Article contents
Determination of Diffusion Parameters for Arsenic
Published online by Cambridge University Press: 25 February 2011
Abstract
This paper deals with an analysis of the oxynitridation and direct nitridation experiments of Fahey et al.(1984[1]). The inconsistencies in the evaluation of the fiactional component of diffusion for aisenic are discussed. Using a recently developed diffusion model an explanation for the diffusion data for arsenic is given. The standard equation 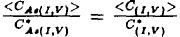 for analyzing diffusion under nonequilibrium conditions is shown to lead to erroneous results. A consistent treatment must account for the concentrations of arsenic, point defects and arsenic point defect pairs. Pair formation kinetics must be included. Values are presented for the diffusion constants DIAs , DVAS , and the reaction constants kIAs , kVAs depending on the fractional interstitial component of diffusion
for analyzing diffusion under nonequilibrium conditions is shown to lead to erroneous results. A consistent treatment must account for the concentrations of arsenic, point defects and arsenic point defect pairs. Pair formation kinetics must be included. Values are presented for the diffusion constants DIAs , DVAS , and the reaction constants kIAs , kVAs depending on the fractional interstitial component of diffusion 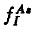 .
.
Information
- Type
- Research Article
- Information
- Copyright
- Copyright © Materials Research Society 1990
References
- 2
- Cited by

