No CrossRef data available.
Article contents
Contactless precision steering of particles in a fluid inside a cube with rotating walls
Published online by Cambridge University Press: 30 June 2025
Abstract
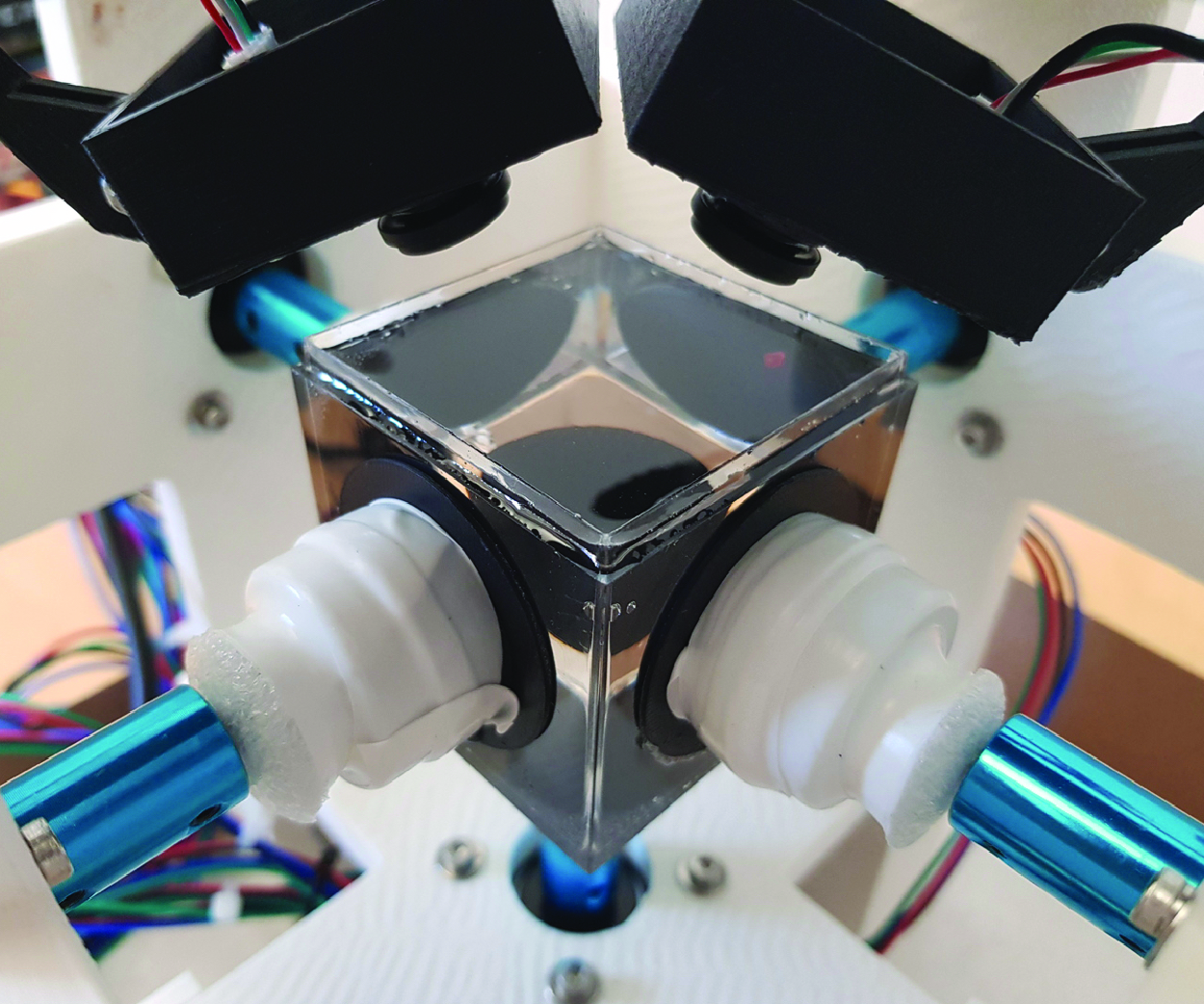
Contactless manipulation of small objects is essential for biomedical and chemical applications, such as cell analysis, assisted fertilisation and precision chemistry. Established methods, including optical, acoustic and magnetic tweezers, are now complemented by flow control techniques that use flow-induced motion to enable precise and versatile manipulation. However, trapping multiple particles in fluid remains a challenge. This study introduces a novel control algorithm capable of steering multiple particles in flow. The system uses rotating disks to generate flow fields that transport particles to precise locations. Disk rotations are governed by a feedback control policy based on the optimising a discrete loss framework, which combines fluid dynamics equations with path objectives into a single loss function. Our experiments, conducted in both simulations and with the physical device, demonstrate the capability of the approach to transport two beads simultaneously to predefined locations, advancing robust contactless particle manipulation for biomedical applications.
Information
- Type
- JFM Papers
- Information
- Copyright
- © Harvard University, 2025. Published by Cambridge University Press
Footnotes
Petr Karnakov and Lucas Amoudruz contributed equally.


