Article contents
Nanoscale TiO2 coating improves water stability of Cs2SnCl6
Published online by Cambridge University Press: 12 November 2020
Abstract
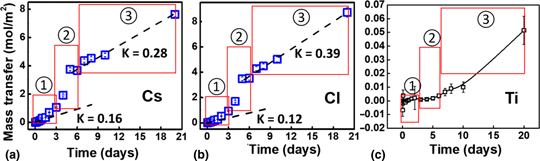
To improve the stability of Cs2SnCl6 under aqueous/moisture environments, we applied a concept of artificial passivation by depositing a protective TiO2 coating of 10 nm on the surface of Cs2SnCl6. Static leaching experiments results indicate that the initial release rates of Cs+ and Cl− are decreased by 20–30 times with TiO2 coating, suggesting its possibility to improve the short-term water/environmental stability of Cs2SnCl6. An amorphous-to-crystalline phase transition in TiO2 film was observed, possibly resulting in degradation of Cs2SnCl6. However, the crystalline TiO2 film still remains after 21 days water exposure and can still act as an effective passivation layer to reduce the release rates of Cs+ and Cl- by as much as about 17 and 7 times, respectively, relative to static leaching without artificial coatings. Therefore, the water/environmental stability of metal halide perovskite Cs2SnCl6, which is a highly soluble molecular salt, can be enhanced by the nanoscale TiO2 coating as an artificial passivation film.
Information
- Type
- Research Letters
- Information
- Copyright
- Copyright © The Author(s), 2020, published on behalf of Materials Research Society by Cambridge University Press
References
- 1
- Cited by


