empirically. Reference Starcevic and Brakoulias41 Although our results are informative, the interdependence of obsessions and compulsions should warrant careful interpretations because these subscales do not measure fully separate phenomena.
We further showed that the effect size of SSRIs in OCD is independent of baseline patient characteristics. This is especially notable for duration of illness, which is associated with worse outcomes of pharmacological treatment in naturalistic follow-up studies and retrospective studies. Reference Albert, Barbaro, Bramante, Rosso, De Ronchi and Maina42–Reference Dell’Osso, Buoli, Hollander and Altamura44 A difference might be that these studies subdivided their samples in long and short duration of illness using a cut-off of 2–3 years, which is short compared to the mean duration of illness in our sample of 17 years. When considered as a continuous variables, the same studies did not find a predictive effect of duration of illness on treatment outcomes. Reference Dell’Osso, Buoli, Hollander and Altamura44 Furthermore, our meta-analysis uses a placebo control arm, controlling for natural fluctuations in symptom severity that could be more present in patients with a short duration of illness. It should be noted that, even though all studies were double-blind, reporting on unblinding caused by apparent side-effects was absent. The presence of unblinding in double-blind SSRI RCTs is debated and in SSRI trials in MDD the evidence regarding bias caused by blinding issues on the effect size is scarce. Reference Hieronymus, Lisinski, Nilsson and Eriksson45–Reference Scott, Sharpe and Colagiuri47 To our knowledge, evidence for the influence of blinding success on efficacy is currently lacking for trials on antidepressants in OCD.
Overall, our findings suggest that SSRIs are generally effective, encouraging their prescription regardless of baseline characteristics. However, the fact that only one-third of patients with active treatment showed response is worrying and emphasises the need for novel solutions to prevent non-response and treatment delay. Response prediction might be achieved using non-linear machine learning algorithms based on clinical patient characteristics or a combination of clinical and non-clinical factors. Reference Rajpurkar, Yang, Dass, Vale, Keller and Irvin48
Our study has strengths. To our knowledge, this is the first IPDMA of placebo-controlled RCTs with SSRIs for OCD in adults. Using crude patient data allowed for a consistent and robust meta-analysis, and for within-study regression with potential, patient-level effect modifiers. In this unique sample of proprietary data, we were able to include 79% of all relevant premarketing trials that were submitted to the Dutch regulatory authority. There are some limitations, however. One important limitation is the fact that two of the three excluded trials were conducted outside the USA. This increases the overrepresentation of the US population in our efficacy findings. Although, to our knowledge, there is no evidence to suggest that effect sizes differ between geographic regions, it does potentially impede generalisability of meta-regression findings and effect estimates. Also, we were unable to mention specific studies and pharmacological compounds because of confidentiality agreements with the trial sponsors. Even though efficacy does differ between SSRIs, the fact that we could not examine the drugs individually may conceal relevant differences between drugs regarding modifying patient characteristics. In addition, our sample size may not have been large enough to reliably detect or precisely estimate potential effect modifications.
As pre-specified in our protocol, we used LOCF as the primary method for handling missing data to align with published OCD trials, ensuring comparability with both original studies and aggregate data meta-analyses. Reference Mavridis, Salanti, Furukawa, Cipriani, Chaimani and White49 However, LOCF assumes data are missing completely at random, which is rarely the case, and may introduce bias by distorting treatment effects and artificially inflating precision. Reference Rioux and Little50 To address this, we conducted a secondary analysis using MICE. Reference Azur, Stuart, Frangakis and Leaf51 Notably, overall efficacy estimates remained consistent across both imputation methods, supporting the robustness of our findings. Nevertheless, our reliance on LOCF for meta-regression remains a limitation, as biases inherent to this approach may have influenced these outcomes. Also, although MICE with auxiliary data from intermediate measurements can capture information relevant to certain mechanisms of missingness (e.g. drop-out caused by non-response), we were unable to include data on side-effects. Consequently, any drop-out driven by adverse events may not be fully captured by our imputation model.
Regarding outcomes, not all potential modifiers were present in each study, which might have decreased the power of our analysis. OCD subtypes, such as contamination, harm or sexual obsessions, which have been found to influence the success of standard clinical care, were wholly unavailable. Reference Herzog, Osen, Stierle, Middendorf, Voderholzer and Koch4 We were unable to include risk of harm or adverse events as these data were unavailable from our database, even though this is an important factor in risk–benefit assessment. Finally, we did not include patient-reported outcome measures (PROMs) or quality of life measures, as these were not conducted in the included trials. Including PROMs as a clinical outcome could add a novel dimension to assessment of efficacy, which more directly reflects patient experience. Reference Weldring and Smith52
In conclusion, we found that SSRIs are effective for OCD compared to placebo, with a small effect size of 2.7 YBOCS points and a NNT of seven, which was not modified by studied patient characteristics. These findings emphasise the need for novel approaches to improve the success of pharmacological treatments for OCD and to identify characteristics that can help allocate interventions more effectively to improve patient outcomes.
Supplementary material
The supplementary material can be found at https://doi.org/10.1192/bjp.2025.87
Data availability
All data was retrieved form the database of the Medicines Evaluation Board (MEB). As this pertains to proprietary data, this data is not publicly available.
Acknowledgement
We gratefully acknowledge the help of L.F. Loomans, BSc, for assisting in retrieving, preparing and cleaning the crude participant data.
Author contributions
S.E.C. is guarantor of the manuscript. S.E.C. retrieved, cleaned and analysed the data. T.K.M. supervised data retrieval and preparation. S.E.C., B.W.S. and J.B.Z. created the first draft of the manuscript. A.d.B. and D.D. supervised the writing process. S.E.C., B.W.S., J.B.Z., T.K.M., A.d.B. and D.D. interpreted the findings and critically revised and approved the manuscript. All authors had final responsibility for the decision to submit for publication.
Funding
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Declaration of Interest
None.
Analytic code availability
Code used for analysis of our data is available from the corresponding author, upon reasonable request.









eLetters
No eLetters have been published for this article.