Introduction
The processing of starchy plant foods to render them edible often demands a variety of implements and methods depending on the context and palatability and/or toxicity of the plant taxa in question (Pedley, Reference Pedley1992, Reference Pedley1993; Cosgrove et al., Reference Cosgrove, Field and Ferrier2007; Messner, Reference Messner2011:16). Numerous investigations around the globe have focussed on grinding and/or pounding stones to better understand function and use of both raw material and plant exploitation (Fullagar et al., Reference Fullagar, Field, Kealhofer, Rowan and R2008; Piperno et al., Reference Piperno, Holst, Weiss and Nadel2004, Reference Piperno, Ranere, Holst, Iriarte and Dickau2009; Liu et al., Reference Liu, Field, Fullagar, Zhao, Chen and Yu2010; Shaw et al., Reference Shaw, Field, Summerhayes, Coxe, Ford, Arifeae, Coster, Jacobsen, Fullagar, Hayes and Kealhofer2020; Hayes et al., Reference Hayes, Field, Coster, Fullagar, Matheson, Florin, Nango, Djandjomerr, Marwick, Wallis, Smith and Clarkson2020, Field et al., Reference Field, Coster, Shaw, Hayes, Fullagar, Lovave, Haro, Summerhayes, Shaw, Gaffney and Ford2024). In Australia and New Guinea, ethnographic studies have been important for gaining insights to starchy plant use across different environments and cultural groups, e.g. Bulmer Reference Bulmer1964; Watson, Reference Watson1964; O’Connell et al., Reference O’Connell, Latz and Barnett1983; Sillitoe, Reference Sillitoe1983. Processing methods and the associated stone tool technologies can show great variation. The keen observations of various researchers have provided a foundation on which ‘ancient starch’ studies have developed (e.g., Reichert, Reference Reichert1913; Tindale, Reference Tindale and Wright1977; O’Connell et al., Reference O’Connell, Latz and Barnett1983; Sillitoe, Reference Sillitoe1983; Smith, Reference Smith1985; Pedley Reference Pedley1992).
The physical remains from the processing of starchy plants can persist for millennia, as shown in the discovery of use-related residues on the surface of flaked and ground stone artefacts (Van Peer et al., Reference Van Peer, Fullagar, Stokes, Bailey, Moeyersons, Steenhoudt, Geerts, Vanderbeken, de Dapper and Geus2003, Birarda et al., Reference Birarda, Badetti, Cagnato, Sorrentino, Pantyukhina, Stani, Dal Zilio, Khlopachev, Covalenco, Obada, Skakun, Sinitsyn, Terekhina, Marcomini, Lubritto, Cefarin, Vaccari and Longo2023). These use-related residues can sometimes be found alongside other plant fossils including macrobotanical remains such as nutshells (Fairbairn and Swadling, Reference Fairbairn and Swadling2005; Fairbairn et al., Reference Fairbairn, Hope and Summerhayes2006; Cosgrove et al., Reference Cosgrove, Field and Ferrier2007; Field et al., Reference Field, Kealhofer, Cosgrove and Coster2016).
The study of wear traces and use-related residues on stone tools from archaeological contexts have the potential to reveal activities and plant food choices that may otherwise remain unknown (Fullagar et al., Reference Fullagar, Field, Denham and Lentfer2006, Reference Fullagar, Field, Kealhofer, Rowan and R2008; Shaw et al., Reference Shaw, Field, Summerhayes, Coxe, Ford, Arifeae, Coster, Jacobsen, Fullagar, Hayes and Kealhofer2020; Field et al., Reference Field, Coster, Shaw, Hayes, Fullagar, Lovave, Haro, Summerhayes, Shaw, Gaffney and Ford2024). Plant-related use residues occur as starch grains, raphides (calcium oxalate crystals), phytoliths and other plant cell tissues (Van Peer et al., Reference Van Peer, Fullagar, Stokes, Bailey, Moeyersons, Steenhoudt, Geerts, Vanderbeken, de Dapper and Geus2003; Fullagar et al., Reference Fullagar, Field, Kealhofer, Rowan and R2008; Piperno et al., Reference Piperno, Ranere, Holst, Iriarte and Dickau2009; Field et al., Reference Field, Kealhofer, Cosgrove and Coster2016, Reference Field, Summerhayes, Luu, Coster, Ford, Mandui, Fullagar, Hayes, Leavesley, Lovave and Kealhofer2020), providing the opportunity for a multifaceted approach to identifying specific plant taxa.
Our interest has principally been in starch grain identification using objective criteria to develop a diagnostic tool, as highlighted by Cortella and Pochettina (Reference Cortella and Pochettino1994). Now over a century ago, Reichert (Reference Reichert1913) published the first images of starch grains from an array of plants and plant parts. The discipline of ancient starch studies has grown quickly, with reports of starch grains as residues on archaeological finds and in calculus on teeth, now representing an important and routine component of archaeological research (Pearsall, Reference Pearsall1989; Piperno, Reference Piperno2006; Torrence and Barton, Reference Torrence and Barton2006; Tromp et al., Reference Tromp, Matisoo-Smith, Kinaston, Bedford, Spriggs and Buckley2020, Hayes et al., Reference Hayes, Field, Coster, Fullagar, Matheson, Florin, Nango, Djandjomerr, Marwick, Wallis, Smith and Clarkson2021).
Attributing unknown starch grains to plant taxa has proven to be a significant challenge (Wilson et al., Reference Wilson, Hardy, Allen, Copeland, Wrangham and Collins2010). Various approaches have claimed success in this endeavour, mainly through the identification of diagnostic grain shapes, comparison of gross morphologies, and maximum dimensions through the hilum (growth centre of the starch grain). Some approaches have involved qualitative assessments supported in part by starch grain metrics and visual confirmation (Louderback et al., Reference Louderback, Wilks, Herzog, Brown, Joyce and Pavlik2022), while others have applied statistical methods, such as Discriminant Function Analysis (DFA) (Allen and Usher, Reference Allen and Ussher2013) as well as Geometric Morphometric approaches (GMA) (Coster and Field, Reference Coster and Field2015, Reference Coster, Field, Anderssen, Broadbridge and Fukumoto2018). More recently, Birarda et al. (Reference Birarda, Badetti, Cagnato, Sorrentino, Pantyukhina, Stani, Dal Zilio, Khlopachev, Covalenco, Obada, Skakun, Sinitsyn, Terekhina, Marcomini, Lubritto, Cefarin, Vaccari and Longo2023) have combined optical and scanning electron microscopy with Fourier Transform Infrared Spectroscopy (FTIR) to assess ancient starch residues.
Robust statistical methods such as GMA, have successfully attributed unknown starch grains to plant taxa in different contexts and environments (Field et al., Reference Field, Kealhofer, Cosgrove and Coster2016, Reference Field, Summerhayes, Luu, Coster, Ford, Mandui, Fullagar, Hayes, Leavesley, Lovave and Kealhofer2020; Owen et al., Reference Owen, Field, Luu, People, Stephenson and Coster2019; Hayes et al., Reference Hayes, Field, Coster, Fullagar, Matheson, Florin, Nango, Djandjomerr, Marwick, Wallis, Smith and Clarkson2021). Our methodology for identifying unknown starch grains has been evolving over the last decade (Coster and Field Reference Coster and Field2015, Reference Coster, Field, Anderssen, Broadbridge and Fukumoto2018, Field et al., Reference Field, Kealhofer, Cosgrove and Coster2016, Reference Field, Coster, Shaw, Hayes, Fullagar, Lovave, Haro, Summerhayes, Shaw, Gaffney and Ford2024; Hayes et al., Reference Hayes, Field, Coster, Fullagar, Matheson, Florin, Nango, Djandjomerr, Marwick, Wallis, Smith and Clarkson2021) and has now moved beyond a strictly GMA approach. While the long-term aim has been to develop a quantitative approach to starch grain identification in concert with expert input, a diverse approach to the topic remains across different research groups.
The primary aim of this paper is to present the most recent developments in our approach to starch grain identification by revisiting the analysis of material from Kuk Swamp in the PNG highlands (Denham et al., Reference Denham, Haberle, Lentfer, Fullagar, Field, Therin, Porch and Winsborough2003; Fullagar et al., Reference Fullagar, Field, Denham and Lentfer2006). We also examine whether it is possible to discriminate between starch grains of similar size and morphology from different plant species and discuss the implications for starch analyses going forward.
Background
Quantitative approaches to starch grain analysis have expanded our understanding of the breadth of plant use in different environments where starch is preserved (see also Field et al., Reference Field, Summerhayes, Luu, Coster, Ford, Mandui, Fullagar, Hayes, Leavesley, Lovave and Kealhofer2020, Reference Field, Coster, Shaw, Hayes, Fullagar, Lovave, Haro, Summerhayes, Shaw, Gaffney and Ford2024; Hayes et al., Reference Hayes, Field, Coster, Fullagar, Matheson, Florin, Nango, Djandjomerr, Marwick, Wallis, Smith and Clarkson2021). Establishing a consensus on a clear and systematic approach to investigating starch as use-related residues on artefacts remains one of the significant problems in ancient starch studies from discovery to analysis, including handling methods and the laboratories in which starches are analysed (Haslam, Reference Haslam2004, Crowther et al., Reference Crowther, Halsam, Oakden, Walde and Mercader2014). In our laboratory at the University of New South Wales (UNSW), careful handling of artefacts and the stringent use of controls during sampling and processing has been effective in identifying and eliminating potential contamination that may affect our analyses.
The ability to correctly identify plant taxa in use-related residues or sediments has important implications for discussions of plant use in any context. The timing of introductions into new environments, the adoption of plant processing strategies, including complex processing, and the antiquity of certain plant species use, all feed into global debates about plant domestication and exploitation, as well as technological developments through time (e.g. Denham et al., Reference Denham, Fullagar and Head2009; Matthews et al., Reference Matthews, Nguyen and Smith2018; Ahmed et al., Reference Ahmed, Lockhart, Agoo, Naing, Nguyen, K and Matthews2020; Shaw et al., Reference Shaw, Field, Summerhayes, Coxe, Ford, Arifeae, Coster, Jacobsen, Fullagar, Hayes and Kealhofer2020).
The first human arrivals in Northern Sahul (New Guinea) occurred around 50,000—65,000 years ago and was followed by a rapid human dispersal across Sahul (Pleistocene Australia-New Guinea landmass) (Bowler et al., Reference Bowler, Johnston, Olley, Prescott, Roberts, Shawcross and Spooner2003; Denham et al., Reference Denham, Fullagar and Head2009; Clarkson et al., 2017). The rapidity of colonisation has been attributed, in part, to the dynamic nature of human adaptation to new environments which, in Sahul, included a suite of previously unknown flora and fauna (Denham et al., Reference Denham, Fullagar and Head2009).
Over the last few hundred years, sweet potato (Ipomoea batatas) has dominated the diets of people in the New Guinea highlands due in part to its potential for cultivation at higher altitudes. Developing an understanding of the exploitation of plant resources in the time periods preceding the introduction of sweet potato has largely relied on inferences from pollen and phytolith records (Denham et al., Reference Denham, Haberle, Lentfer, Fullagar, Field, Therin, Porch and Winsborough2003; Haberle, Reference Haberle2003; Hope, Reference Hope2009). Macrobotanical remains have also provided empirical support for the antiquity of specific plant use such as bananas and Pandanus (Denham et al., Reference Denham, Haberle, Lentfer, Fullagar, Field, Therin, Porch and Winsborough2003; Fairbairn et al., Reference Fairbairn, Hope and Summerhayes2006; Summerhayes et al., Reference Summerhayes, Leavesley, Mandui, Fairbairn, Field, Fullagar and A2010; Lentfer et al., Reference Lentfer, Matthews, Gosden, Lindsay and Specht2013).
Ancient starch studies have now confirmed the widespread exploitation of tree nuts across the highlands, along with a range of subterranean storage organs such as tubers and yams. The evidence is derived from formally manufactured mortars and pestles from the PNG highlands that were dated to the mid-Holocene. These included Joe’s Garden in the Ivane Valley, and at Waim (Shrader Range) and Imbiben in the Upper Kaironk Valley of the Madang Province (Field et al., Reference Field, Summerhayes, Luu, Coster, Ford, Mandui, Fullagar, Hayes, Leavesley, Lovave and Kealhofer2020, Reference Field, Coster, Shaw, Hayes, Fullagar, Lovave, Haro, Summerhayes, Shaw, Gaffney and Ford2024; Shaw et al., Reference Shaw, Field, Summerhayes, Coxe, Ford, Arifeae, Coster, Jacobsen, Fullagar, Hayes and Kealhofer2020). Speculation about the use of other starch-producing plants first proposed in the early 1960s can now be addressed through these new methodologies (Bulmer, Reference Bulmer1964; Field et al., Reference Field, Summerhayes, Luu, Coster, Ford, Mandui, Fullagar, Hayes, Leavesley, Lovave and Kealhofer2020; Hayes et al., Reference Hayes, Field, Coster, Fullagar, Matheson, Florin, Nango, Djandjomerr, Marwick, Wallis, Smith and Clarkson2021).
The Kuk Swamp artefact K/76/S29B and ancient starch
Artefact K/76/S29B is the distal end of a broken flake tool, recovered from Phase 1 of the Kuk Swamp sediments dated to c. 10,000 cal yr BP (Figure 1). Usewear traces and residue extractions for Artefact K/76/S29B were first studied more than three decades ago—initially by Tom Loy, then by Richard Fullagar and later with Judith Field, Michael Therin and Carol Lentfer (Denham et al., Reference Denham, Haberle, Lentfer, Fullagar, Field, Therin, Porch and Winsborough2003; Fullagar et al., Reference Fullagar, Field, Denham and Lentfer2006; Fullagar and Golson, Reference Fullagar, Golson, Golson, Denham, Hughes, Swadling and Muke2020).
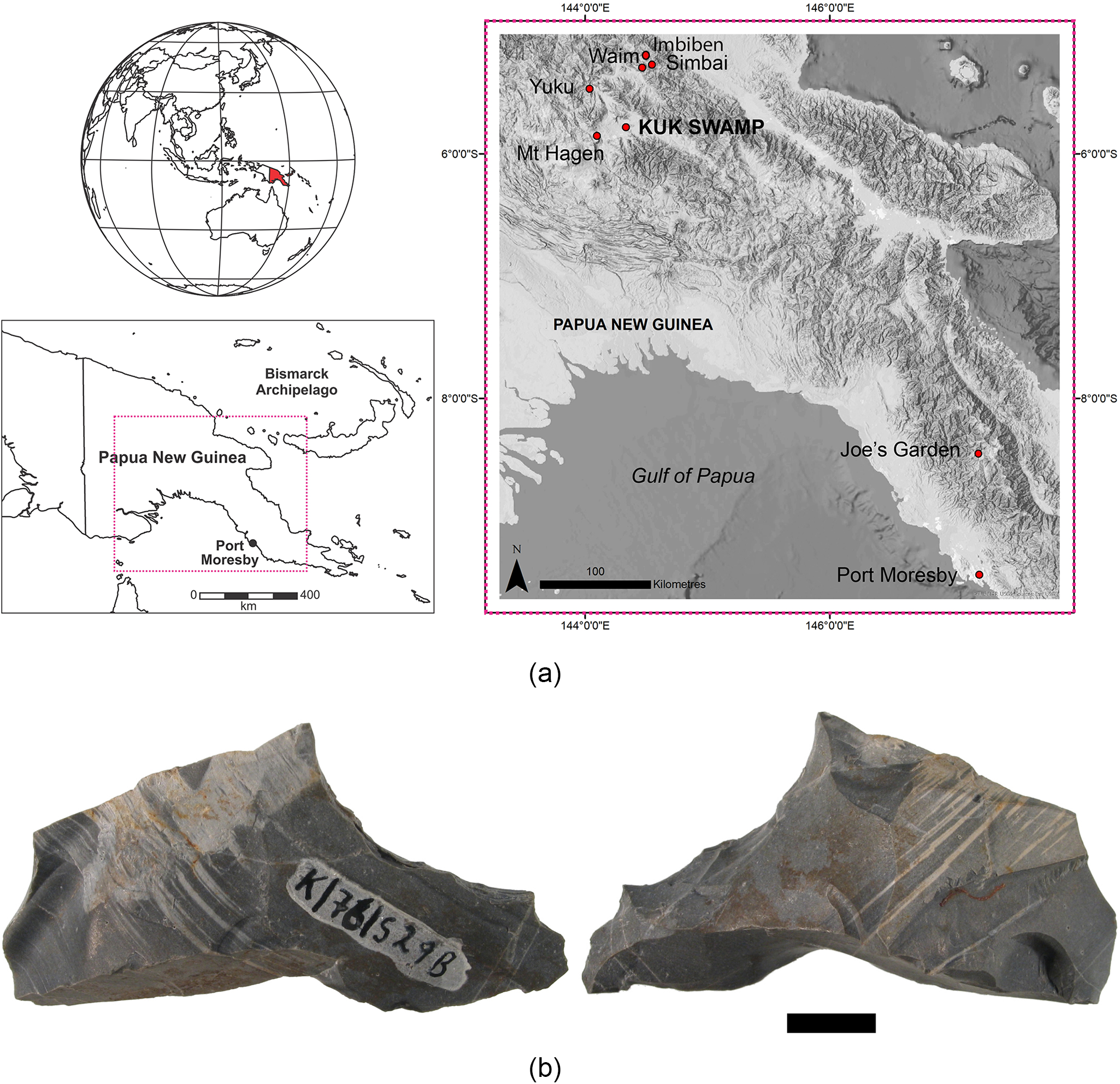
Figure 1. A. Location of Kuk Swamp in the Paua New Guinea Highlands and other sites mentioned in text. B. Stone artefact K/76/S29B from the Phase I context (see Golson et al., Reference Denham, Golson, Hughes, Golson, Denham, Hughes, Swadling and Muke2017).
Fullagar identified retouch and use-scars along two edges, striations oriented perpendicular to the edge, and a reticular polish typical of woodworking. The level of confidence in interpreting tool function is high: definite use for scraping hard siliceous woody tissue (possibly palm). Woody plant fibres, starch grains, raphides and phytoliths were observed under incident light on the artefact surface. The stone raw material was identified by David Ellis, Philip Hughes and Marjorie Sullivan as ignimbrite, a pyroclastic igneous rock (hardened tuff) (Fullagar and Golson, Reference Fullagar, Golson, Golson, Denham, Hughes, Swadling and Muke2020:396).
To facilitate the identification of starch grains to plant taxa, the residue study required access to a modern comparative reference collection. For the Fullagar et al., (Reference Fullagar, Field, Denham and Lentfer2006) study we were able to access the reference material collected by Carol Lentfer, Lisa Kealhofer and Michael Therin from Garu village, West New Britain. The 165 plant specimens in that collection had their identifications confirmed/verified at the Botanical Gardens in Lae, PNG (Therin et al., Reference Therin, Torrence and Fullagar1997).
The 2006 study determined that the use of Artefact K/76/S29B (perhaps after the tool broke), was to slice or scrape raw taro (C. esculenta) (Fullagar et al., Reference Fullagar, Field, Denham and Lentfer2006:605). Other starch residues of different morphologies documented in the original residue preparation were never attributed to a plant taxa, reflecting the limitations of the original qualitative study.
Methods
The original slide preparation was mounted in Karo syrup and has been stored for 20 years. To enable a re—examination of the slide preparation, the original nail polish that had sealed the coverslip was removed with a scalpel blade and distilled water was introduced to the sample by capillary action. The slide was resealed with clear nail polish.
The methodological approach to identifying unknown starch has undergone significant changes since the studies described above. For the present study, an algorithm was developed for the analysis of the unknown starch assemblage isolated from the K/76/S29B artefact surface (Figure 2). The five main stages of the analysis were to:
1. Establish a modern comparative reference set from the UNSW starch database.
2. Compile previously unpublished data on the technology and usewear traces of artefact K/76/29B that was analysed by RF.
3. Examine the original K/76/29B slide preparation (Fullagar et al., Reference Fullagar, Field, Denham and Lentfer2006) and re-photograph the starch assemblage.
4. Execute a Geometric Feature Analysis (GFA) of the unknown starch grains observed on the slide preparation.
5. Review the archaeology, residues, archaeobotany and ethnobotany (e.g., Pardoe et al., Reference Pardoe, Fullagar and Hayes2019).

Figure 2. Ancient starch analysis algorithm used in the study of starch of Kuk Swamp Artefact K76/S29B.
The starch database
The UNSW modern comparative starch reference collection has been developed over the last 20 years with material collected during field trips, from herbarium collections or material shared by other researchers. Plant material was cut as hand sections and/or partially pulverised with water in a glass mortar and pestle before mounting on a slide.
The comparative reference collection sub—set used in this study was compiled based on our current knowledge of the flora of the New Guinea highlands, ethnographic reports, local informants and herbaria records (e.g., Paijmans, Reference Paijmans1976; Gardner, Reference Gardner2010) (Table 1, Figure 3). Note that some plant species are not currently in our UNSW database (including Elaeocarpus womersleyi and Finschia chloroxantha and we have not yet established if they are starchy).
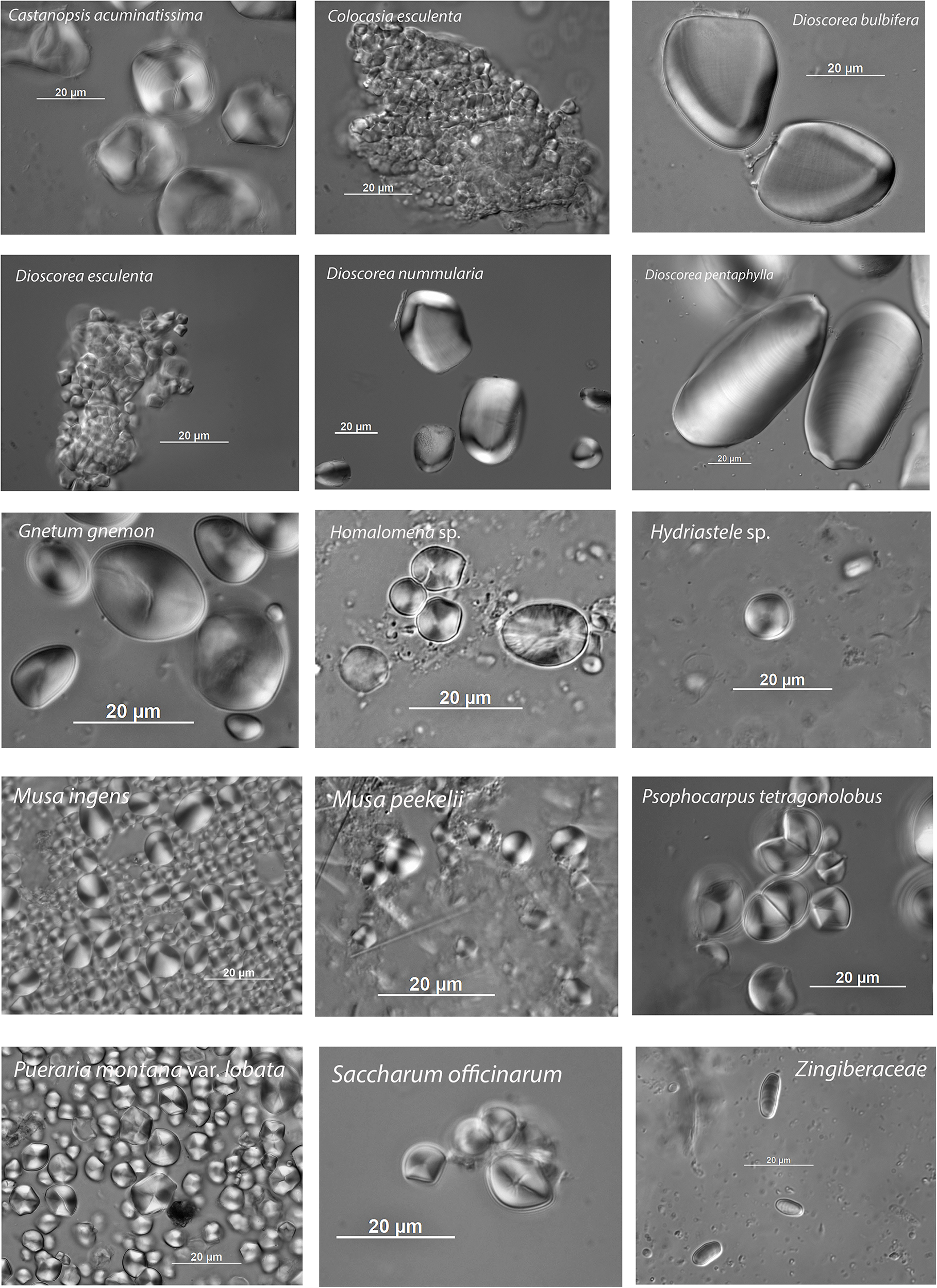
Figure 3. Examples of starch grains from plant taxa included in the comparative reference set, as listed in Table 1. Images were photographed using the Differential Interference Contrast (DIC) method.
Table 1. Plants known to be used and those that were possibly scraped or pounded in the Papua New Guinea highlands, with starch-producing species known from the Wahgi Valley, Western Highlands Province, Papua New Guinea, also included.a
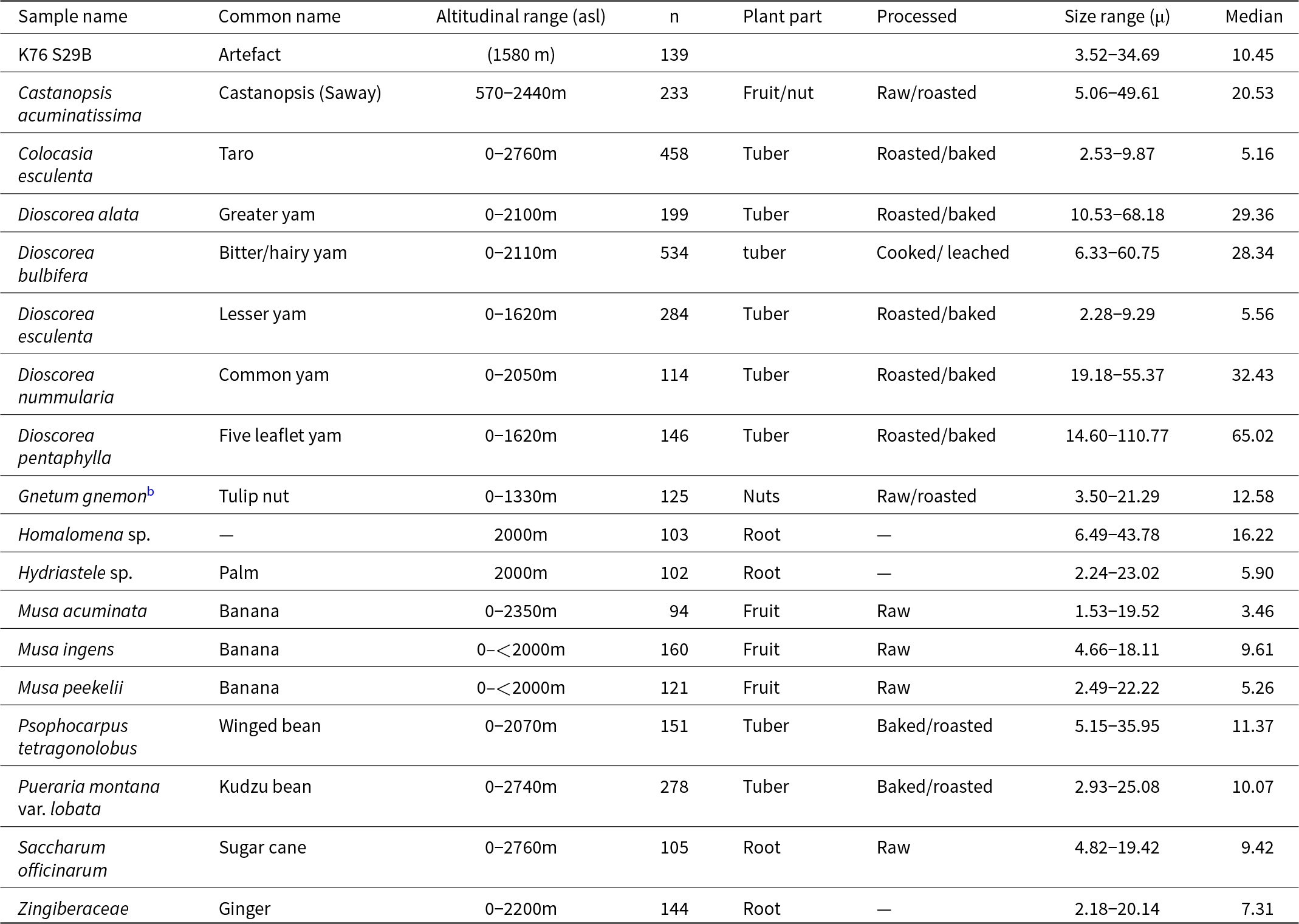
a Some of the plant species are common across the highlands, although other species such as D. esculenta are rarely grown above 900 m asl (Bourke, Reference Bourke, Haberle, Stephenson and Prebble2010). The two species discussed in this paper are shaded in the table.
b Cultivated in the present day as a supplementary food (Paijmans, Reference Paijmans1976).
Starch grains were viewed with a Zeiss Axioskop II transmitted brightfield microscope with Nomarski optics and images collected with a Zeiss HRc camera. Images were stored as .zvi files using Zeiss Axiovision software. Starch grain outlines and hilum positions were digitised from micrographs using either a graphical user interface (GUI) developed in MATLAB (MATLAB Release 2014b, The MathWorks, Inc., Natick, MA, USA), and a WACOM Intuos Pen Tablet (CTH-480) or a mouse (Logitech Pebble M350). A minimum of 100 starch grains were digitised per sample. Other features that were noted include presence/absence of lamellae, fissures/cracks at hilum, vacuoles and the presence of faceting. The individual starch grain outline is referred to as the region of interest (ROI) (see Coster and Field, Reference Coster and Field2015, Reference Coster, Field, Anderssen, Broadbridge and Fukumoto2018; Field et al., Reference Field, Summerhayes, Luu, Coster, Ford, Mandui, Fullagar, Hayes, Leavesley, Lovave and Kealhofer2020).
The starch grains in the residue extraction from the Artefact K/76/S29/B were photographed and digitised using the same method as for the comparative reference set. Examples of starch grains from Artefact K/76/S29/B are presented in Figure 4.
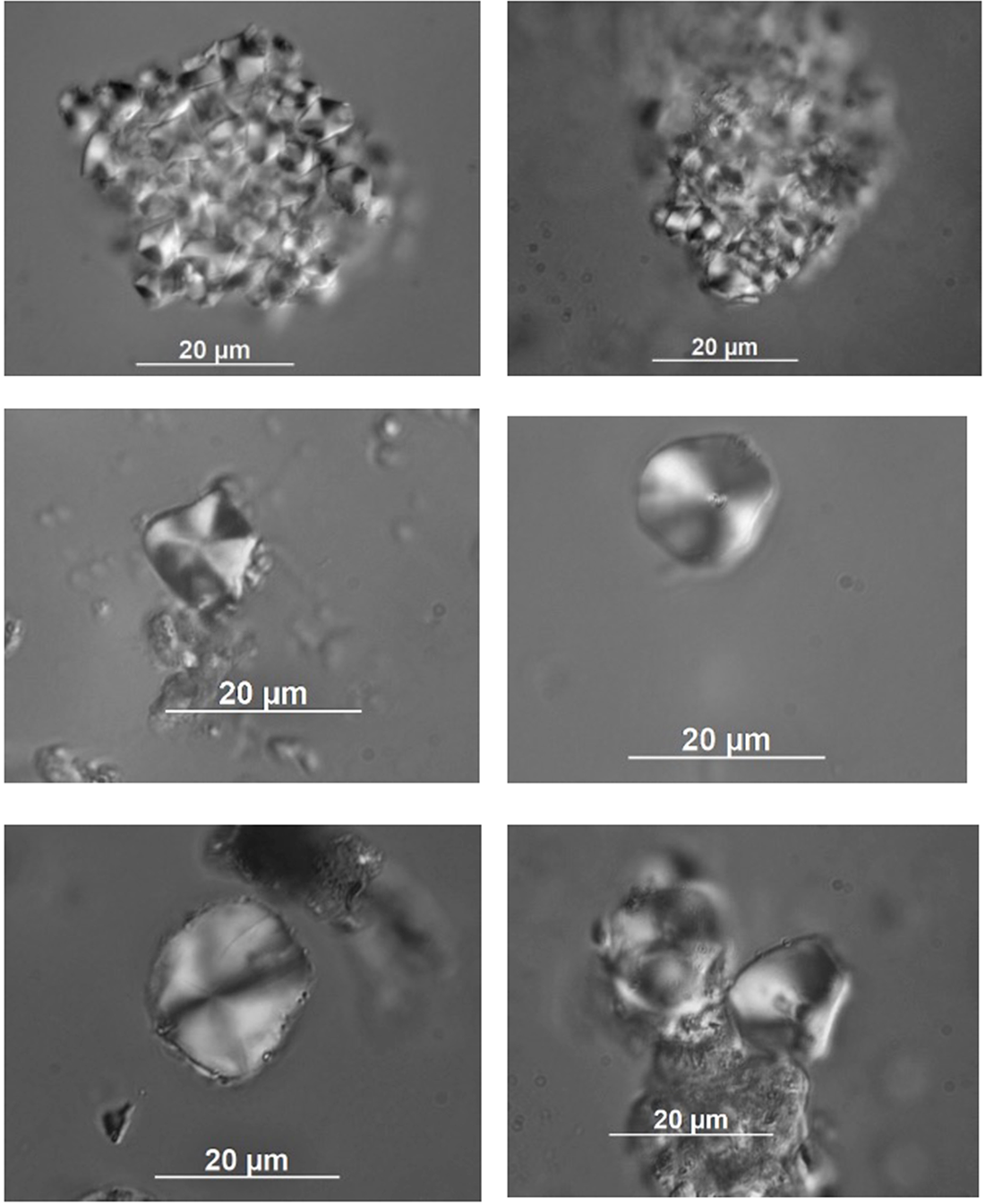
Figure 4. Examples of starch grains recovered in the aqueous sample extracted from the surface of ignimbrite flake K/76/S29B from Phase I at Kuk Swamp.
Geometric feature extraction and analysis
The method used here is fundamentally a ‘shape analysis’ (microscopic geometric features) determined from the traced starch grain outlines (ROI) and the hilum position. Starch grain metrics extracted from the ROI include the hilum position within the shape, perimeter, area, maximum length through the hilum (maxD), and the Fourier signature of the grain outline (i.e. a radial decomposition of the shape) (see Coster and Field, Reference Coster and Field2015). Histograms of the metrics and the distances between the artefact sample and the reference set were compared for overlaps.
The measures used for the Artefact K/76/S29B starch assemblage were maxD (maximum length through the hilum), area, perimeter, and hilum position. The two strongest discriminatory metrics for this process were maxD and hilum position. Grain shapes are examined at their original size and as ‘normalised’ shapes. Normalised shapes are determined by scaling the size of individual grains such that the average radius around the hilum is one. The shapes are then rotated and aligned around the hilum, and a distance measure between the shape’s proportional to the absolute area difference was determined (Figure 5). The aligned normalised shapes were clustered hierarchically into self-similar groups based on their average pairwise distance. A common cut-off between the comparative reference and archaeological assemblages was used to identify sub-groups and to ensure the same levels of self—similarity between the different sub—groups.
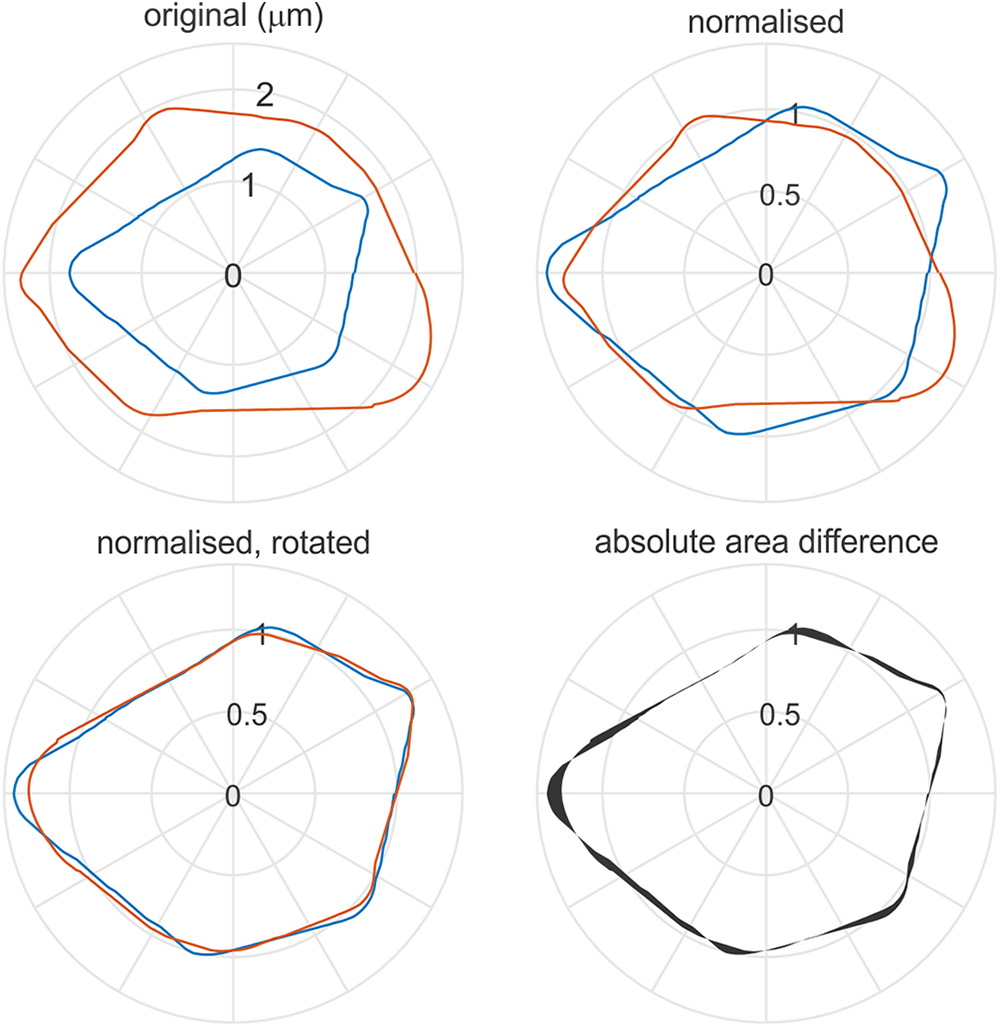
Figure 5. Traced outlines of two D. esculenta starch grains from the reference collection. The grain shapes are centred at the hilum. The starch grain outlines are shown, left to right, at their original size and orientation as extracted from the micrographs; normalised in size such that their average radius about their hilum is one; rotated so that they best align, then the difference between the normalised gains is shown as an absolute area difference, which is proportional to the distance measure.
The distributions of the metrics (maxD, area, perimeter, hilum position) were then determined for each of the sub-groups to analyse the overlaps with those of the archaeological assemblage.
The categorical features of the grain shapes of the overlapping reference sets were compared to the archaeological assemblage grains and ranked in distance in normalised shape. The categorical features and visual assessment of grain shape correspondence were used to classify whether the reference species could match each grain from the artefact. The correspondence to each reference was then quantified, noting that the grains could align with more than one reference species.
Expert input is essential in these analyses as the geometric feature extraction basically works on a 2-dimensional basis. A review of the starch grains and correlated attribution to plant taxa was important to assess the other features (3D shape, vacuoles etc) not captured in the digital tracing.
Results
Artefact K/76/S29B starch grain assemblages
A range of starch grain shapes and sizes were identified in the rehydrated sample, with a total of 139 grains documented and digitised. The clumps of very small compound grains observed (see Figure 3) were similar in size and morphology to those illustrated in Fullagar et al. (Reference Fullagar, Field, Denham and Lentfer2006: 606). Boxplots of the metrics for all the artefact and reference set starch grains are presented in Figure 6. The reference sets that overlapped with the artefact sample are shown in Figure 7, and those that did not in Figure 8. Note: it would be expected that the within-reference and within-artefact distances would be similar to the between-sample distances if the reference and artefact grains showed similar normalised shape characteristics.
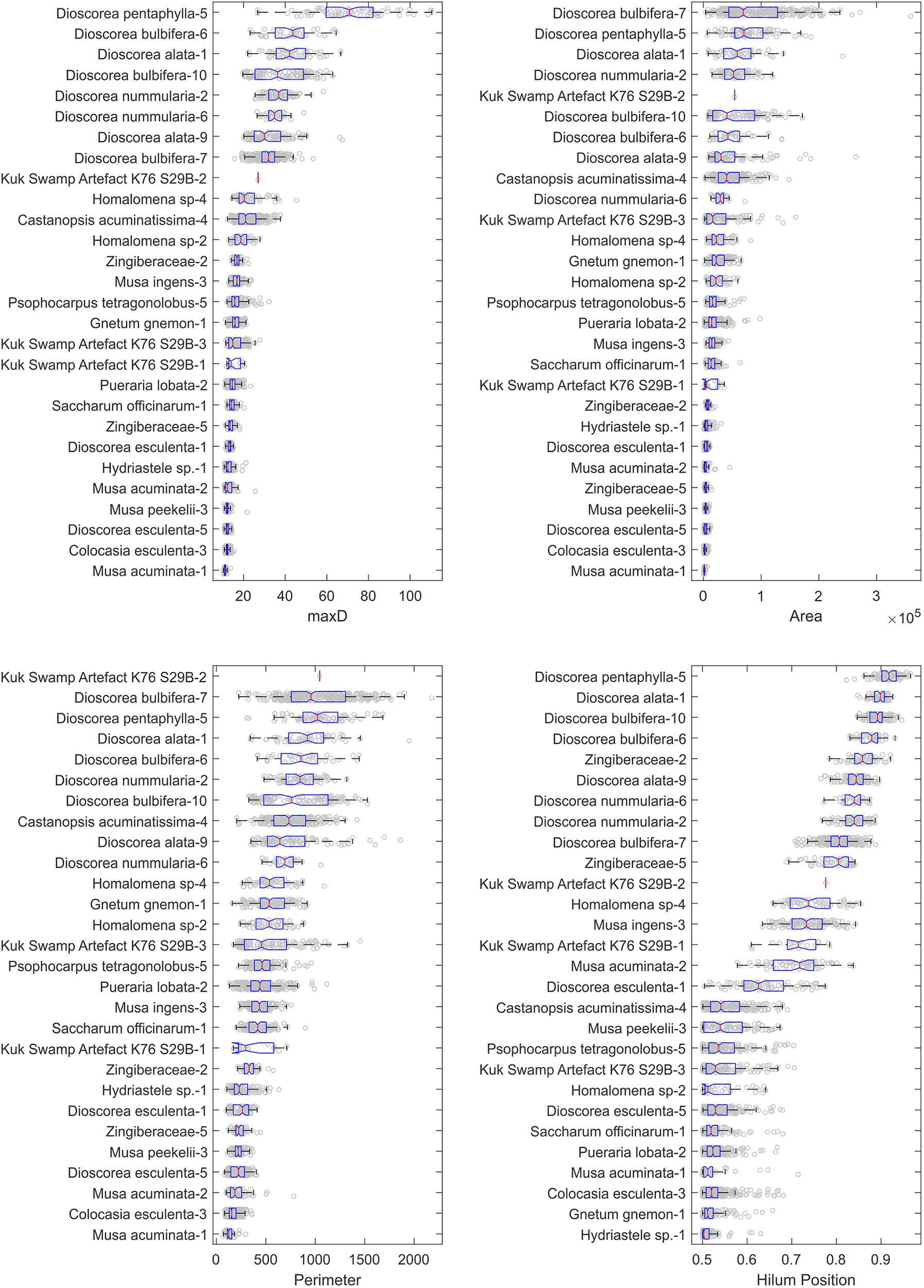
Figure 6. Boxplot graphs of archaeological and reference set grains for the metrics used in this study: maxD, area, perimeter and hilum position. The data is presented as subgroups which are determined following the normalisation of the starch grains (see Field et al., Reference Field, Summerhayes, Luu, Coster, Ford, Mandui, Fullagar, Hayes, Leavesley, Lovave and Kealhofer2020).
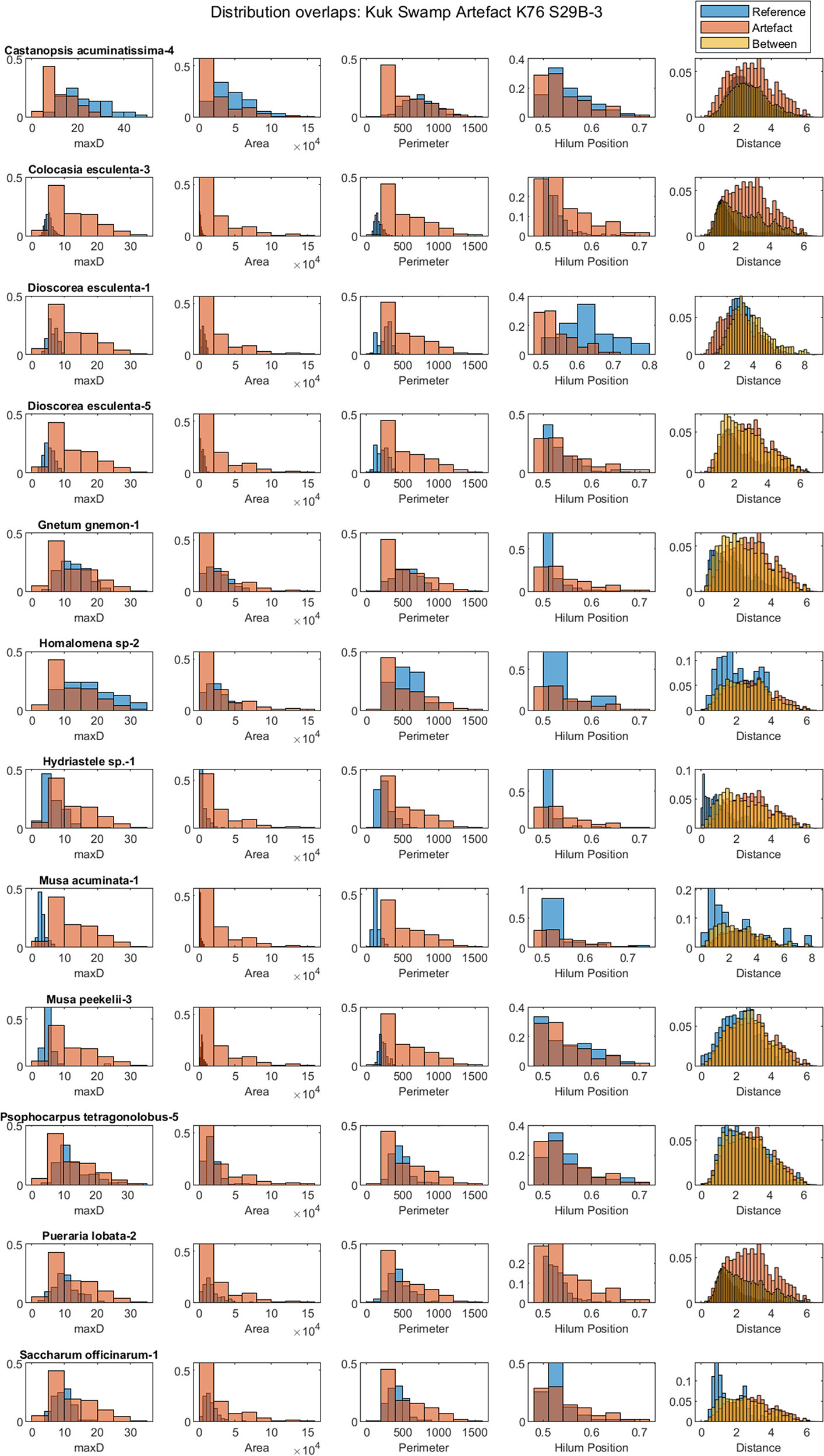
Figure 7. Histograms for the reference set samples plotted against the entire archaeological sample with the four metrics used in the analysis. The correspondence of the normalised grains shapes is also shown. The reference sets shown in this figure overlap with the unknown archaeological assemblage under consideration.
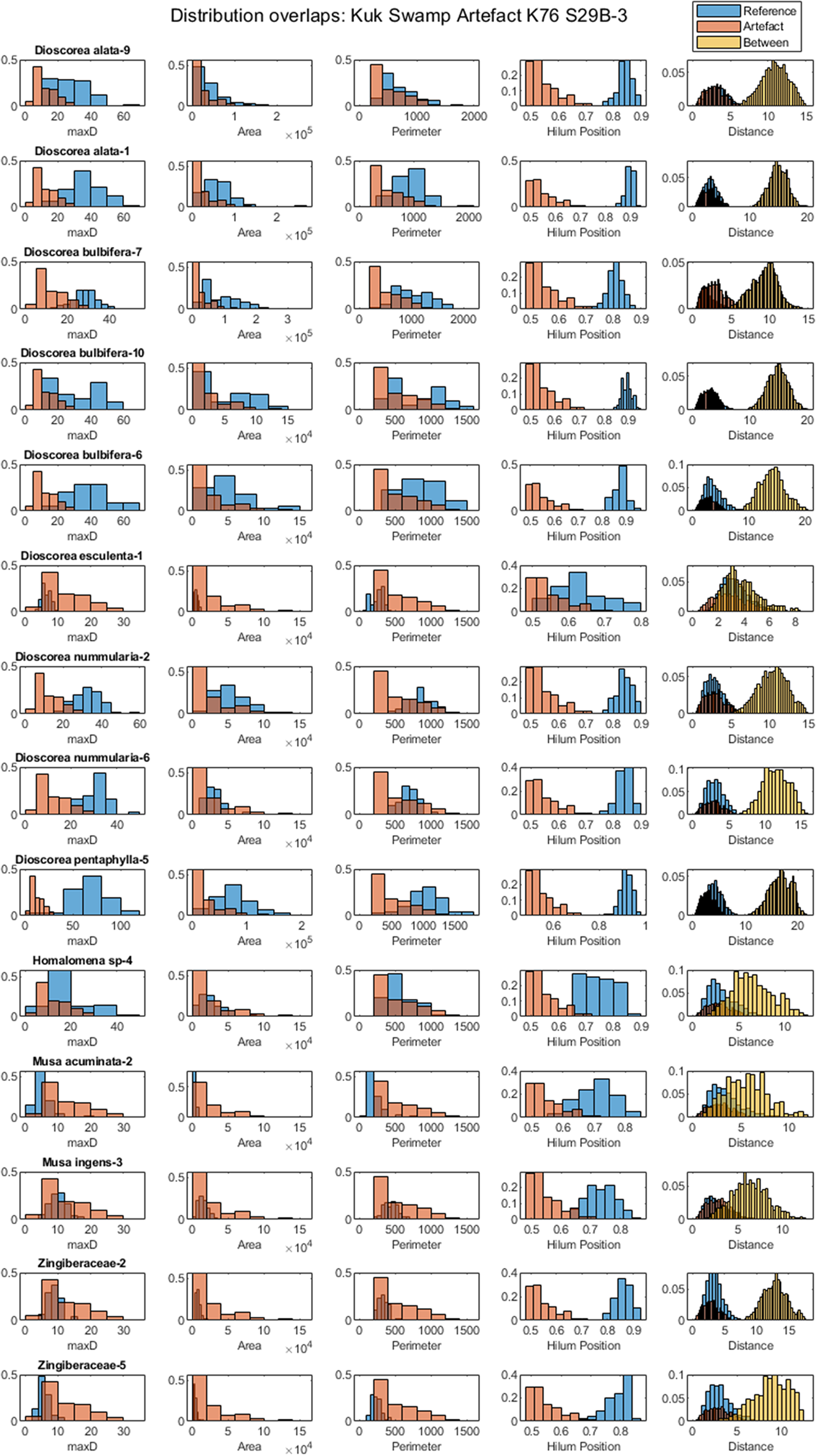
Figure 8. Examples of reference sets that did not overlap with the unknown archaeological assemblage under consideration. For instance, in the last row, while the maxD, area and perimeter overlapped, the hilum position did not correspond.
It was found that 40% (n=48) of the 120 unknown grains could correspond to D. esculenta-5, 39% (n=47), to C. esculenta-3, and 18% (n=22) to P. montana var. lobata-2. An example of the correspondence of the unknown grains to P. lobata-2 is shown in Figure 9. Of the 48 grains that could correspond to D. esculenta-5 and the 47 grains corresponding to C. esculenta-3, 34 were in common. An example of the micrographs and overlapping outlines of the grains is shown in Figure 10. Thus, the likely contributing species for 64% of the sample (77 grains) was reduced to just 2: C. esculenta-3 and D. esculenta-5.
Based on the ‘geometric feature’ approach undertaken in this study, we cannot distinguish between these two species. Other characteristics of the two species need to be considered, and may include geography, altitudinal limits, and growing requirements.
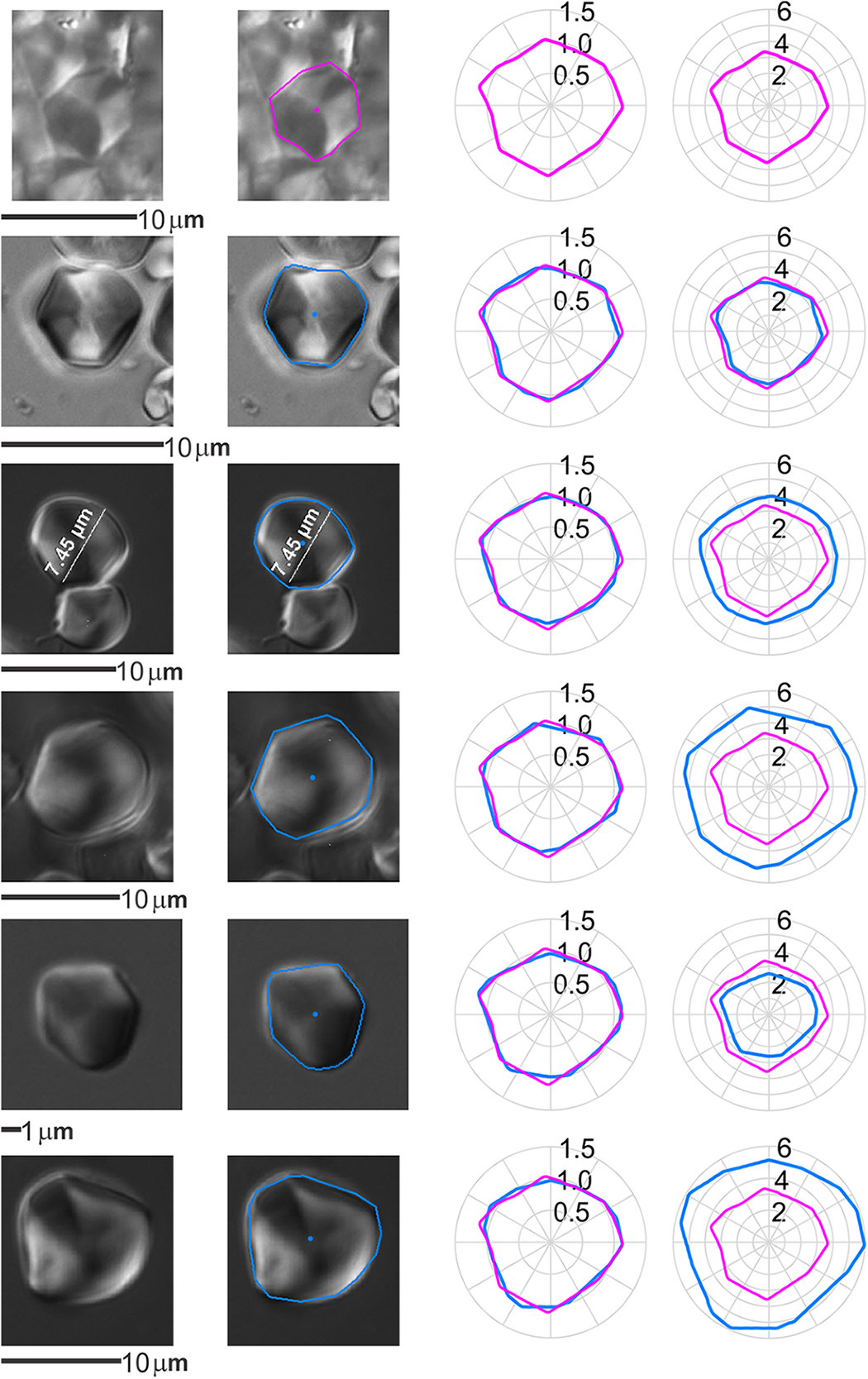
Figure 9. An example of the shape of an individual grain from K/76/S29B (first row, magenta) associated with P. lobata 2 (remaining rows, blue). The first column shows the micrographs, second column the grain outline as traced over the micrograph, third column overlaid and rotationally aligned normalised grain outlines, fourth column overlaid and rotationally aligned grain outlines at original scale (μm). Note that there is no significant difference between the starch grain sizes or shapes and this was repeated across the component of the assemblage associated with Pueraria montana var. lobata.
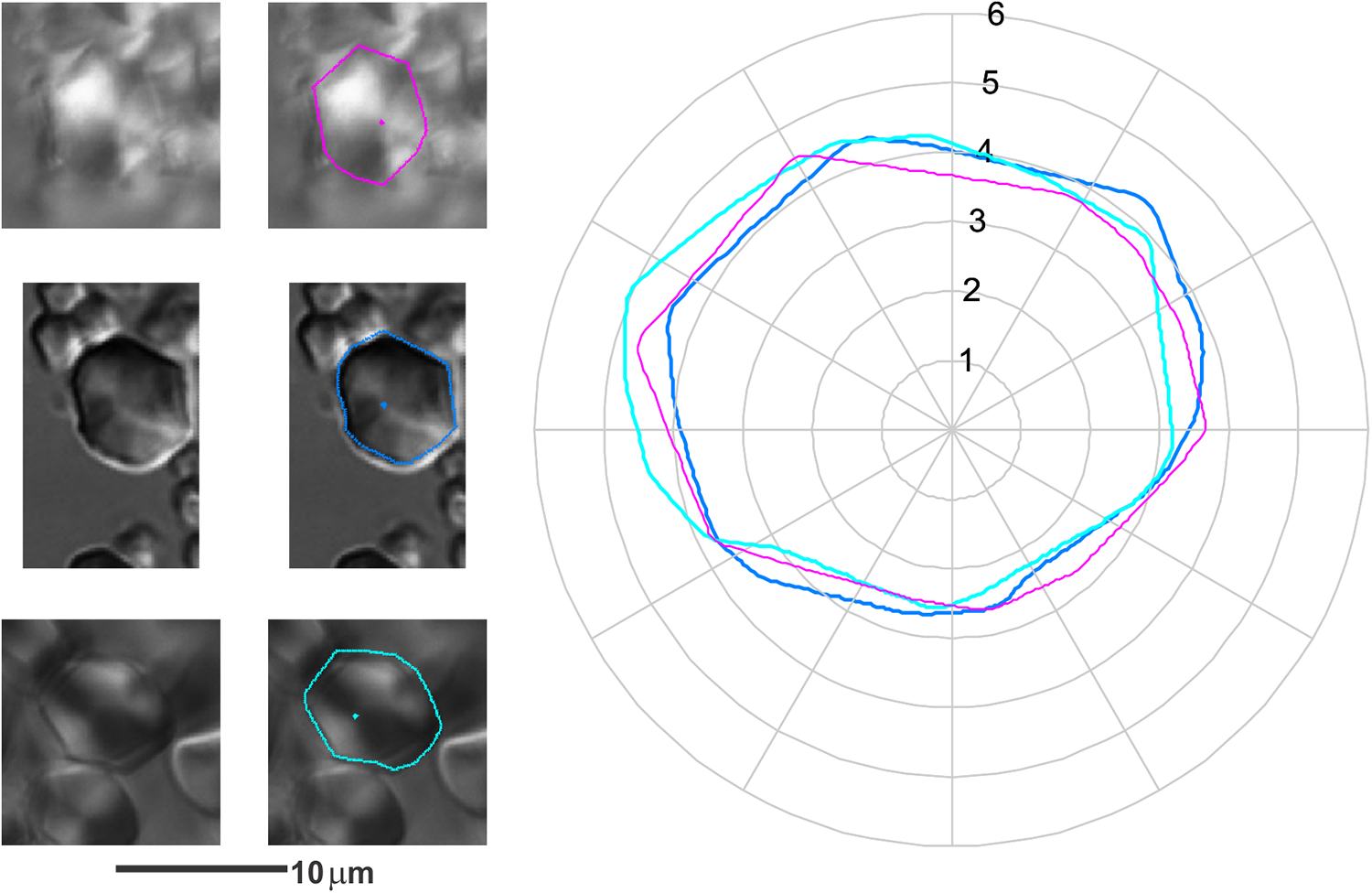
Figure 10. An example of the shapes of individual starch grains from K/76/S29B (first row), C. esculenta (second row) and D. esculenta (third row). Note that there is no significant difference between the two starch grain size or shapes and this was repeated across the component of the assemblage associated with these reference species. The overlaid and rotationally aligned grain outlines are shown at the right (magenta K/76/S29B, blue C. esculenta, cyan D. esculenta; scale in μm).
Discussion
There are three important findings in the study of starch residues from Artefact K/76/S29B:
1. We can confidently conclude that P. montana var. lobata (kudzu bean) was exploited in the Phase I Kuk Swamp sequence dated to c. 10 kya;
2. Notably, the lesser yam (D. esculenta), and taro (C. esculenta) grow in very different habitats but are effectively indistinguishable microscopically, as demonstrated by the metrics and correspondence analysis (Table 2); and,
Table 2. Comparative reference material and archaeological sample subgroups.a

a Groups (in italics) and subgroups after hierarchical clustering with a cutoff equal to 0.25 maximum distance. Note that the archaeological starch assemblage effectively sorted into three subgroups, of which two comprised 17 and 121 grains.
3. The species identification of C. esculenta (taro) from Phase 1 sediments at Kuk Swamp has been confirmed, following consideration of the various growing requirements, altitudinal limits and other factors discussed below.
P. montana var. lobata/kudzu bean (previously P. lobata)
Kudzu bean is considered a famine food by modern day highlanders, though numerous researchers had flagged the potential for it as an important staple of some antiquity (Watson, Reference Watson1964, Reference Watson1968; Barrau, Reference Barrau1965; Paijmans, Reference Paijmans1976; Sillitoe, Reference Sillitoe1983). Strathern (Reference Strathern1969) suggested that the term ‘oka’ which can apply to both kudzu bean and sweet potato may signify the importance of kudzu bean in some highland diets prior to sweet potato. Kudzu bean tubers are known to be longer lasting than I. batatas both prior to and after harvesting. It is a semi-woody vine and produces a large edible (but fibrous) tuber up to 1 m long that grows laterally from the main stem just below the surface. Paijmans (Reference Paijmans1976) has noted that the tubers may weigh up to 32 kg.
Sillitoe (Reference Sillitoe1983) reported that, in the present day, only men (in the Wola clan) grow kudzu bean, and this ‘male concern’ is reflected in planting practices elsewhere (Watson, Reference Watson1964). It is propagated vegetatively (see also Strathern, Reference Strathern1969), “taking the tuberous apex where the roots join, together with 15cm or so of stem…they plant it at the same times as beans…following the planting of sweet potato, taro and pitpit” (Sillitoe, Reference Sillitoe1983:48). In some places it is also planted near houses as it only needs a tree trunk (or pole) to grow around. Strathern (Reference Strathern1969) also observed that Kudzu Bean may have been interspersed with taro, yam and other garden plants. It was seen as a journey food and kept well after being cooked (Watson, Reference Watson1968). Furthermore, the large size of the tubers meant that kudzu bean was suitable for feeding large congregations of people and is perhaps why it has been associated with large ceremonies.
The discovery of kudzu bean starch grains in the Phase I context at Kuk Swamp supports the suggestion that it was an important staple prior to the introduction of sweet potato, around 300 years ago (Roullier et al., Reference Roullier, Kambouo, Paofa, McKey and Lebot2013). Like taro, kudzu bean was easily replanted and is likely to have been a significant adjunct to taro during the Early Holocene in the New Guinea Highlands.
C. esculenta vs D. esculenta in ancient starch assemblages
The ability to discriminate between starch grains of similar size and morphology is challenging. In the past few decades, reports have claimed to be able to attribute starch grains to specific plant taxa, notably C. esculenta (taro) and D. esculenta (purple yam). Taro starch grains have been described as having a diameter of 1–6 µm, ‘more-round’ in shape, and with ‘shorter’ raphides that are square in cross-section and pointed at one end. Purple yam starch grains have been described as 2–10 µm in diameter, ‘more oval’ in shape, with ‘longer’ raphides that are circular in cross-section with tapered points at both ends (see also Loy et al., Reference Loy, Spriggs and Wickler1992; Fullagar et al., Reference Fullagar, Loy, Cox and Fullagar1998).
Horrocks and Weisler (Reference Horrocks and Weisler2006:1190) (citing Loy et al., Reference Loy, Spriggs and Wickler1992) argued that C. esculenta starch has a mean size of <5 μm with most grains <3 μm, then asserted that the identification of D. esculenta could be distinguished from C. esculenta on size, shape, and the morphology of the xylem elements (bordered ellipsoid pits), as well as the shape and size of raphides. The same rationale was used to identify D. esculenta in deposits at Yuku Rockshelter in the PNG highlands, C. esculenta and D. esculenta at Viti Levu Island in Fiji, and in cave deposits in New Caledonia (Horrocks and Nunn, Reference Horrocks and Nunn2007; Horrocks et al., Reference Horrocks, Grant-Mackie and Matisoo-Smith2008a, Reference Horrocks, Bulmer and Gardner2008b:293—294). Allen and Ussher (Reference Allen and Ussher2013) also reported the presence of C. esculenta based on this rationale, though their measurement of starch grains showed a large overlap in size between D. esculenta and C. esculenta.
Starch grains from C. esculenta and D. esculenta are indistinguishable from each other based on their size and morphology. The identification of starch grains as C. esculenta in this study was not dependent just on the grain morphology but followed consideration of a range of other factors (Fullagar et al., Reference Fullagar, Field, Denham and Lentfer2006) as outlined below:
• Context: Artefact K/76/S28B was recovered from the Phase I palaeosurface which was consistent with cultivation (including digging and staking), and inter-cropping as proposed by Denham et al. (Reference Denham, Golson, Hughes, Golson, Denham, Hughes, Swadling and Muke2017: 190—196).
• Archaeobotany: Macrobotanical remains identified as C. esculenta in previous studies included putative seeds of an aroid recovered from the same channel near to where the artefact was recovered (Lentfer and Denham, Reference Lentfer, Denham, Golson, Denham, Hughes, Swadling and Muke2017:166—167). At least one raphide was observed (by Fullagar) on the original slides, though none were found on the rehydrated preparations.
• Habitat: Taro prefers growing in wetland environments, in contrast to D. esculenta which prefers dry and well-drained soils. Furthermore, D. esculenta while having been recorded as high as 1620 m asl, is rarely found growing above 900 m asl (Bourke, Reference Bourke, Haberle, Stephenson and Prebble2010). The altitudinal trends for growing D. esculenta do not preclude yams being transported into the Wahgi Valley from lower elevations, though we think it is unlikely in this context.
The study undertaken here has conclusively shown that D. esculenta and C. esculenta are indistinguishable at the microscopic level. Contrary to earlier qualitative descriptions which suggested size-shape differentiation, our study shows that the starch grains of both taxa overlap in shape and size and we suggest that previous claims may need review and revision.
Questions remain over the identification of D. esculenta and C. esculenta in other published studies from across the Pacific region. The widespread distribution of both plant taxa and their indistinguishable morphologies does mean that other factors must be considered before attributing confident identifications. Misidentification has the potential to skew our view of plant use over time, as both taxa are known from contrasting environmental contexts and require different growing conditions. We would argue that the accurate attribute measurement data that was collected, with the methods described here, bring into question the utility of qualitative measurements and subsequent conclusions drawn from those datasets.
In summary, it is perhaps unsurprising that both taro and kudzu bean starch grains were identified on the small, flaked tool recovered from Phase I sediments at Kuk Swamp. It has been well documented that taro plants are trimmed by scraping prior to re-planting, as is the kudzu vegetative apex (e.g., Sillitoe, Reference Sillitoe1983:39, 48). Our interpretation is that the flake tool was used on taro and kudzu in preparation for replanting in the Kuk Swamp sediments.
Conclusion
The identification of P. montana var. lobata in the starch assemblage from Artefact K/76/S28 is an important addition to the range of plants known to have been exploited at Kuk Swamp in the Early Holocene. The presence of P. montana var. lobata in a 10 kyka cal BP context is the oldest known age in Papua New Guinea and provides support for earlier arguments that kudzu bean was a significant staple in highland New Guinea diets. The identification of P. montana var. lobata builds on previous discoveries at the Joe’s Garden site in the Ivane Valley in the Mid-Holocene and highlights what appears to be a long history of exploitation in highland contexts.
Of considerable methodological importance is demonstrating that the identification of C. esculenta and D. esculenta in starch assemblages cannot rely on quantitative size-shape criteria alone. Consideration of other features such as xylem element and raphide morphology, habitat requirements and cultural activities is critical to the correct attribution of species identification. A detailed understanding of the archaeological context from which these finds are recovered is essential.
Acknowledgments
We are grateful for support from the University of New South Wales, the Forest Research Institute in Lae, and to Tim Denham for permission to re—examine the Kuk Swamp artefact studies here and for reading a previous draft. JF is grateful to Carol Lentfer for the reference slides of Musa spp. We dedicate this paper to the late Dr Beth Gott AM (1922—2022) who was instrumental in assisting JF in the early stages of her starch research. We also thank the two anonymous reviewers for their detailed comments. A huge thank you to Dr Anthea Mitchell for preparing the topographic map in Figure 1A.
Competing Interests
The authors declare that they have no competing interests that could interfere with the objectivity or integrity of this publication.
















