Introduction
In situ observation of cephalopods in their natural habitat plays a fundamental role in advancing their study since their complex behaviours (Hanlon and Messenger, Reference Hanlon and Messenger2018) can be minimized or poorly recognized in artificial environments such as aquariums and laboratories. The ability of these organisms to efficiently camouflage themselves in their environment and explore crevices and tight spaces like dens also makes their detection, observation, and consequently their study and collection challenging.
To better understand the ecology, biology, and behaviour of these animals and their interactions with the environment, it is crucial to observation in the natural environment. Scientific diving, which involves the immersion of scientists/divers, is an effective tool for this purpose. This approach has been widely used to study various groups of marine animals, from large marine mammals (Skrovan et al., Reference Skrovan, Williams, Berry, Moore and Davis1999) and fishes (Hixon, Reference Hixon2011; Kampouris et al., Reference Kampouris, Pappou, Erga, Kouloumperis and Batjakas2023; Pitts, Reference Pitts2017) to invertebrates (Eggleston et al., Reference Eggleston, Herrnkind, Hines, Lang, Marinelli, Roberts and Taylor2013; Lessios et al., Reference Lessios, Lasker, Levitan, Lang, Marinelli, Roberts and Taylor2013; Mcintyre, Reference Mcintyre1969), including cephalopods (Norman et al., Reference Norman, Finn and Tregenza2001; Leite et al., Reference Leite, Haimovici, Mather and Oliveira2009; Mather and Alupay, Reference Mather and Alupay2016; Naud and Havenhand, Reference Naud and Havenhand2005).
Brock (Reference Brock1954) was among the first to implement a quantitative approach to estimating fish abundance using SCUBA, pioneering underwater transects as a systematic method for assessing population size and distribution. By enabling direct in situ observations, SCUBA surveys facilitated non-destructive assessments of fish populations, reducing reliance on techniques like netting and the use of ichthyocides (Brock, Reference Brock1954). These early contributions helped establish the foundation for the development of scientific diving methodologies, with studies involving fishes and corals becoming some of the earliest and most widely documented applications of this research tool (Hixon, Reference Hixon2011; Squires, Reference Squires1966). Much of the methodology and monitoring protocols have been developed with a focus on these organisms, such as the Atlantic and Gulf Rapid Reef Assessment (Lang et al., Reference Lang, Marks, Kramer, Kramer and Ginsburg2010), the Roving Diver Technique, originally developed by the Reef Environmental Education Foundation (Bohnsack, Reference Bohnsack, Crosby, Gibson and Potts1995), and the Reef Check Survey (Hodgson, Reference Hodgson1997).
While SCUBA-based studies of fishes and corals have long been established, advancements in specialized diving equipment, such as closed-circuit rebreathers (Norro, Reference Norro2016; Sieber and Pyle, Reference Sieber and Pyle2010), have facilitated research extending into deeper and mesophotic reef ecosystems to study deep-sea fishes and coral reefs (Pinheiro et al., Reference Pinheiro, Goodbody-Gringley, Jessup, Shepherd, Chequer and Rocha2016; Pyle, Reference Pyle1996; Rocha et al., Reference Rocha, Pinheiro, Shepherd, Papastamatiou, Luiz, Pyle and Bongaerts2018). However, research on cephalopods in natural environments has developed more recently, with field studies dating back to the late 1970s (Hurley, Reference Hurley1977; Ward et al., Reference Ward, Stone, Westermann and Martin1977). Unlike the well-established methodologies used for fishes and corals, cephalopod studies have often adapted protocols originally designed for these taxa (Leite et al., Reference Leite, Haimovici, Mather and Oliveira2009). Furthermore, due to the challenges of studying cephalopods in situ, most research has remained restricted to shallow waters, relying on snorkelling (Mather, Reference Mather1982) or SCUBA diving (Hanlon and Hixon, Reference Hanlon and Hixon1980; Hanlon et al., Reference Hanlon, Hixon and Hulet1983).
Despite the physiological limitations associated with scientific diving, which vary depending on the diving method used (freediving, autonomous diving, and technical diving) (Joiner, Reference Joiner2001), it still has certain advantages compared to other methodologies such as the use of remotely operated underwater vehicles and autonomous underwater vehicles from research vessels (Yakhontov, Reference Yakhontov2021). SCUBA diving enables the execution of complex tasks, rapid adaptation and decision-making in changing circumstances, reduced operating costs, and its flexibility and logistical ease, being able to be used in small boats or even from the shoreline (Yakhontov, Reference Yakhontov2021). The use of diving thus enables the observation and performance of less invasive procedures on aquatic ecosystem fauna and flora in their natural environment, while also providing greater sampling accuracy (Kur and Mioduchowska, Reference Kur and Mioduchowska2018).
In the study of cephalopods, scientific diving has been applied in different areas, from collecting specimens for taxonomy (Hochberg et al., Reference Hochberg, Norman and Finn2006) to observations of their behaviour of inter- and intraspecific relationships in their natural environment (Madin et al., Reference Madin, Hamner, Haddock, Matsumoto, Lang, Marinelli, Roberts and Taylor2013). An example of the versatility of the use of diving is clear in the case of Octopus insularis, where diving was initially used for specimen sampling for morphological studies and species description (Leite et al., Reference Leite, Haimovici, Molina and Warnke2008) and subsequently for observation purposes, video recording, collection of food remains, generating important results on its ecology, feeding habits, trophic relationships, behavioural patterns, and body patterns (Batista and Leite, Reference Batista and Leite2016; Leite et al., Reference Leite, Haimovici, Mather and Oliveira2009; O’Brien et al., Reference O’Brien, Di Miccoli and Fiorito2021). In situ behavioural study allows, for example, the elaboration of ethograms, as done for Sepioteuthis sepioidea through video recordings obtained in freediving (Mather et al., Reference Mather, Griebel and Byrne2010), and studies on their different colour presentations and camouflage, as in Sepia apama through photographs obtained by SCUBA divers (Schnell et al., Reference Schnell, Hanlon, Benkada and Jozet-Alves2016; Zylinski et al., Reference Zylinski, How, Osorio, Hanlon and Marshall2011).
The relevance of diving as a tool in the development of marine science is still understudied and lacks quantitative and qualitative information regarding its methods and results obtained, making the recognition of its scientific impact diluted and unrecognized (Leite et al., Reference Leite, Pinheiro, Berchez, Bertoncini, Cima, Demetrescu, Francini-Filho, Kikuchi, Machado, Maia-Nogueira and Martins2023; Sayer, Reference Sayer2007).
A systematic review provides a broader view of a target subject, where the identification, evaluation, and synthesis of the results obtained assist in the comprehensive understanding of the topic and the recognition of possible information gaps that, once identified, can be used as a basis for studies that will address future questions and studies areas (Crowther and Lim, Reference Crowther and Lim2010). Thus, this study aims to conduct a systematic review of an underwater study tool, scientific diving, employed in cephalopod research, perform trend analyses and evaluations of applications, areas of study, and therefore provide a broader understanding of the methods and results obtained in the research of these molluscs over time.
Material and methods
A systematic search of published articles that used scientific diving as a research tool in the study of cephalopods was conducted. Two search platforms were used: SciVerse Scopus (http://scopus.com/) and Portal Capes – Web of Science (WoS) (http://periodicos.capes.gov.br/). The searches were conducted using the terms ‘coleoidea’, ‘octopod*’, ‘cuttlefish*’, ‘octopus*’, ‘squid*’, ‘cephalopod*’, ‘sepioidea’, ‘teuthoidea’, ‘nautilus’, and ‘nautilidae’. Each of these terms was combined with the following set of words using the AND operator: ‘scuba OR diving OR dive OR snork* OR diver OR divers OR freediv* OR underwater method* OR scientific div*’, with the document type filter set to ‘article’.
Following an initial screening of article titles and/or abstracts on the search platform, those addressing the theme of the class Cephalopoda were selected. Subsequently, the articles were individually analysed, selecting only those that mentioned the use of scientific diving as a research tool during the study. The selected articles were added to an Excel spreadsheet with information including title, first author, last author, year, journal, subclass, order, species, mention of the term ‘SCUBA’, diver experience (when provided), knowledge area, geographical area, dive site coordinates, methods, dive depth, temperature, and visibility. Literature review articles were not included. For accuracy estimation, the ResearchGate platform of Professor Dr Tatiana S. Leite (https://www.researchgate.net/profile/Tatiana-Leite-5), an authority in Brazil for the use of scientific diving in cephalopod studies, was used as a reference. On this platform, 21 articles from 2008 to 2022 that used scientific diving in cephalopod studies were found and used to estimate the accuracy of the method, which was adapted from Sayer (Reference Sayer2007).
Based on the accuracy calculated for the first evaluation, we decided to do a second search to broaden the results. The search was conducted on the WoS platform combining the acronym ‘TI = ’ (title search) with the terms from the first search: ‘coleoidea’, ‘octopod*’, ‘cuttlefish*’, ‘sepioidea’, ‘Teuthoidea’, ‘nautilus’, and ‘nautilidae’, with the document type filter set to ‘article’, totalling 1,933 additional publications analysed. The use of the ‘TI’ acronym limited the search to articles necessarily containing the searched term in the title, with the aim of obtaining a greater number of relevant publications for the study. All results from the second search were evaluated following the same selection criteria applied in the first search. The new articles found were added to those from the first search and used as a basis for estimating the current number of studies employing scientific diving in cephalopod studies, as well as to assess the accuracy of this review.
Subsequently, a third search was conducted on the WoS platform, employing the terms ‘coleoidea’, ‘octopod’, ‘cuttlefish’, ‘octopus*’, ‘squid*’, ‘cephalopod*’, ‘sepioidea’, ‘teuthoidea’, ‘nautilus’**, and ‘nautilidae’, in combination with the ‘TI’ acronym, covering the period from 1973 to 2022 (the year the first publication included in this study was identified). The number of articles per year was extracted and incorporated into the dataset to assess whether the increasing use of scientific diving in cephalopod studies reflects the overall growth in cephalopod-related research.
To examine temporal trends, we fitted linear regression models to the number of total cephalopod-related publications and to the subset of publications using scientific diving (SCUBA and freediving) from 1973 to 2022. To test whether scientific diving publications are increasing at a significantly different rate than total cephalopod publications, we conducted an analysis of covariance (ANCOVA) using the stats package in RStudio (R version 4.3.2; R Core Team, 2023a, 2023b). The model included year (as a continuous variable), publication type (scientific diving vs. total cephalopod), and their interaction as predictors. A significant interaction term indicated a difference in slopes between the two publication types. All figures were produced using the ggplot2 package (version 3.4.0; Wickham Reference Wickham2016).
Additionally, the country/region of each publication was retrieved using the ‘Analyze Results’ tool in WoS, which categorizes publications based on the country of the affiliated institution of the authors. This information was used to compare the global distribution of cephalopod research with the distribution of scientific diving studies based on author affiliations.
Diver experience categories were defined based on designations mentioned in the studies. These categories included Researcher, Author, Scientific Diver, Fisherman, Citizen Scientist, Commercial Diver, Technical Diver, and Volunteer. The Researcher, Author, and Scientific Diver categories were consolidated into a single ‘Researcher’ category since they all involve divers from the scientific community. The purpose of scientific diving was grouped into different categories according to the methodology used, with the ‘Observation’ category comprising different methodologies aimed at observation such as Roving diver, Underwater visual census, Animal focal methodology, and Underwater visual transect, and the ‘Filming and Photography’ category encompassing methodologies using cameras for documentation.
First and last author data were analysed together to determine the number of publications per country, using the institutions linked to the authors to identify the country where the studies were conducted. Geographic coordinates were used to create a map in ArcGIS 10.5 software. An analysis of maximum depths reached on the dives in relation to the taxonomic order studied from the first search and diver experience was performed in order to create a dive profile.
Results
In the first search, the use of the keyword set on the platforms generated a total of 2,159 articles, of which 96 met the search criteria. Among these 96 articles, only 6 (28% of accuracy) belonged to the 21 articles published by Leite, T.S. In the second search, 129 articles that met the criteria were found, which, added to the first search, amounted to 225 publications. Of these, 12 articles belonged to the 21 articles used as a reference by the author Leite, T.S., increasing the search accuracy to 57%, an increase of 29% compared to the first search.
Among the 205 articles found that addressed SCUBA diving in both searches, it was found that 166 of them (81%) mentioned the term ‘SCUBA’. However, only 70 of these articles (42%) were identified in the first search, which relied solely on the presence of the term in titles, abstracts, or keywords. The use of SCUBA has increased at an annual rate of 4.05% following a moderate linear trend (R 2 = 0.511). The remaining 20 articles (9% of the total) referred to freediving or similar activities, but these studies used a range of terms – such as ‘snorkeling’, ‘skin diving’, ‘breath-holding’, or ‘freediving’ – to describe the activity, making it inconsistent for direct comparison. The use of freediving has grown at a slower rate of 2.27% with a weaker linear trend (R 2 = 0.403).
The earliest article found was from 1973, ‘Vitesse de Digestion d’ Octopus cyanea (Cephalopoda: Octopoda)’ (Boucher-Rodoni, Reference Boucher-Rodoni1973), reporting freediving for octopus collection. In 1977, the first articles using SCUBA were published, studying the species Nautilus pompilius in ‘Notes on Animal Weight, Cameral Fluids, Swimming Speed, and Color Polymorphism of the Cephalopod Nautilus pompilius in the Fiji Islands’ (Ward et al., Reference Ward, Stone, Westermann and Martin1977) and Loligo opalescens in ‘Mating Behavior of the Squid Loligo opalescens’ (Hurley, Reference Hurley1977). The first work used SCUBA for releasing the specimens used in the study, and the second conducted observations of L. opalescens spawns.
The number of cephalopod-related publications obtained in the third search from 1973 to 2022 (N = 14,022) exhibited a consistent upward trend, following a linear model of y = 6.832x − 13,353.9 (R 2 = 0.794), indicating an average annual growth rate of approximately 3.00%. Publications using scientific diving (N = 225) also increased over time, following a linear model of y = 0.275x − 535.4 (R 2 = 0.596), with a higher growth rate of 4.34%. To assess whether scientific diving publications are increasing at a significantly different rate than total cephalopod research, we performed an ANCOVA with publication type and year as predictors. The analysis revealed a significant interaction between year and publication type (F(1, 98) = 7.50, p = 0.0075), indicating that scientific diving use is increasing at a significantly faster rate than overall cephalopod publications (Figure 1).
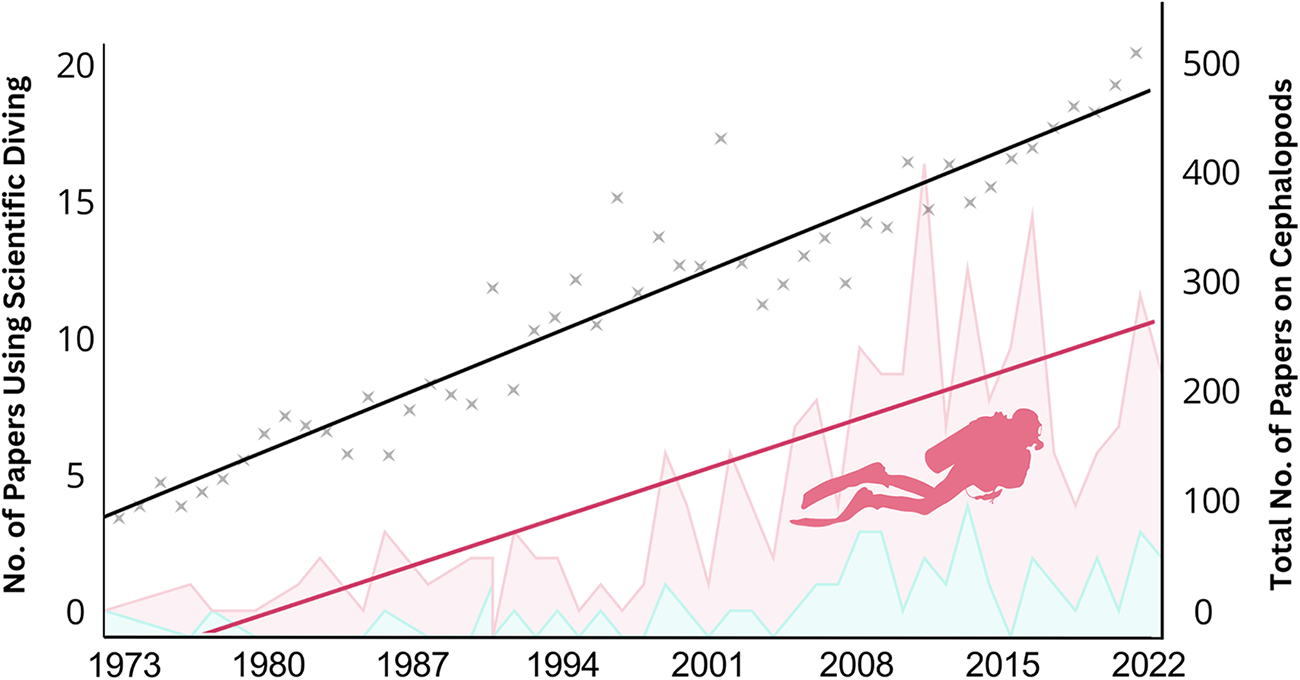
Figure 1. Trends in the use of scientific diving in cephalopod research from 1973 to 2022. The left y-axis represents the number of publications that used scientific diving, which increased over time following y = 0.275x − 535.4 (R 2 = 0.596), with the red line showing its linear increase. The right y-axis represents the total number of cephalopod-related publications per year, following a linear growth model y = 6.832x − 13,353.4 (R 2 = 0.794), with the black line representing this trend. Asterisks indicate the total number of cephalopod-related publications per year. The red-shaded area represents the number of studies utilizing SCUBA, while the blue-shaded area represents those conducted using snorkelling.
The United States ranked first in both total cephalopod research output and scientific diving utilization, contributing 3,727 publications (27%) of all cephalopod-related studies and 89 publications (21%) of those employing scientific diving. Japan and Germany followed in overall cephalopod research output, with Japan producing 2,006 publications (14%) and Germany 1,160 publications (8%), yet their use of scientific diving was comparatively lower. Japan ranked 11th in scientific diving use with 14 publications (3%), while Germany ranked 14th with 8 publications (2%) (Table 1).
Table 1. Information on the total number of publications using scientific diving in cephalopod studies (left) and the total number of cephalopod-related publications (right) from 1973 to 2022
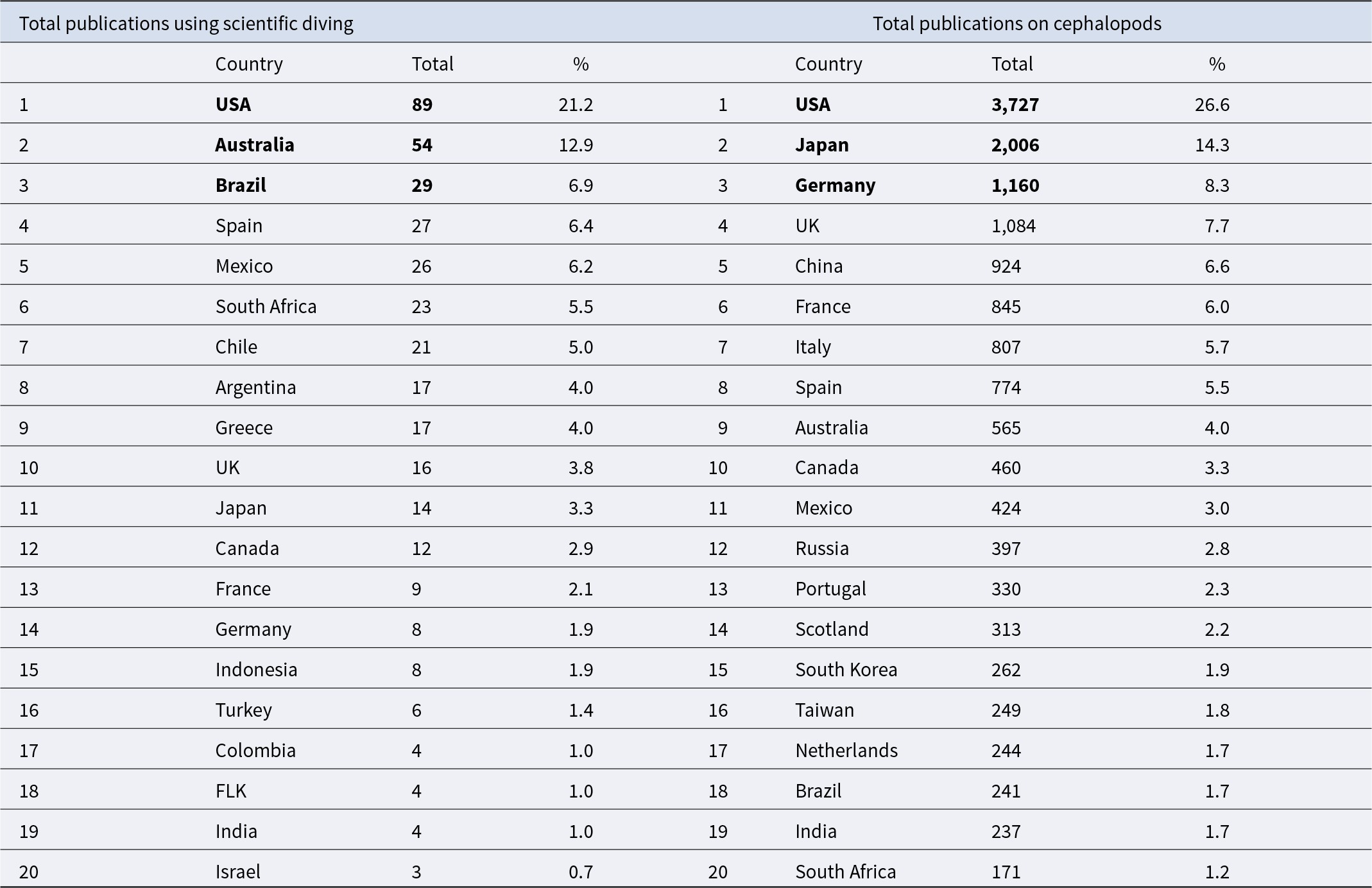
The table presents the 15 countries with the highest number of publications in each category, ordered by their total number of publications. The first three countries in each category are highlighted in bold.
In contrast, Australia and Brazil exhibited relatively higher proportions of scientific diving use despite their lower rankings in cephalopod research productivity. Australia, which ranked ninth in total cephalopod research with 565 publications (4%), was the second-highest contributor to scientific diving publications with 54 publications (13%). Similarly, Brazil ranked 18th in cephalopod research with 241 publications (2%) yet held the third position in scientific diving use with 29 publications (7%). These disparities highlight the varying degrees to which scientific diving is utilized across countries, suggesting that some nations employ scientific diving in cephalopod research more frequently despite having a lower overall research output. Spain and Mexico followed a similar pattern. Spain ranked eighth in cephalopod research with 774 publications (6%) but ranked fourth in scientific diving use with 27 publications (6%). Mexico ranked 11th in cephalopod research with 424 publications (3%) but ranked fifth in scientific diving use with 26 publications (6%).
On the other hand, China, despite ranking fifth in total cephalopod research with 924 publications (7%), did not appear among the countries utilizing scientific diving. Our search did not identify any cephalopod-related studies from China that used scientific diving. This absence highlights how the reliance on scientific diving varies significantly across nations, suggesting that its use is influenced not only by research volume but also by other underlying factors (Figure 2).
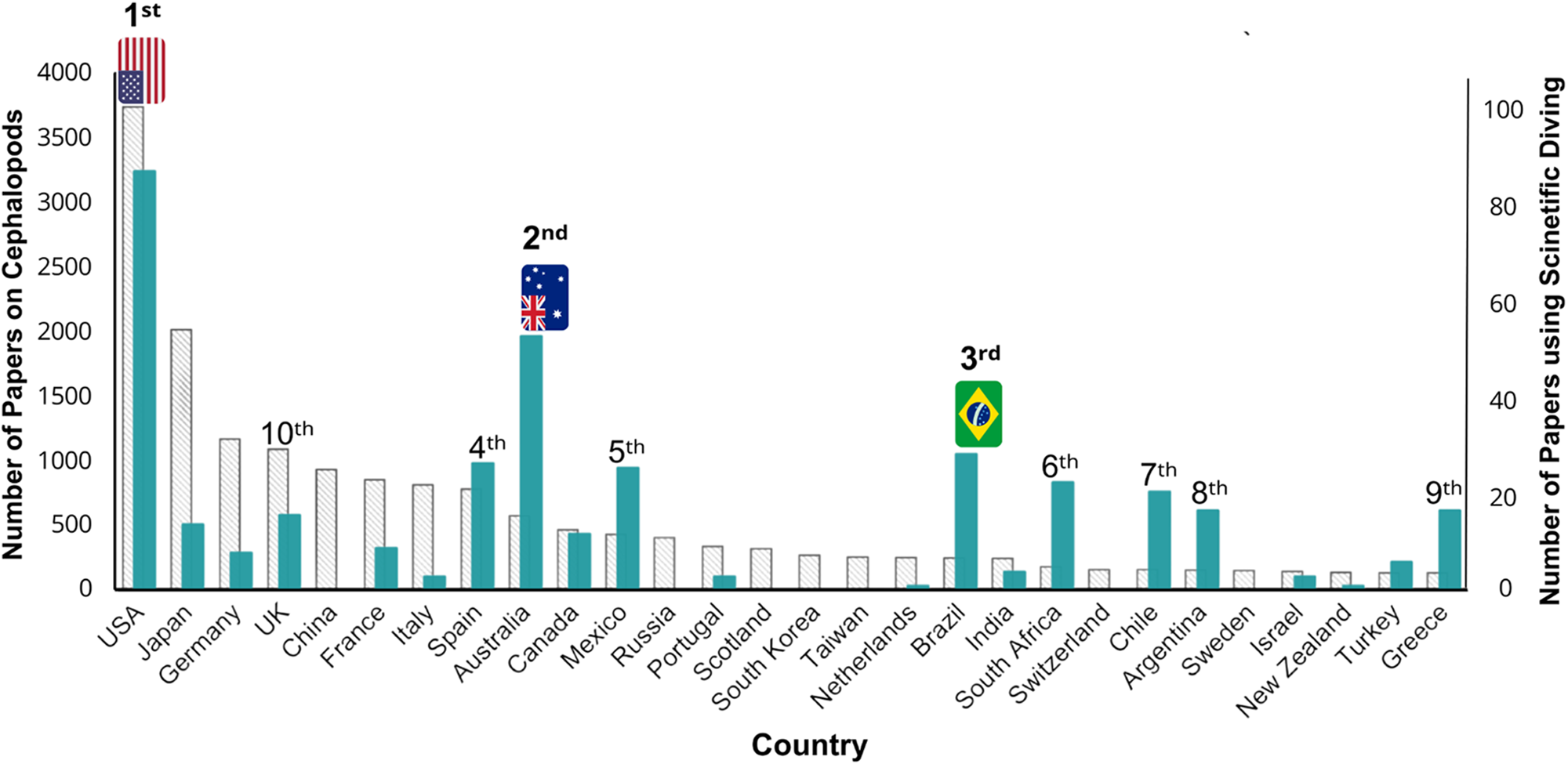
Figure 2. Total publications per country based on the institutional affiliation of authors using scientific diving in cephalopod studies from 1973 to 2022. Blue bars represent the number of cephalopod studies that utilized scientific diving, while grey bars indicate the total number of cephalopod-related publications per country. Countries are ranked according to the number of publications that specifically used scientific diving in cephalopod studies.
Of the 225 articles analysed, a total of 238 geographic coordinates of dive locations were obtained, as some studies were conducted in multiple locations (Figure 3). The distribution of these sites shows a strong clustering along the West Coast of North and South America and the Caribbean (47.5%), particularly in Mexico, Chile, the United States, Brazil, and Peru. The East Coast of Australia and the broader Oceania region (17.7%) also had a substantial number of dive sites, with Australia, Papua New Guinea, and Palau being the most frequently reported.
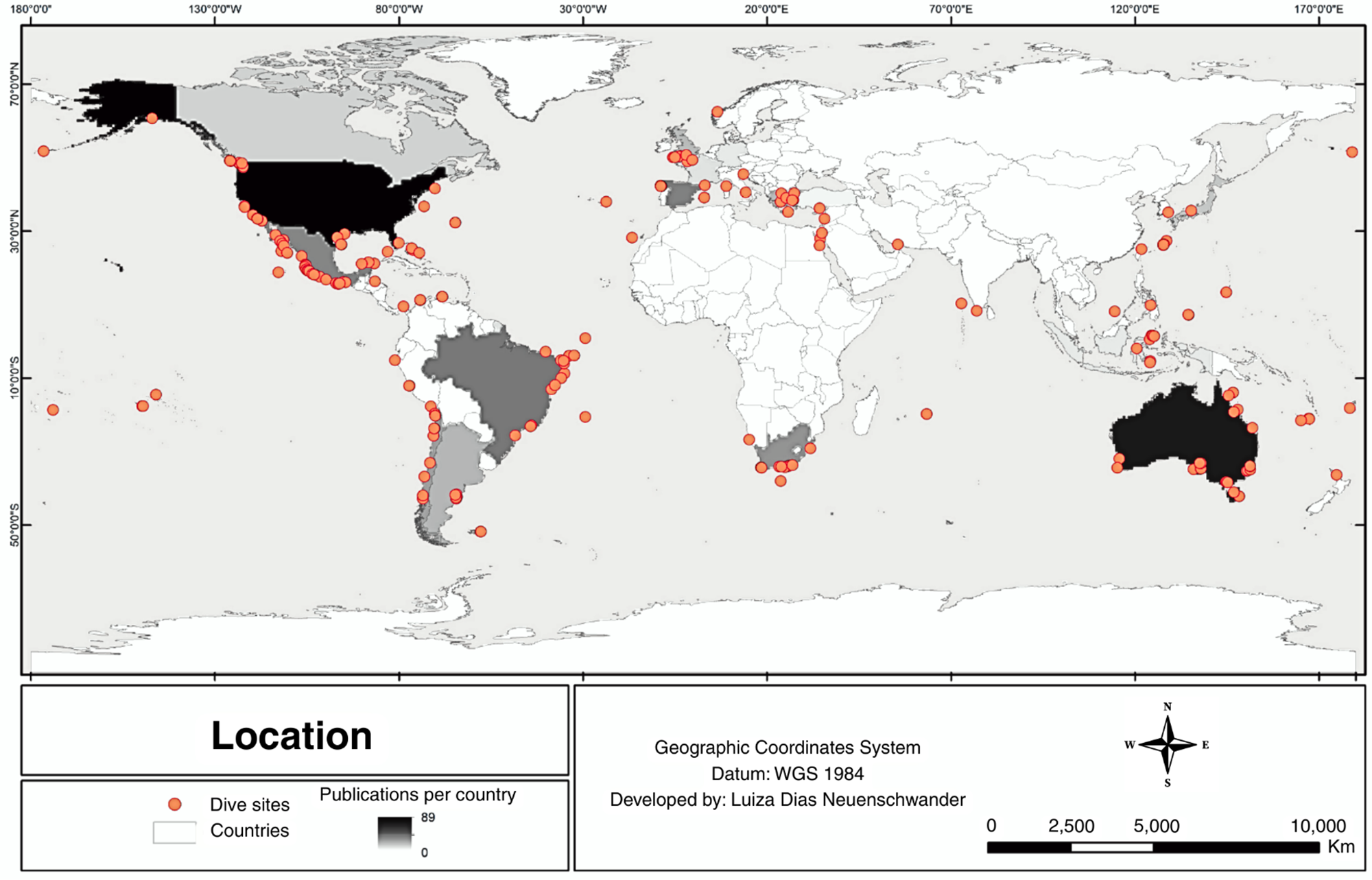
Figure 3. Map showing locations where scientific diving was conducted in cephalopod studies, derived from geographic coordinates listed in publications. The colour gradient indicates publication density per country. Elaborated by Luiza D. Neuenschwander.
The Mediterranean and European waters (16.3%) accounted for a significant portion of dive locations, with Greece, Spain, France, and Italy leading in reported sites. Meanwhile, South Africa (6.4%) stood out as the primary representative for the African continent. In contrast, Asia (11.7%), including Japan, Indonesia, and Israel, showed a moderate number of reported locations, while the Eastern Atlantic and Indian Ocean (4.3%) had relatively few records, with only Mauritius, Namibia, the Falkland Islands, and French Polynesia contributing (Figure 3).
Concerning the taxa studied in the articles from the first search (N = 96), the order Octopoda was the most studied, accounting for more than half (52.7%) of the publications obtained, followed by the orders Sepiida (19.8%), Myopsida (18.7%), Nautilida (4.9%), and Oegopsida (3.8%).
As for the species studied, the articles covered a total of 83 different species and 5 identified at the genus level (Supplementary Table S1). The Octopus vulgaris complex Cuvier, 1797 was the most recurrent species in the systematic review with a total of 25 publications, followed by S. apama Gray, 1849 (n = 18) and O. insularis Leite & Haimovici, 2008 (n = 15).
Ecology was the field of knowledge with the highest percentage of publications, totalling 50.9%, followed by behaviour (19.5%) and embryology (10.9%) (Figure 4).
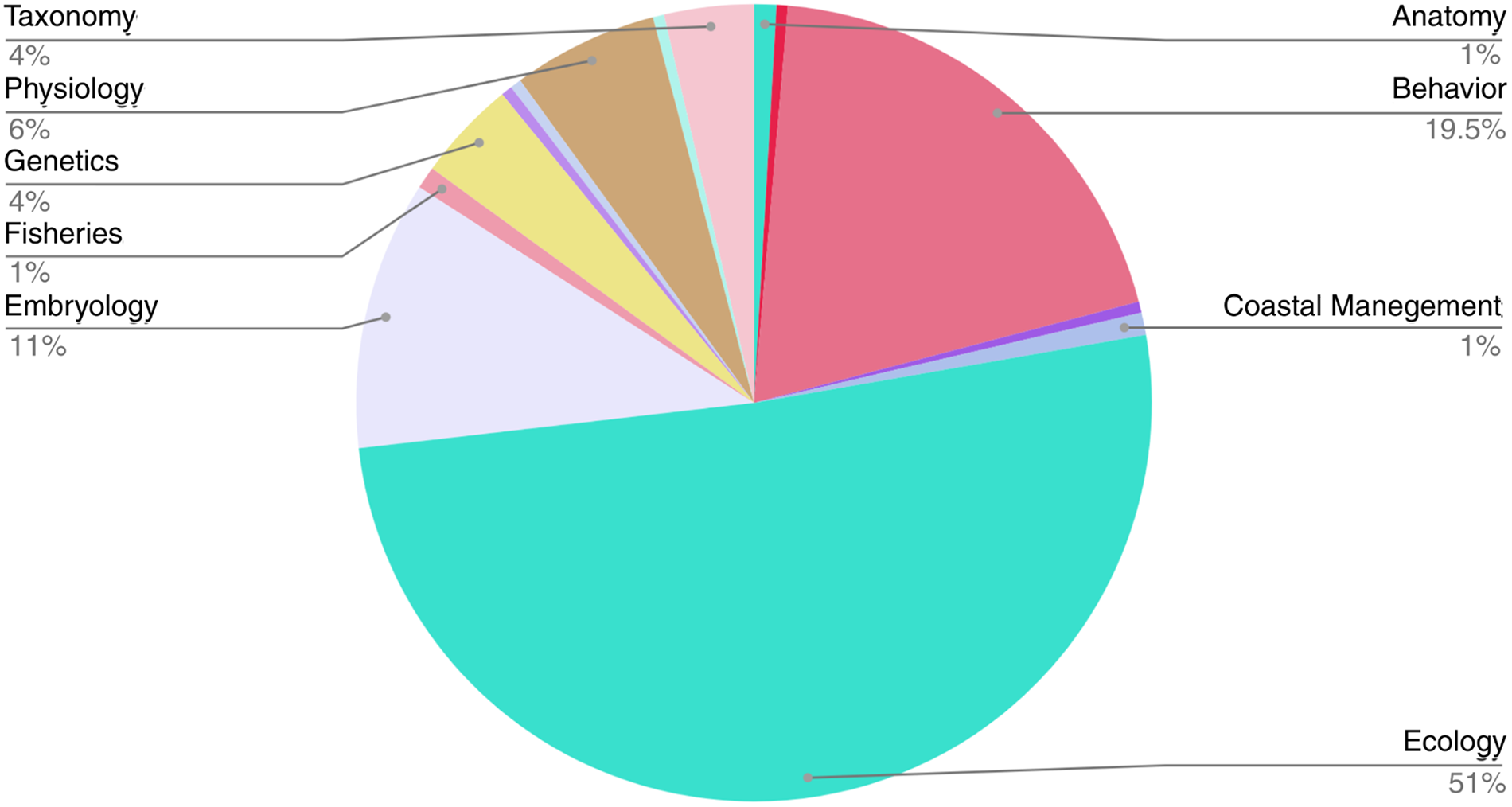
Figure 4. Percentage of publications that used scientific diving in the study of cephalopods in the period 1973–2022 by the area of knowledge studied.
The primary use of scientific diving was for manual collection purposes (44%), with the most common technique being the use of a hook in this context. Subsequently, diving was applied for direct observations (35%) and for recording videos and photographs (15%). It is important to note that the total data regarding the goal of use sum up to 247, as the same study may have used more than one methodology. Among the 108 studies that used scientific diving for collection purposes, 78 (72.2%) focused on specimen collection, followed by 19 (17.6%) on egg collection, 7 (6.5%) on diet analysis, and 2 (1.9%) each for sediment sampling and tissue sampling.
Each studied order was analysed separately with data related to depths, where Octopoda and Sepiida had higher frequency in the intervals of 0–9 and 10–19 m, while Myopsida and Nautilida in the group of >30 m. The Oegopsida order had a higher frequency in the 20–29 m group with no record of 0–9 m (Figure 5).

Figure 5. Distribution of Cephalopoda orders in evaluated articles and their relationship with depth intervals. Elaborated by Athila Bertoncini.
In relation to the oceanographic conditions where the dives were conducted, 45 articles (20%) provided information on water temperature, with the lowest recorded at 2°C in the United States and the highest at 38°C in India. A total of 159 articles (70.2%) reported the depth of the dives, ranging from 0 to 54 m. The SCUBA category had the majority of its dives performed in the depth group of 10–19 m (34%), while the Freediving category was in the group of 0–9 m (81%). There were 31 dives recorded in the depth group of >30, where all the dives were performed through SCUBA diving, and 8 of them were at depths greater than 30 m, with the deepest being 54 m.
The review included 57 (25%) publications that informed the experience/category of the divers participating in the studies, with the majority (64.9%) being the authors themselves. Depth intervals were used to determine the diving profile according to experience, with the ‘Author’ group being the only one present in all depth intervals with a predominance in the 0–9 m interval and a maximum depth reached of 40 m. The ‘Citizen Science’ and ‘Volunteer’ groups have a similar profile with dives performed in the intervals of 0–9 m and 10–19 m. The ‘Commercial Diver’ group did not perform dives in depth intervals less than 20 m, while the ‘Technical Diver’ group performed only dives in >30 m. Professional diver groups such as ‘Technical Diver’ and ‘Commercial Diver’ had the highest average maximum depths (44 m and 20 m, respectively), followed by the ‘Fisherman’ and ‘Researcher’ groups. The ‘Citizen Science’ and ‘Volunteer’ groups had average maximum depths of 8.3 and 8 m, respectively (Figure 6).
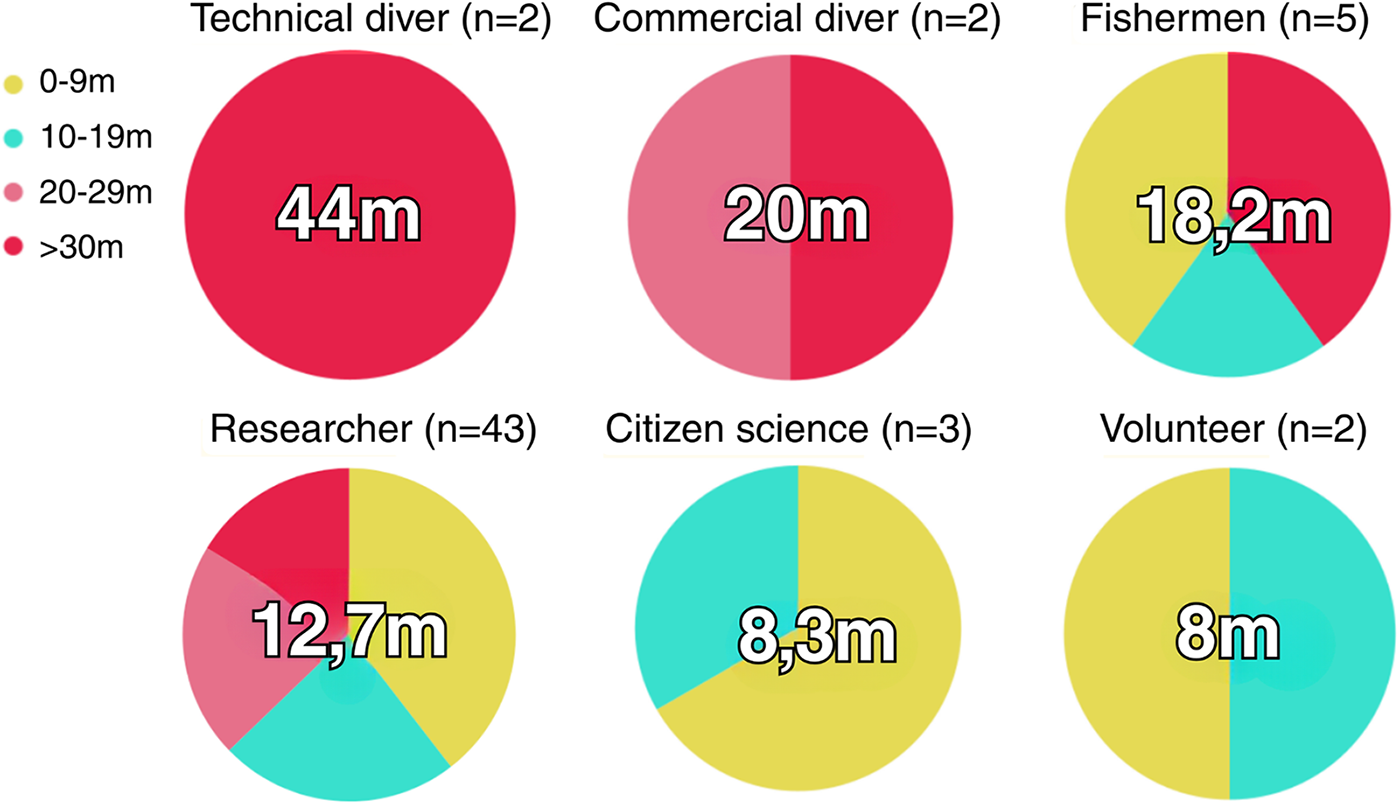
Figure 6. Relationship between diver categories/experience (SCUBA and freediving) and depth profile in cephalopod studies.
Discussion
This work is the first review of the use of scientific diving in cephalopod research. It provides insight into the trends, effectiveness, and versatility of this tool across different taxa of the class Cephalopoda and in various areas of knowledge. The results highlight the important role that diving-based approaches play, particularly in studies focused on the ecology and behaviour of octopuses, squids, and cuttlefish.
Over the past five decades, the use of SCUBA diving in scientific research – especially within biology and oceanography – has expanded significantly, supporting major advances in marine biodiversity (Leite et al., Reference Leite, Pinheiro, Berchez, Bertoncini, Cima, Demetrescu, Francini-Filho, Kikuchi, Machado, Maia-Nogueira and Martins2023; Witman et al., Reference Witman, Dayton, Arnold, Steneck, Birkeland, Lang, Marinelli, Roberts and Taylor2013). Our findings show that this growth is also reflected in cephalopod research, where scientific diving has become increasingly valuable, particularly over the last two decades. Although diving-based studies still represent a small fraction of the total cephalopod literature, their growth rate has outpaced that of overall cephalopod research. This trend suggests a growing recognition of the advantages that in situ methods offer, especially for observing cephalopods in their natural environments.
In parallel, freediving (snorkelling) in cephalopod research has shown slower but steady growth over time. Despite its limitations in depth and bottom time, freediving remains an essential method for accessing shallow environments that are inaccessible to SCUBA, such as coral reefs and tide pools. Additionally, it provides an alternative for researchers with physiological or anatomical limitations that prevent them from using SCUBA, ensuring that they can continue their scientific work.
The use of scientific diving was not consistent when studying the various taxa of the class Cephalopoda. Species from the orders Octopoda, Sepiida, and Myopsida collectively made up 91% of the total publications found, most of which live in shallow waters in benthic/demersal environments (Norman and Hochberg, Reference Norman and Hochberg2005; O’Dor et al., Reference O’Dor, Adamo, Aitken, Andrade, Finn, Hanlon and Jackson2002). Because these species are found in coastal and shallow areas, they are more likely to be within the reach of SCUBA divers and sometimes even freedivers, with approximately 63% of dives conducted in the depth range of 0–19 m. On the other hand, species with pelagic/oceanic lifestyles are harder to access through autonomous diving, like species from the orders Nautilida and Oegopsida (Finn and Norman, Reference Finn and Norman2010; Lindgren, Reference Lindgren2010).
Nautiluses live beyond the limits of non-decompression diving, but articles show that this method is used to release individuals after laboratory research. Divers take the animals to depths of up to 30 m to reduce the risks of predation and physiological complications post-release (Dunstan et al., Reference Dunstan, Bradshaw and Marshall2011; Saunders and Spinosa, Reference Saunders and Spinosa1978; Woodruff et al., Reference Woodruff, Mulvey, Saunders and Carpenter1983). On the other hand, Oegopsida have fully adapted to the pelagic environment, and unlike other cephalopods that attach their eggs to substrates, Oegopsida egg clusters have neutral buoyancy and float freely in the ocean (Vijai, Reference Vijai2016), which are collected and documented through videos and photos (Staaf et al., Reference Staaf, Camarillo-Coop, Haddock, Nyack, Payne, Salinas-Zavala, Seibel, Trueblood, Widmer and Gilly2008).
Scientific diving activities involve inherent risks associated with autonomous diving, combined with the ability to perform underwater tasks (Leite et al., Reference Leite, Pinheiro, Berchez, Bertoncini, Cima, Demetrescu, Francini-Filho, Kikuchi, Machado, Maia-Nogueira and Martins2023). Upon evaluating the skills of the divers in the published studies, it was observed that professional divers (technical and commercial) were mainly responsible for deep dives (>30 m), while volunteers and citizen science divers tended to stick to shallower depths of up to 8 m likely due to insufficient training. Despite the controversy surrounding the role of citizen science, it has proven to be a viable alternative for marine science and cephalopods (Hughes et al., Reference Hughes, Hughes and Smith2014; Jesus et al., Reference Jesus, Sales, Martins, Ready, Costa, Ablett and Schiavetti2021; Thiel et al., Reference Thiel, Hong, Jambeck, Gatta-Rosemary, Honorato-Zimmer, Kiessling, Knickmeier, Kruse, Cigliano and Ballard2017).
According to Gleadall et al. (Reference Gleadall, Moustahfid, Sauer, Ababouch, Arkhipkin, Bensbai, Elegbede, Faraj, Ferreiro-Velasco, González-Gómez and González-Vallés2024), SCUBA divers, with proper training and experience, can assist in conducting surveys and improving data collection efforts. When trying to evaluate cephalopod stocks, traditional fisheries sampling methods may not be the most effective approach. For instance, octopuses are typically den-occupying ambush predators, often hiding in rock clefts, crevices, and small caves that are difficult to sample. With practice, SCUBA divers can learn how to locate octopus’ dens, but conducting scientific surveys using this method is not practical for most researchers. Volunteer-based citizen science, on the other hand, is an ideal approach for these types of surveys.
The distribution of cephalopod research utilizing scientific diving varies across countries and regions. While nations with high overall cephalopod research output are often among those employing scientific diving, their representation in diving-based studies is not necessarily proportional to their total research productivity. The United States, for instance, emerges as a dominant contributor in both cephalopod research and scientific diving studies, a trend also observed by DI Cosmo et al. (Reference DI Cosmo, Pinelli, Scandurra, Aria and D’Aniello2021) in their assessment of octopus research productivity. However, other highly research-active countries, such as Japan and Germany, show limited use of scientific diving in comparison to their overall research output. This suggests that factors beyond publication volume influence the adoption of diving-based methodologies.
Conversely, Australia, Brazil, Spain, Mexico, and South Africa demonstrate a higher reliance on scientific diving relative to their overall cephalopod research output, further supporting the idea that scientific diving use is shaped by factors beyond research activity alone. Geographic accessibility may also play a role, as dive sites are clustered along the coastlines of countries with higher scientific diving output, including the United States, which ranks first in both cephalopod research and scientific diving use. Additionally, coastal environments more suitable for SCUBA diving may naturally facilitate the integration of diving-based methods.
At a broader scale, disparities in the use of scientific diving may also stem from factors beyond geographic suitability for diving. Africa and much of Asia remain under-represented in studies using scientific diving despite their extensive coastlines. In Africa, South Africa and Egypt account for a disproportionate share of the continent’s scientific output (Sooryamoorthy, Reference Sooryamoorthy2018), aligning with our findings where South Africa stood out as the primary representative in scientific diving use. This could be attributed to the lack of resources for this type of research (Gaillard, Reference Gaillard1992), which requires technical training, specialized equipment, training routines, and appropriate regulations (Leite et al., Reference Leite, Pinheiro, Berchez, Bertoncini, Cima, Demetrescu, Francini-Filho, Kikuchi, Machado, Maia-Nogueira and Martins2023). While Africa presents these limitations that may contribute to this gap in Africa, other factors may play a role in Asia.
China and South Korea are major players in the global cephalopod trade, particularly in fresh and processed seafood markets, while Japan has historically been an important trader (Ospina-Alvarez et al., Reference Ospina-Alvarez, de Juan, Pita, Ainsworth, Matos, Pita and Villasante2022). The lower use of scientific diving in cephalopod research across the region may be shaped by national research priorities, particularly in countries where cephalopods hold high economic value. The absence of diving-based studies from China, despite its significant contribution to cephalopod research, highlights that scientific diving is not uniformly adopted, even in highly productive research nations. While these nations significantly contribute to cephalopod science, their well-established fisheries industries may influence a stronger reliance on fisheries-dependent data over direct in situ approaches. This could, in turn, contribute to the lower use of scientific diving, reinforcing disparities in its application.
Regarding the limitations of this study, it was confirmed that conducting an accuracy test when performing a systematic review is important. The first search yielded 28% accuracy, indicating the need for a new search with different criteria. The second search, with supplementation, achieved 57% accuracy, showing an improvement of more than half compared to the first search. However, there is still a need for improvement in the search criteria. An explanation could be that articles using scientific diving as a research tool may not clearly specify the terms related to the use of scientific diving in their scope. Sayer (Reference Sayer2007) suggests using the terms ‘SCUBA’ and ‘scientific diving’ to create consistency in SCUBA and/or freediving activities for scientific purposes across various areas. However, many references in publications simply use the term ‘diver’ without providing further explanation, making it difficult to identify the specific type of diving, the methods used, the equipment utilized, and the characteristics of the divers involved.
The set of keywords selected in this study intended to encompass various descriptions of scientific diving (SCUBA and freediving); however, terms such as ‘scientific diving’, ‘scientific dive’, and ‘underwater method*’ did not yield relevant results when searched with the set of terms, indicating a lack of direct reference to this activity in publications. In contrast, the words ‘diver’ and ‘divers’ yielded more results in the initial search, highlighting the absence of uniformity in mentioning scientific diving in academic works, contributing to its under-representation. In addition to what has been mentioned, scientific search platforms usually conduct searches based on titles, keywords, and abstracts (Sayer, Reference Sayer2007), which corresponds to the results of the first search. Therefore, any mention of diving that is not present in these elements would not be found. Most articles using autonomous diving included the word ‘SCUBA’, but less than half of them were identified in the first search, since this mention was not in the titles, abstracts, or keywords. The decision not to perform a similar search with the term ‘Freediving’, as it was made with the term ‘SCUBA’, was deliberate. The lack of standardized terminology describing the activity makes it challenging to conduct a comprehensive search using the term ‘Freediving’ alone. As a result, the decision was made to focus solely on articles that explicitly mentioned SCUBA diving. The second, more comprehensive search revealed that scientific diving, when mentioned, is typically found in the methods, introduction, or even acknowledgments sections of studies. It is essential to establish standards and regulations to ensure the replicability and reliability of these activities, making the information more accessible to the scientific community.
Despite the importance of scientific diving in underwater research, its detailed inclusion in the methods and results of scientific publications is still incipient, compromising the standardization of research, as observed in this review focusing on the class Cephalopoda. The technological evolution and advancements in diving equipment necessitate continual evaluation and comparison of methods, offering a broader perspective on the results in each field of study. Moving forward, it is crucial to integrate the details of scientific diving techniques into the methodologies of research, thus emphasizing the significance of this tool for data collection within this group. These changes will strengthen the role of scientific diving in significantly contributing to the advancement of knowledge concerning cephalopods and their natural environment.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0025315425100180.
Acknowledgements
We would like to thank Dra. Barbara Segal and Dr Athila Bertoncini for their valuable insights into this study. A.K.P. is especially grateful to Mariana O. Côrtes for her unwavering support and to Luiza D. Neuenschwander for her contribution to the elaboration of the map depicting the geographic locations of dive sites used in this study. We also extend our gratitude to LAMECE and the Universidade Federal de Santa Catarina for the academic foundation and supportive environment that greatly contributed to the success of this work. Finally, we thank the anonymous reviewers for their constructive comments and suggestions, which greatly improved the quality of this manuscript.
Authors contributions
T.S.L. conceptualized the study and critically reviewed the manuscript. A.K.P. developed the study, performed the analysis, and drafted the manuscript. Both authors contributed significantly to the development of the review, including revising it for intellectual content and ensuring its relevance.
Funding
This work was not supported by any external funding.
Competing interests
The authors declare no conflicts of interest.
Data availability
All data used in this study were obtained from previously published sources. The compiled dataset generated during the systematic review is available from the corresponding author upon request.









