Introduction
Glycine-rich proteins (GRPs) comprise many biomolecules, usually arbitrarily defined as proteins containing at least 20% glycine (Gly) in their composition. Typically, Gly amino acid residues are distributed in a glycine-rich domain that can be arranged randomly or in repeating patterns. Accordingly, GRPs are categorised into subtypes based on the pattern motifs they exhibit, which influence the protein structure and can allow for secondary structures such as alpha-helix, glycine loops or β-sheets. This affects the stability of proteins and may influence protein interactions and functions. Additionally, they facilitate the binding of other molecules, such as nucleotides (Bossemeyer, Reference Bossemeyer1994; Sachetto-Martins et al., Reference Sachetto-Martins, Franco and De Oliveira2000; López-Llano et al., Reference López-Llano, Campos and Sancho2006; Dong et al., Reference Dong, Sharma, Zhou and Cross2012). The functions of plant GRPs have been extensively studied, influencing plant development, physiology, and defence against abiotic and biotic stresses (Mangeon et al., Reference Mangeon, Junqueira and Sachetto-Martins2010; Ma et al., Reference Ma, Cheng, Li, Deng, Zhang and Zhu2021). In spiders and silkworms, GRPs are components of silk fibers, conferring flexibility with elastomericity and strength (Parkhe et al., Reference Parkhe, Seeley, Gardner, Thompson and Lewis1997; Asakura et al., Reference Asakura, Yao, Yamane, Umemura and Ulrich2002; Dicko et al., Reference Dicko, Porter, Bond, Kenney and Vollrath2008; Malay et al., Reference Malay, Sato, Yazawa, Watanabe, Ifuku, Masunaga, Hikima, Guan, Mandal, Damrongsakkul and Numata2016, Reference Malay, Arakawa and Numata2017). In hemipteran insects, they exhibit antimicrobial and RNA binding activities (Futahashi et al., Reference Futahashi, Tanaka, Tanahashi, Nikoh, Kikuchi, Lee and Fukatsu2013; Meraj et al., Reference Meraj, Dhari, Mohr, Lowenberger and Gries2024), while in spider mites and mosquitoes, GRPs have been implicated in feeding functions (Jariyapan et al., Reference Jariyapan, Choochote, Jitpakdi, Harnnoi, Siriyasatein, Wilkinson and Bates2006; Sun et al., Reference Sun, Li, Shi, Zhang, Chai, Chen, Niu and Wang2023). As a common structural component, GRPs are also found in insect cuticles (Zhong et al., Reference Zhong, Mita, Shimada and Kawasaki2006) and molluscan shells (Zhang and Zhang, Reference Zhang and Zhang2006).
Ticks are hematophagous ectoparasites that are widely distributed across the globe and have evolved to thrive in a range of biotic and abiotic conditions (Ogden et al., Reference Ogden, Ben Beard, Ginsberg and Tsao2021). They have a detrimental impact on both veterinary and human health, as they can transmit more pathogens than any other arthropod, including mosquitoes (Jongejan and Uilenberg, Reference Jongejan and Uilenberg2004). Moreover, the phenomenon of climate change has been suggested as the main cause for the spread of tick species to wider areas (Gilbert, Reference Gilbert2021). Currently, tick control remains heavily reliant on the use of chemicals, an approach that has become increasingly unfavourable due to the emergence of resistant populations, environmental contamination, and rising costs (Obaid et al., Reference Obaid, Islam, Alouffi, Khan, da Silva Vaz, Tanaka and Ali2022). Vaccines have been considered the most desirable alternative for tick control, and, although commercial vaccines were already occasionally successfully used, their development are still challenging (De La Fuente and Ghosh, Reference de la Fuente and Ghosh2024). In fact, anti-tick vaccine strategies must deal with caveats such as genetic diversity, immunodominant regions among protective antigens, development of long-lasting immunity and redundancy of functions among different molecules that can be recognized by the immune system (Leal and Ferreira, Reference Leal and Ferreira2021; De La Fuente and Ghosh, Reference de la Fuente and Ghosh2024). Therefore, a cocktail of molecules may be more likely to achieve significant anti-tick protection (Ndawula Jr and Tabor, Reference Ndawula and Tabor2020), and some GRPs exhibit properties compatible with such desired multicomponent immunogens (Trimnell et al., Reference Trimnell, Hails and Nuttall2002, Reference Trimnell, Davies, Lissina, Hails and Nuttall2005; Leal et al., Reference Leal, Alzugaray, Seixas, Vaz and Ferreira2018). The modulatory interplay between ticks and their hosts is likely to be the primary factor contributing to the parasite's success. A multitude of salivary proteins compose the biochemical dialogue between the two species, which undergoes changes over time, rendering tick saliva probably the most complex animal saliva (Ribeiro and Mans, Reference Ribeiro and Mans2020).
Ticks possess a wide variety of GRPs and their presence has been identified primarily in saliva and salivary glands (SGs), although their functions are not totally understood. Tick GRPs have been described as being involved in the formation of the cement, a hard glue structure secreted to attach to the host skin and hypothesized to help in the evasion of the host immune response, influencing pathogen transmission (Kemp et al., Reference Kemp, Stone, Binnington, Frederick and Rachel1982; Havlíková et al., Reference Havlíková, Roller, Koči, Trimnell, Kazimírová, Klempa and Nuttall2009; Hart et al., Reference Hart, Ribeiro, Kazimirova and Thangamani2020). Ticks are ectoparasites that can be divided into longirostrate (long) and brevirostrate (short) according to the size of their mouthparts, which influences the amount of cement needed at the attachment site. Furthermore, the strategy employed by ticks to survive while feeding on a host can also vary according to the type of host, the number of hosts within the life cycle, or the duration of feeding on the host. In this context, the quantity and variability of GRPs appear to be influenced by the strategies of each tick species and developmental stage, in addition to the size of the tick mouthparts (Anderson et al., Reference Anderson, Sonenshine and Valenzuela2008; Maruyama et al., Reference Maruyama, Anatriello, Anderson, Ribeiro, Brandão, Valenzuela, Ferreira, Garcia, Szabó, Patel, Bishop and De Miranda-Santos2010). This review focuses on the structural aspects and dynamics of GRP production in ticks as well as discusses the possible roles these proteins may play in response to certain stimuli, such as feeding, infection and stress.
GRP structural characteristics
The content of Gly in the composition of GRPs varies between proteins, and Gly-rich motifs can be arranged in specific patterns or not. Glycine-rich regions can form loops, giving the region a flexible characteristic (Steinert et al., Reference Steinert, Mack, Korge, Gan, Haynes and Steven1991; Högel et al., Reference Högel, Götz, Kuhne, Ebert, Stelzer, Rand, Scharnagl and Langosch2018). Gly residues themselves can also be displaced randomly or in patterns. Among ticks, the most common patterns described are tripeptides, such as GXG, GXX and GGX, but Gly-rich repeats can also be found in longer arrangements and in varying patterns, as shown in Fig. 1 (Bishop et al., Reference Bishop, Lambson, Wells, Pandit, Osaso, Nkonge, Morzaria, Musoke and Nene2002; Francischetti et al., Reference Francischetti, Sa-Nunes, Mans, Santos and Ribeiro2009; Maruyama et al., Reference Maruyama, Anatriello, Anderson, Ribeiro, Brandão, Valenzuela, Ferreira, Garcia, Szabó, Patel, Bishop and De Miranda-Santos2010; Leal et al., Reference Leal, Alzugaray, Seixas, Vaz and Ferreira2018). For instance, the glycine-rich region of P29 of Haemaphysalis longicornis is present in the middle of the sequence and has been described as a collagen-like domain, as the GXX repeat that predominates resembles those present in vertebrate collagen proteins (Mulenga et al., Reference Mulenga, Sugimoto, Sako, Ohashi, Musoke, Shubash and Onuma1999). The classification of GRPs within ticks has been primarily based on motif similarity, although it also been categorized by function, such as the cement GRPs, or by orthology, such as some Rhipicephalus GRPs, which are further categorized into distinct subclasses (GYG, expansion of large GYG, and superlarge GYG) (Francischetti et al., Reference Francischetti, Sa-Nunes, Mans, Santos and Ribeiro2009; Ribeiro et al., Reference Ribeiro, Anderson, Manoukis, Meng and Francischetti2011; Ribeiro and Mans, Reference Ribeiro and Mans2020).
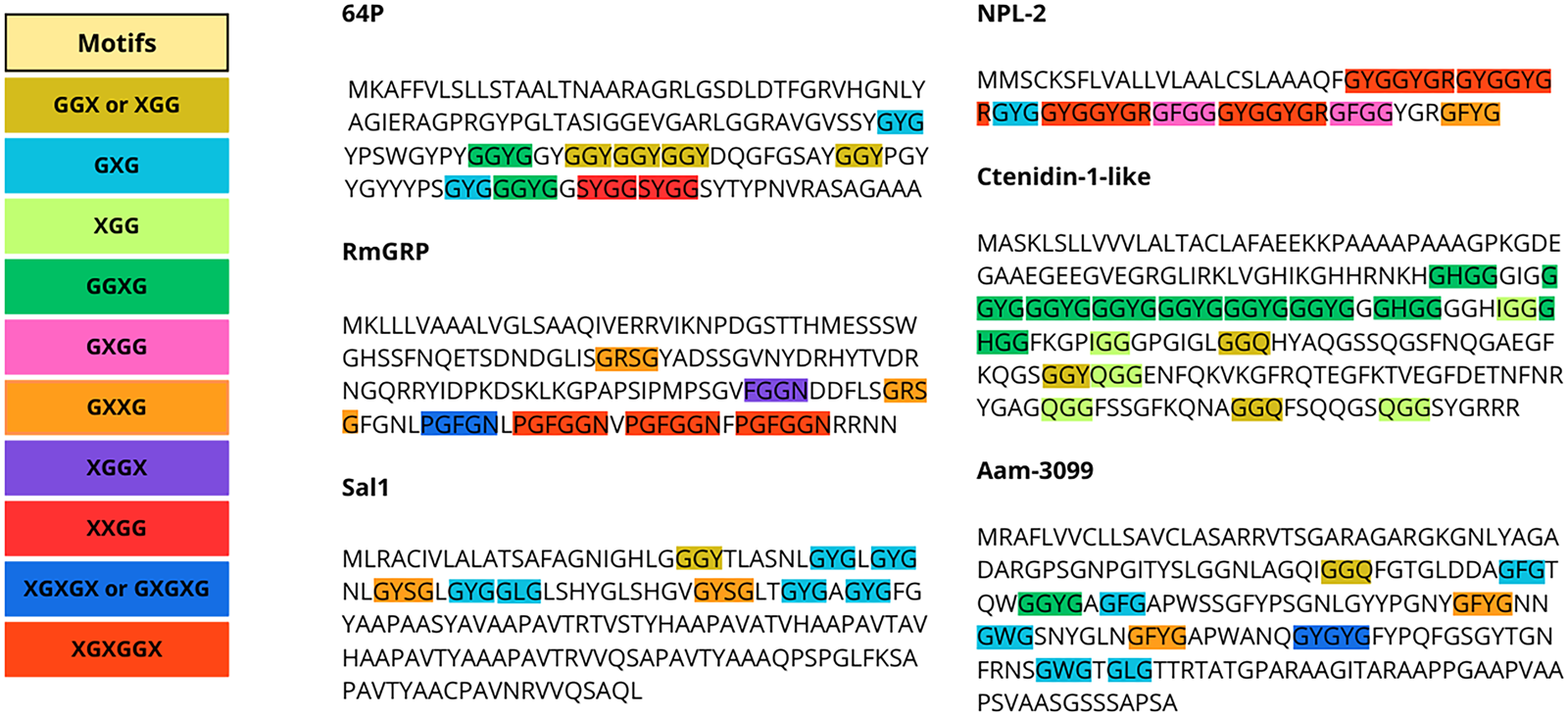
Figure 1. Gly-repeat patterns in tick GRP sequences. Each repeat is represented with a different color, and pattern variations within sequences are highlighted with the respective color. Sequences presented are: 64P of Haemaphysalis longicornis (AAM09648.1), RmGRP of Rhipicephalus microplus (AQX36208.1), Sal 1 of Rhipicephalus annulatus (AGR45924.1), NPL-2 of Ixodes scapularis (EEC15723.1), Ctenidin-1-like of Ixodes scapularis (XP_029830867.1), and Aam-3099 of Amblyomma americanum (JAG92486.1).
As previously described, Gly residues are involved in the helical conformation of proteins, influencing the helix-helix packing, and, for instance, conferring greater stability to tertiary structures in transmembrane domains (Dong et al., Reference Dong, Sharma, Zhou and Cross2012). Furthermore, the flexibility of proteins may be influenced by the position of Gly residues. Gly can lead to more flexible termini or rigid centres, which in turn alters the hydration and H-bonding of the helix (Högel et al., Reference Högel, Götz, Kuhne, Ebert, Stelzer, Rand, Scharnagl and Langosch2018).
At least three Metastriate ticks present GRPs containing GGY or GGY-related motifs, which, interestingly, present similarities with silk sequences of spiders (Mulenga et al., Reference Mulenga, Blandon and Khumthong2007; Maruyama et al., Reference Maruyama, Anatriello, Anderson, Ribeiro, Brandão, Valenzuela, Ferreira, Garcia, Szabó, Patel, Bishop and De Miranda-Santos2010). In dragline spider silks, these motifs are associated with mechanical characteristics, such as fibre strength, despite the glycine-rich region usually corresponds to a non-elastic domain (Brooks et al., Reference Brooks, Stricker, Joshi, Kamerzell, Middaugh and Lewis2008; Malay et al., Reference Malay, Sato, Yazawa, Watanabe, Ifuku, Masunaga, Hikima, Guan, Mandal, Damrongsakkul and Numata2016 and, Reference Malay, Arakawa and Numata2017). In the insects Bombyx mori and Antheraea pernyi, the GGY sequence appears to be associated with cuticle hardening and plays a fundamental role in humidity-induced behaviour of silks (Suzuki et al., Reference Suzuki, Matsuoka, Iimura and Fujiwara2002; Futahashi et al., Reference Futahashi, Okamoto, Kawasaki, Zhong, Iwanaga, Mita and Fujiwara2008; Wang et al., Reference Wang, Lin, Ren, Pei, Chen, Qi, Shao and Ling2020).
The intrinsically disordered nature of many GRPs seems to facilitate liquid-liquid phase separation (LLPS) in the process of forming adhesive structures. In a salivary GRP from Ixodes ricinus, the N-terminal portion is more negatively charged, while the C-terminal portion has more aromatic residues regularly spaced by glycine-rich regions, which allows for extensive cation-π interactions and therefore the accumulation of particles around the GRP, leading to coacervation. Furthermore, it was demonstrated that the mature GRP (without the signal peptide), referred to as Tick-GRP77, undergoes LLPS and forms viscoelastic solid structures, as a transition to adhesive forms, what corroborates with a cement formation function (Ganar et al., Reference Ganar, Turbina, Nandy, Chen, Suylen, van der Beelen, Louise Pascoe, Koenraadt, Dijkgraaf and Deshpande2023).
Tissue and ontogenetic distribution in ticks
GRPs have been frequently identified in the tick cement, and their tissue localization has been studied in several tick species (Kemp et al., Reference Kemp, Stone, Binnington, Frederick and Rachel1982). The components of cement are secreted in saliva, and the host species, feeding, and mouthparts morphology influence its composition (Tabor et al., Reference Tabor, Ali, Rehman, Garcia, Zangirolamo, Malardo and Jonsson2017). Furthermore, GRPs presence is also modulated by biotic and abiotic factors (see Table 1). The following subsections present, compare, and discuss data on when and where these proteins are found and expressed in ticks, as well as the known conditions that may affect them.
Table 1. Distribution of glycine rich proteins in different tissues and developmental stages of ticks, and their response to feeding
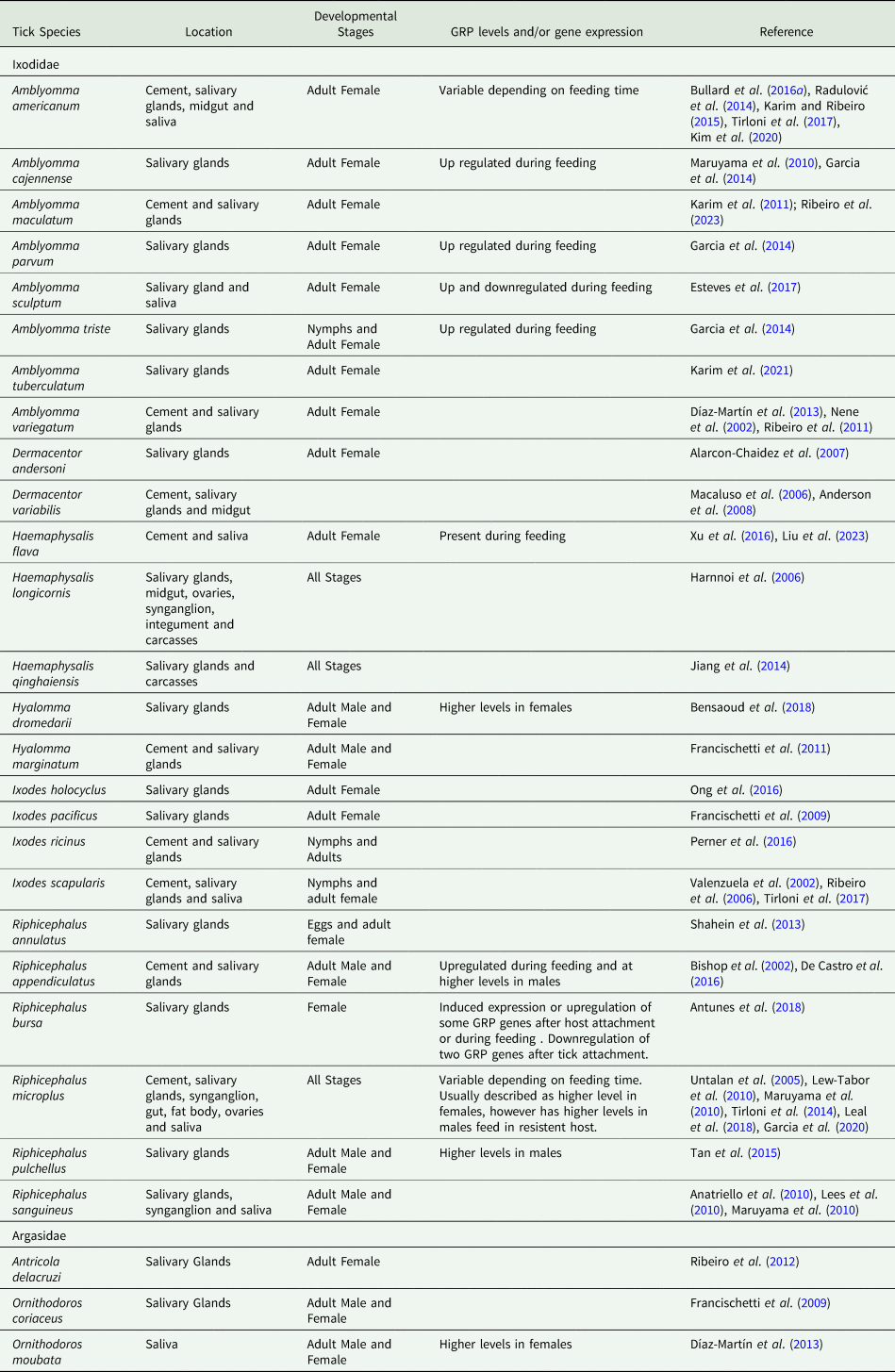
Ontogenetic distribution
The GRPs RmGRP and Rm39 of Rhipicephalus microplus and Hq15 of Haemaphysalis qinghaiensis were detected in all stages of development (Maruyama et al., Reference Maruyama, Anatriello, Anderson, Ribeiro, Brandão, Valenzuela, Ferreira, Garcia, Szabó, Patel, Bishop and De Miranda-Santos2010; Jiang et al., Reference Jiang, Gao, Wang, Xu, Li, Qi, Yang, Ji, Zhang, Luo and Yin2014; Leal et al., Reference Leal, Alzugaray, Seixas, Vaz and Ferreira2018). Also, a substantial number of transcripts encoding cuticle-like GRPs were identified in R. microplus nymphs and females during feeding. These transcripts showed considerable heterogeneity, including the presence of GGY family transcripts exclusively in nymphs (Garcia et al., Reference Garcia, Chaves Ribeiro, Maruyama, Gardinassi, Nelson, Ferreira, Andrade and de Miranda Santos2020). Shahein et al. (Reference Shahein, Abouelella, Hussein, Hamed, El-Hakim, Abdel-Shafy and Tork2013) identified open reading frames (ORFs) for three putative salivary GRPs in Rhipicephalus annulatus (RaSal1, RaSal2 and RaSal3). RaSal1 and RaSal3 were present in eggs after 12 days of oviposition, while RaSal2 was weakly recognized after 6 days (Shahein et al., Reference Shahein, Abouelella, Hussein, Hamed, El-Hakim, Abdel-Shafy and Tork2013). In Haemaphysalis longicornis, anti-sera against the GRP P29 reacted with larval and adult extracts, indicating its presence (or of immune-related proteins) in immature and mature ticks, while HLMLP (H. longicornis muscle LIM protein) was present in all developmental stages (Mulenga et al., Reference Mulenga, Sugimoto, Sako, Ohashi, Musoke, Shubash and Onuma1999; Luo et al., Reference Luo, Shen, Ren, Guan, Zhao, Yin, Chen, Zhao, Luo, Li and Liu2020). Expressed sequence tags (ESTs) of GRPs have been identified in nymphs and adults of Ixodes scapularis, exhibiting considerable similarity to the Ixodes pacificus profile (Ribeiro et al., Reference Ribeiro, Alarcon-Chaidez, Ivo, Mans, Mather, Valenzuela and Wikel2006). In Amblyomma americanum, five profiles of GRPs were detected in larvae and nymphs, with upregulation observed following ecdysis (Hollmann et al., Reference Hollmann, Kim, Tirloni, Radulović, Pinto, Diedrich, Yates, da Silva Vaz and Mulenga2018).
The profile of the GRP sialotranscriptome in ticks differs between males and females. This can be explained by the distinctive characteristics of attachment presented by each biological sex and presuming a higher necessity for variability and abundance of these proteins in females (Díaz-Martín et al., Reference Díaz-Martín, Manzano-Román, Valero, Oleaga, Encinas-Grandes and Pérez-Sánchez2013; Tan et al., Reference Tan, Francischetti, Slovak, Kini and Ribeiro2015). This assumption is corroborated by the findings that Hyalomma dromedarii (Bensaoud et al., Reference Bensaoud, Nishiyama, Ben Hamda, Lichtenstein, Castro De Oliveira, Faria, Loiola Meirelles Junqueira-De-Azevedo, Ghedira, Bouattour, M'Ghirbi and Chudzinski-Tavassi2018, Reference Bensaoud, Aounallah, Sciani, Faria, Chudzinski-Tavassi, Bouattour and M'ghirbi2019) and Ornithodoros moubata (Díaz-Martín et al., Reference Díaz-Martín, Manzano-Román, Valero, Oleaga, Encinas-Grandes and Pérez-Sánchez2013) present a greater number of GRPs in females than males. In R. microplus, females exhibit a greater abundance of cuticle-like GRP transcripts, whereas two subtypes of GRPs were found almost exclusively in males (elastin-like and glue-like) (Garcia et al., Reference Garcia, Chaves Ribeiro, Maruyama, Gardinassi, Nelson, Ferreira, Andrade and de Miranda Santos2020). On the other hand, this does not seem to be a universal fact. The transcriptome of Rhipicephalus pulchellus males shows almost threefold more transcripts of GRPs, while females exhibit greater variability in sex-exclusive GRPs (Tan et al., Reference Tan, Francischetti, Slovak, Kini and Ribeiro2015). Additionally, Rhipicephalus appendiculatus males also presented twice the number of GRPs transcripts than females, suggesting that these proteins play roles beyond the cement formation, as males typically exhibit a shorter period of attachment to the same host point (de Castro et al., Reference De Castro, de Klerk, Pienaar, Latif, Rees and Mans2016).
Distribution within ticks
GRPs may be present in many tissues and organs of ticks. GRPs were identified in the ovaries, fat body, synganglia, and midgut of R. microplus (Lew-Tabor et al., Reference Lew-Tabor, Moolhuijzen, Vance, Kurscheid, Valle, Jarrett, Minchin, Jackson, Jonsson, Bellgard and Guerrero2010; Leal et al., Reference Leal, Alzugaray, Seixas, Vaz and Ferreira2018) and I. scapularis (Lees et al., Reference Lees, Woods and Bowman2010). In H. longicornis, Hlim2 was present in all organs investigated, except for the midgut. In contrast, Hlim3 was identified in the SG, synganglia, and carcass (Harnnoi et al., Reference Harnnoi, Watchabunsook, Sakaguchi, Xuan and Fujisaki2006). Therefore, GRPs have a highly heterogeneous distribution, with some GRPs being present or uniquely associated with the SG (see Table 1) and the cement (Lees et al., Reference Lees, Woods and Bowman2010; Leal et al., Reference Leal, Alzugaray, Seixas, Vaz and Ferreira2018; Ribeiro et al., Reference Ribeiro, Bayona-Vásquez, Budachetri, Kumar, Frederick, Tahir, Faircloth, Glenn and Karim2023).
The SG transcriptome of different tick species (Dermacentor andersoni, R. appendiculatus, R. microplus, Amblyomma variegatum, Amblyomma tuberculatum and Antricola delacruzi) and the proteome of A. americanum saliva reveal that GRPs transcripts and putative GRP proteins are abundantly present (Alarcon-Chaidez et al., Reference Alarcon-Chaidez, Sun and Wikel2007; Karim et al., Reference Karim, Singh and Ribeiro2011; Ribeiro et al., Reference Ribeiro, Anderson, Manoukis, Meng and Francischetti2011, Reference Ribeiro, Labruna, Mans, Maruyama, Francischetti, Barizon and de Miranda Santos2012; de Castro et al., Reference De Castro, de Klerk, Pienaar, Latif, Rees and Mans2016; Kim et al., Reference Kim, Tirloni, Pinto, Diedrich, Moresco, Yates, da Vaz and Mulenga2020). The transcriptome of Amblyomma maculatum SG also encodes 12 types of GRPs (Ribeiro et al., Reference Ribeiro, Bayona-Vásquez, Budachetri, Kumar, Frederick, Tahir, Faircloth, Glenn and Karim2023). However, Ixodes holocyclus presents less than 20% of their SGs transcripts for putative secreted proteins and therefore also shows a lower abundance of GRPs compared to other ticks (Ong et al., Reference Ong, Rodriguez-Valle, Moolhuijzen, Barrero, Hunter, Szabo, Bellgard and Lew-Tabor2016).
Influence of feeding
The feeding process induces ticks to alter the composition of proteins secreted in saliva (Radulović et al., Reference Radulović, Kim, Porter, Sze, Lewis and Mulenga2014; Tirloni et al., Reference Tirloni, Kim, Pinto, Yates, da Silva Vaz and Mulenga2017). In this sense, GRPs of A. americanum are more abundant in the SG of partially fed females than in unfed females (Jaworski et al., Reference Jaworski, Muller, Simmen and Needham1990). During the feeding period, nine GRP genes of adult A. americanum ticks showed differential expression that varied according to the time since the onset of feeding. Among them, the gene of the GRP AamerSigP-41539 was almost exclusively expressed in unfed and 24 h post-feeding ticks. However, the genes of Aam-36909 and Aam-41235 reached their maximal expression after 48 h of feeding, whereas Aam-41540 and Aam-3099 did so after 72 h and 120 h, respectively (Bullard et al., Reference Bullard, Williams and Karim2016a). Kim et al. (Reference Kim, Tirloni, Pinto, Diedrich, Moresco, Yates, da Vaz and Mulenga2020) detected a highly cumulative relative abundance of GRPs in the saliva of A. americanum during the feeding process. Most of the GRP genes (90%) were shown to be upregulated after 48 h of feeding, with a maximum peak at 72 h. In R. appendiculatus, GRP 64P gene transcripts were not detected in unfed ticks, whereas in fed females, the maximum expression was observed after 24 h, with a subsequent decrease in expression levels following detachment. In males, by contrast, the expression reached maximum levels seven days after the beginning of feeding (Havlíková et al., Reference Havlíková, Roller, Koči, Trimnell, Kazimírová, Klempa and Nuttall2009).
In a proteome analysis of Haemaphysalis flava, the GRPs identified were shared between partially and fully engorged females (Liu et al., Reference Liu, Cheng, Mao, Duan, Feng and Cheng2023). Conversely, 17 GRP transcripts were detected in the SG transcriptome of Amblyomma sculptum. Of these, nine were up-regulated and two down-regulated after 24 h of feeding, while only three GRPs were identified in the saliva proteome, with only one present at higher levels in fed ticks (Esteves et al., Reference Esteves, Maruyama, Kawahara, Fujita, Martins, Righi, Costa, Palmisano, Labruna, Sá-Nunes, José and Fogaça2017). In the saliva of R. microplus females, the proteome of saliva showed a higher abundance of secreted GRPs in partially than in fully engorged females (Tirloni et al., Reference Tirloni, Seixas, Mulenga, Da Silva Vaz and Termignoni2014). However, Leal et al. (Reference Leal, Alzugaray, Seixas, Vaz and Ferreira2018) identified higher transcript levels of RmGRP in the SG of fully engorged females. In unfed larvae of R. microplus, ORFs of different putative GRPs were also identified (Untalan et al., Reference Untalan, Guerrero, Haines and Pearson2005). The presence of different levels and diversity of GRPs pre-, during and post-feeding may imply additional roles for these proteins beyond cement formation.
Bullard et al. (Reference Bullard, Sharma, Das, Morgan and Karim2019) demonstrated that the silencing of the GRP AamerSigP-41539 gene resulted in a 20-fold increase in the bacterial load during the feeding of A. americanum. Also, after 5 and 8 days of feeding, the bacterial load was two-fold higher when the GRPs AamerSigP-41539 and Aam-40766 were depleted, respectively, suggesting that, within the SG, GRPs are involved in maintaining microbiota homeostasis (Bullard et al., Reference Bullard, Sharma, Das, Morgan and Karim2019).
The type of host also affects the expression of GRPs. R. microplus fed on susceptible bovine hosts presented a higher secretion of GRPs, including more variability in GRPs (Maruyama et al., Reference Maruyama, Anatriello, Anderson, Ribeiro, Brandão, Valenzuela, Ferreira, Garcia, Szabó, Patel, Bishop and De Miranda-Santos2010; Garcia et al., Reference Garcia, Chaves Ribeiro, Maruyama, Gardinassi, Nelson, Ferreira, Andrade and de Miranda Santos2020). A. americanum showed a higher presence of GRPs in the saliva when fed on rabbits compared to humans or dogs (Tirloni et al., Reference Tirloni, Kim, Pinto, Yates, da Silva Vaz and Mulenga2017). In I. holocyclus, GRPs contigs were detected at lower levels in ticks feeding on domestic animals than when feeding on bandicoots (wild marsupials) (Ong et al., Reference Ong, Rodriguez-Valle, Moolhuijzen, Barrero, Hunter, Szabo, Bellgard and Lew-Tabor2016). Furthermore, artificial feeding has been shown to alter the levels of GRPs in the midgut of I. ricinus and the cement of A. americanum (Bullard et al., Reference Bullard, Allen, Chao, Douglas, Das, Morgan, Ching and Karim2016b; Perner et al., Reference Perner, Provazník, Schrenková, Urbanová, Ribeiro and Kopáček2016).
Maruyama et al. (Reference Maruyama, Anatriello, Anderson, Ribeiro, Brandão, Valenzuela, Ferreira, Garcia, Szabó, Patel, Bishop and De Miranda-Santos2010) identified more transcripts of GRPs in R. microplus and R. sanguineus (brevirostrata) than in A. cajennense (longirostrata), and the monoxenic tick, R. microplus, presented more GRPs contigs than heteroxenous ticks (R. sanguineus and A. cajennense). Also, de Castro et al. (Reference De Castro, de Klerk, Pienaar, Latif, Rees and Mans2016) compared GRP transcripts of R. appendiculatus, R. punchellus and Amblyomma spp., where Rhipicephalus spp. presented GRPs in greater abundance than Amblyomma spp. (de Castro et al., Reference De Castro, de Klerk, Pienaar, Latif, Rees and Mans2016). Therefore, biological aspects of ticks influence the saliva protein diversity, including GRPs. It was suggested that brevirostrata ticks, which possess smaller mouthparts than longirostrata, secrete a greater amount of cement in order to remain attached to the host, and ticks of a single host (monoxenic) must produce a more diverse repertoire of GRPs than ticks that change hosts during their life cycle (Maruyama et al., Reference Maruyama, Anatriello, Anderson, Ribeiro, Brandão, Valenzuela, Ferreira, Garcia, Szabó, Patel, Bishop and De Miranda-Santos2010).
Microbial interactions
Ticks are well-known vectors for the transmission of animal and human diseases, and the presence of pathogens such as Theileria spp. and Babesia spp. changes the sialotranscriptome and sialoproteome of ticks (Paulino et al., Reference Paulino, Vitari, Rezende, Couto, Antunes, Domingos, Peckle, Massard, Araújo and Santos2021; Schäfer et al., Reference Schäfer, Pfaff, Höper and Silaghi2022). In this context, the tick immune system has been shown to respond to the presence of pathogens, including salivary proteins produced by ticks, the expression of which can be stimulated by the pathogen itself and may aid in its transmission (Kurokawa et al., Reference Kurokawa, Lynn, Pedra, Pal, Narasimhan and Fikrig2020).
With regard to GRPs, the infection of Dermacentor variabilis with Rickettsia montanensis resulted in a reduction in Oi814-GRP gene expression in the ovaries, midgut, and SG, with a 52% decrease compared to uninfected ticks (Macaluso et al., Reference Macaluso, Mulenga, Simser and Azad2003). In contrast, GRP upregulation was detected in the ovaries of Rickettsia-infected D. variabilis (Macaluso et al., Reference Macaluso, Mulenga, Simser and Azad2006). Infection by Theileria parva of R. appendiculatus resulted in a nearly twofold increase in the expression of three GRPs (Nene et al., Reference Nene, Lee, Kang'A, Skilton, Shah, De Villiers, Mwaura, Taylor, Quackenbush and Bishop2004), and R. bursa infected with Babesia ovis showed a GRP associated with the cellular matrix that was also upregulated (Antunes et al., Reference Antunes, Couto, Ferrolho, Rodrigues, Nobre, Santos, Margarida Santos-Silva, de la Fuente and Domingos2018). A flagelliform silk protein (100Silk) was found to be reduced in R. microplus infected with Anaplasma marginale, and, when the respective gene was silenced, the level of tick infection decreased (Zivkovic et al., Reference Zivkovic, Esteves, Almazán, Daffre, Nijhof, Kocan, Jongejan and de la Fuente2010). Accordingly, R. microplus infected with Babesia presented a downregulation of one GRP in ovaries (Rachinsky et al., Reference Rachinsky, Guerrero and Scoles2007). These data provide evidence that tick protein production is modulated by various components of their microbiome, including pathogens (Kurokawa et al., Reference Kurokawa, Lynn, Pedra, Pal, Narasimhan and Fikrig2020). However, the overall interaction of GRPs with tick pathogens remains unclear. As possible immune proteins, GRPs would be expected to be upregulated as part of a tick response to infection, although GRP transcription could be inhibited by the pathogen. Some GRPs may also potentially be upregulated by the pathogen, which could be explained as facilitating transmission to a new host.
Possible roles of GRPs in ticks
As described in the previous sections, tick GRPs may present multiple functions. In a similar manner, four classes of GRPs and more than 12 functions for GRPs in plants have already been described (Mangeon et al., Reference Mangeon, Junqueira and Sachetto-Martins2010). Tick GRPs are often associated with cement formation, especially those expressed in the SG. However, in other tissues they seem to perform alternative and/or unknown functions (Maruyama et al., Reference Maruyama, Anatriello, Anderson, Ribeiro, Brandão, Valenzuela, Ferreira, Garcia, Szabó, Patel, Bishop and De Miranda-Santos2010). The differences described for GRP variability and abundance between adult males and females also suggest additional roles, as males are expected to attach and detach more times than females during feeding and reproduction. In fact, R. appendiculatus and R. microplus showed more total GRP transcripts in males, suggesting potential involvement in other physiological processes (de Castro et al., Reference De Castro, de Klerk, Pienaar, Latif, Rees and Mans2016; Bensaoud et al., Reference Bensaoud, Aounallah, Sciani, Faria, Chudzinski-Tavassi, Bouattour and M'ghirbi2019; Garcia et al., Reference Garcia, Chaves Ribeiro, Maruyama, Gardinassi, Nelson, Ferreira, Andrade and de Miranda Santos2020).
The cement cone of R. microplus was previously described as presenting a high content of glycine (Kemp et al., Reference Kemp, Stone, Binnington, Frederick and Rachel1982). Thereafter, GRPs in the cement were suggested to promote the hardening of the adhesion structure (Bullard et al., Reference Bullard, Allen, Chao, Douglas, Das, Morgan, Ching and Karim2016b). As shown above, this is strongly corroborated by the property of certain GRPs to form a gel-solid structure after LLPS (Ganar et al., Reference Ganar, Turbina, Nandy, Chen, Suylen, van der Beelen, Louise Pascoe, Koenraadt, Dijkgraaf and Deshpande2023).
Another important function that may be performed by tick GRPs is the modulation of the host immune response. The similarity of some GXX/GGX repeats in tick GRPs compared to the Gly-rich motifs of vertebrate GRPs indicates a probable function on evasion of host defence/haemostasis by mimicking host skin components (Mulenga et al., Reference Mulenga, Sugimoto, Sako, Ohashi, Musoke, Shubash and Onuma1999; Bishop et al., Reference Bishop, Lambson, Wells, Pandit, Osaso, Nkonge, Morzaria, Musoke and Nene2002). This hypothesis is endorsed by the increased inflammation at tick attachment sites in GRP-silenced ticks, which also shows increased haemorrhaging at the tick bite site (Hollmann et al., Reference Hollmann, Kim, Tirloni, Radulović, Pinto, Diedrich, Yates, da Silva Vaz and Mulenga2018). Additionally, Gly can be recognized by receptors and when Gly binds to anion channel receptors, the chloride conductance increases, leading to chloride influx, which causes the hyperpolarization of platelets, thereby inhibiting platelet aggregation (Schemmer et al., Reference Schemmer, Zhong, Galli, Wheeler, Xiangli, Bradford, Conzelmann, Forman, Boyer and Thurman2013). Indeed, Ribeiro et al. (Reference Ribeiro, Alarcon-Chaidez, Ivo, Mans, Mather, Valenzuela and Wikel2006) identified one GRP as a probable inhibitor of platelet aggregation.
GRPs are also described to bind nucleic acids and RNA-binding GRPs are associated with the development of embryos in a range of organisms, as well as in the ecdysis of insects (Zhong et al., Reference Zhong, Mita, Shimada and Kawasaki2006; Ciuzan et al., Reference Ciuzan, Hancock, Pamfil, Wilson and Ladomery2015). In zebrafish, GRPs are also involved in spinal cord regeneration (Ma et al., Reference Ma, Cheng, Li, Deng, Zhang and Zhu2021). In ticks, the presence of GRPs in eggs was reported in H. qinghaiensis, H. longicornis, and R. microplus. Furthermore, silencing GRP genes has been shown to reduce hatchability and weight of larvae (Jiang et al., Reference Jiang, Gao, Wang, Xu, Li, Qi, Yang, Ji, Zhang, Luo and Yin2014; Leal et al., Reference Leal, Alzugaray, Seixas, Vaz and Ferreira2018; Luo et al., Reference Luo, Shen, Ren, Guan, Zhao, Yin, Chen, Zhao, Luo, Li and Liu2020). These data indicate that GRPs are important players in tick development and that further studies can elucidate the specific roles that GRPs play in these processes.
In response to stress, insect GRPs in the cuticle protect against temperature changes, allowing them to adapt to new environmental conditions (Zhang et al., Reference Zhang, Goyer and Pelletier2008). Response to environmental stress mediated by A. americanum GRPs has also been suggested, as temperature changes (cold and heat), oxidative stress, and injury positively or negatively modulate the expression of GRP genes. Interestingly, gene expression of the GRP Aam-40766 decreased in the SG but increased when the entire tick was analysed. On the other hand, the expression of the GRP Aam-36909 gene was downregulated in the whole tick when exposed to cold or injury stresses, but upregulated in the SG, which was suggested as a physiological adaptation (Bullard et al., Reference Bullard, Sharma, Das, Morgan and Karim2019). In eggs of H. longicornis, one GRP is also upregulated in response to temperature stress (25°C) (Luo et al., Reference Luo, Shen, Ren, Guan, Zhao, Yin, Chen, Zhao, Luo, Li and Liu2020).
Antimicrobial activity is a known function of GRPs and glycine-rich antimicrobial peptides (GR-AMPs), including silk GRPs from spiders, scorpions, and insects. GR-AMPs are molecules of the innate immune response that act against a wide range of pathogens. In general, GR-AMP sequences contain a signal peptide, a pro-peptide and a glycine-rich region, which usually present domains with repeats containing Gly residues (Yi et al., Reference Yi, Chowdhury, Huang and Yu2014; Wang and Wang, Reference Wang and Wang2016), similar to what can be found in tick GRPs. Attacins, ctenidins, gloverins, diptericins, serrulins, acanthoscurrins, hyastatin, and prolixins are some GR-AMPs that have already been described and characterized (Yi et al., Reference Yi, Chowdhury, Huang and Yu2014; Wang and Wang, Reference Wang and Wang2016; Meraj et al., Reference Meraj, Dhari, Mohr, Lowenberger and Gries2024). Most of assays employing GR-AMPs indicated activity against gram-negative bacteria, although effects against gram-positive bacteria, fungi, protozoa (Meraj et al., Reference Meraj, Dhari, Mohr, Lowenberger and Gries2024) and virus are also reported (Yi et al., Reference Yi, Chowdhury, Huang and Yu2014; Wang and Wang, Reference Wang and Wang2016). For example, serrulin from the scorpion Tityus serrulatus inhibits the growth of E. coli, Pseudomonas aeruginosa (gram-negative bacteria), Micrococcus luteus (gram-positive bacteria), Aspergillus niger (filamentous fungus) and Candida albicans (yeast) at low concentrations and its sequence presented similarity to acanthoscurrins (Acantho 1 and 2) from the spider Acanthoscurria gomesiana, which present a reported antimicrobial activity (Lorenzini et al., Reference Lorenzini, da Silva, Fogaça, Bulet and Daffre2003; de Jesus Oliveira et al., Reference de Jesus Oliveira, Oliveira and da Silva Junior2019). Interestingly, serrulin is also similar to a secreted GRP from the tick I. scapularis (de Jesus Oliveira et al., Reference de Jesus Oliveira, Oliveira and da Silva Junior2019), suggesting a possible antimicrobial role for this tick GRP.
GRPs/Gly-rich motifs may also present different activities not directly responsible for cellular killing. One such example is the GRP hyastatin of Hyas Araneus, which presents three domains: an N-terminal region containing Gly residues, a Pro/Arg-rich region and a C-terminal region containing six cysteine residues. Native hyastatin exhibits antimicrobial activity against bacteria (E. coli and Corynebacterium glutamicum) and yeast (S. cerevisiae and C. albicans), whereas the recombinant N-terminal region does not present the same effect, although both proteins are able to bind chitin. Therefore, it is suggested that the Gly-rich region may be primarily involved in pathogen attachment rather than in cell disruption (Sperstad et al., Reference Sperstad, Haug, Vasskog and Stensvåg2009). Insect attacins, the most extensively studied GR-AMPs, were first identified in the giant silk moth Hyalophora cecropia and are probably the most promising antimicrobials among the GRPs (Yi et al., Reference Yi, Chowdhury, Huang and Yu2014; Wang and Wang, Reference Wang and Wang2016). Attacins may increase outer membrane permeability, bind LPS, and inhibit the synthesis of specific proteins, such as the OMPs, without reaching the internal membrane or cytoplasm (Carlsson et al., Reference Carlsson, Nyström, de Cock and Bennich1998). In terms of mechanism of action, attacins can be compared to polymyxin. The polymyxin-resistant strain of P. mirabilis EH193 was found to be sensitive to an attacin at a concentration 100-fold lower than polymyxin (Carlsson et al., Reference Carlsson, Nyström, de Cock and Bennich1998; Yi et al., Reference Yi, Chowdhury, Huang and Yu2014). Additionally, a peptide with 50% identity to Bactrocera dorsalis attacin B showed antimicrobial activity against methicillin-resistant Staphylococcus aureus (MRSA) (Shin and Park, Reference Shin and Park2019). Although the antimicrobial activity of tick GRPs was not directly tested for tick GRPs, it has been previously suggested that members of the GGY GRP family may possess this activity (Ribeiro et al., Reference Ribeiro, Alarcon-Chaidez, Ivo, Mans, Mather, Valenzuela and Wikel2006; Mulenga et al., Reference Mulenga, Blandon and Khumthong2007; Francischetti et al., Reference Francischetti, Sa-Nunes, Mans, Santos and Ribeiro2009). If tick GRPs do have antimicrobial properties, then modulation of GRP levels by pathogens may represent another component of the arms race in the host-parasite-vector relationship.
Vaccine potential
Tick infestations and tick-borne diseases are emerging global health concerns for both livestock and humans. Conventionally, acaricides are employed to control these ectoparasites, but resistant ticks and the worrisome effects of chemicals emphasizes the need for the development of alternative strategies, such as vaccines (Schetters et al., Reference Schetters, Bishop, Crampton, Kopáček, Lew-Tabor, Maritz-Olivier, Miller, Mosqueda, Patarroyo, Rodriguez-Valle, Scoles and De La Fuente2016). In this context, tick GRPs have already been identified as protective antigens, and the outcomes of immunization protocols can be seen summarized in Table 2 (Trimnell et al., Reference Trimnell, Davies, Lissina, Hails and Nuttall2005; Shahein et al., Reference Shahein, Abouelella, Hussein, Hamed, El-Hakim, Abdel-Shafy and Tork2013; Antunes et al., Reference Antunes, Couto, Ferrolho, Rodrigues, Nobre, Santos, Margarida Santos-Silva, de la Fuente and Domingos2018; Couto et al., Reference Couto, Seixas, Stutzer, Olivier, Maritz-Olivier, Antunes and Domingos2021).
Table 2. Glycine rich proteins from ticks used in immunization protocols, the respective outcomes and organ/tissue distribution
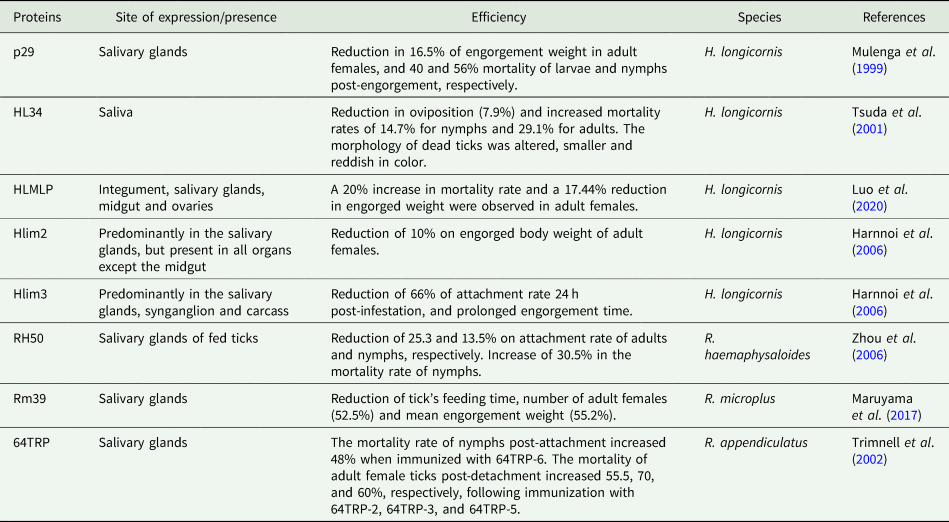
P29, a GRP of H. longicornis, was used for immunization of rabbits, reducing the feeding time and increasing the mortality of larvae and nymphs (Mulenga et al., Reference Mulenga, Sugimoto, Sako, Ohashi, Musoke, Shubash and Onuma1999). A recent study used HLMLP, a cysteine-glycine-rich protein, to immunize rabbits, affecting engorgement (Luo et al., Reference Luo, Shen, Ren, Guan, Zhao, Yin, Chen, Zhao, Luo, Li and Liu2020). Two other H. longicornis GRPs were used to immunize mice. Hlim2 reduced the weight of engorged females, while Hlim3 reduced tick attachment and duration of feeding time (Harnnoi et al., Reference Harnnoi, Watchabunsook, Sakaguchi, Xuan and Fujisaki2006).
Another potential antigen candidate is RH50 from Rhipicephalus haemaphysaloides. In ticks fed to RH50-immunized rabbits, the attachment of nymphs and adults decreased, and nymphal mortality increased (Zhou et al., Reference Zhou, Gong, Zhou, Xuan and Fujisaki2006). Vaccination of Holstein calves with Rm39, a GRP of R. microplus, resulted in weak immune recognition, and significant anti-Rm39 IgG levels were only obtained at the end of the challenge. However, egg weight and hatch rate were reduced in vaccinated calves, while fed adult ticks were capable of full engorgement, albeit with a pale colour (Maruyama et al., Reference Maruyama, Garcia, Teixeira, Brandão, Anderson, Ribeiro, Valenzuela, Horackova, Veríssimo, Katiki, Banin, Zangirolamo, Gardinassi, Ferreira and De Miranda-Santos2017).
Mice immunized with the R. appendiculatus salivary protein 64TRP reduced the transmission of tick-borne encephalitis virus (TBEV) when transmission occurred from infected mice to nymphs and infected ticks to mice. Also, secreted cement proteins demonstrated an anamnestic response after 64TRP vaccination (Labuda et al., Reference Labuda, Trimnell, Ličková, Kazimírová, Davies, Lissina, Hails and Nuttall2006). In guinea pigs, the immunization with 64TRP reduced the attachment rate of R. sanguineus, and the vaccination with three different recombinant protein versions of truncated 64TRP fragments (64TRP2, 64TRP3 and 64TRP5) reduced the attachment of R. appendiculatus. I. ricinus fed in rabbits immunized with 64TRP2 reduced engorged weight and egg mass weight, and increased mortality, whereas immunization with 64TRP2/6 increased mortality of nymphs fed on guinea pigs. The feasibility of a broad-spectrum 64TRP-based vaccine against four ticks (R. appendiculatus, I. ricinus, A. variegatum and R. microplus) has been suggested based on the presence of cross-reactive conserved protective epitopes (Trimnell et al., Reference Trimnell, Hails and Nuttall2002, Reference Trimnell, Davies, Lissina, Hails and Nuttall2005).
Other GRPs have been identified as potential vaccine candidates, but further studies are needed to assess their antigenic and immunogenic effects in the host and tick (de la Fuente and Contreras, Reference de la Fuente and Contreras2022). Proteins present in tick saliva are interesting targets, as they must interact directly with the host immune system. Moreover, an increase in tick-borne disease transmission and tick territorial dispersal, both of which are influenced by climatic changes, highlights the potential health risks that ticks may pose to the animal food chain and public health (Madison-Antenucci et al., Reference Madison-Antenucci, Kramer, Gebhardt and Kauffman2020; Tardy et al., Reference Tardy, Vincenot, Bouchard, Ogden and Leighton2022). This reinforces the necessity for the development of anti-tick vaccines, particularly those of broad-spectrum.
Conclusions
GRPs are a very diverse group of proteins that have been reported to be important cement proteins in ticks, but the amount of data accumulated on them has shown that their roles must be much broader, especially when compared to what has been described in other organisms. This review focused on the features that may make these proteins a hallmark of the tick-host relationship. GRPs can be found with specific domains and repetitions of amino acid residues, often showing similarity to host proteins such as collagen, configuring probable modulators of the haemostatic and immune systems. The intrinsically disordered nature of some GRPs may be essential for LLPS, which seems to be mechanistically involved in the scaffolding of the tick adhesive structure with flexibility plus rigidity. The large variation in the presence of the different GRPs when evaluated ontogenetically, in different sexes, in the localisation of different tissues/organs of the tick, during feeding, and even when comparing genetically closely related species, suggests possible roles in additional physiological processes. The regulation of GRPs in response to injury, infection, oxidative stress and temperature changes also suggests an involvement in adaptation to different conditions or environments. Furthermore, it seems probable that tick GRPs are involved in nucleic acid binding and antimicrobial activities. In light of the aforementioned context, it seems unlikely that GRPs are involved in more than one type of physiological process in ticks. Indeed, it seems reasonable to suggest that many GRPs may be multifunctional proteins. It can therefore be concluded that GRPs are promising candidates for the composition of anti-tick vaccines. Although anti-GRP responses do not appear to increase the mortality of adult ticks, they significantly reduce the number of progeny. In order to improve the design of vaccination approaches and ultimately achieve more effective protective outcomes, it would be beneficial to gain a more comprehensive understanding of the roles and mechanisms of action of GRPs, as well as their dynamics within hosts and pathogens.
Data availability statement
Not applicable.
Author's contributions
Renata Perotto de Souza: Conceptualization, Data curation, Writing – original draft, Writing review & editing, Visualization. Mariana Vieira Dalla Valentina: Writing – original draft, Visualization. Bruna Ferreira Leal: Writing – original draft, Writing review & editing. Sílvia Dias de Oliveira: Data curation, Writing review & editing. Carlos Alexandre Sanchez Ferreira: Conceptualization, Data curation, Writing – original draft, Supervision, Writing – review & editing.
Financial support
Renata Perotto de Souza and Mariana Vieira Dalla Valentina received financial support in the form of a fellowship from CNPq and PUCRS, respectively.
Competing interests
The authors declare no conflicts of interest exist.
Ethical standards
Not applicable.