Introduction
“Patient centricity” is an increasingly important concept for healthcare decisions, although what it means and how it is realized vary considerably. This concept is founded on the tenet that to ensure healthcare decisions meet patients’ needs and expectations, patients must be involved in the process (Reference Weeks, Polisena and Scott1-Reference Wong5). Healthcare systems in the Asia Pacific (APAC) region are diverse. From a financing perspective, some use universal healthcare, some are self-pay, and most combine both. Health Technology Assessment (HTA) principles and methodologies were introduced in many APAC countries to guide funding decisions, especially for new health technologies (Reference Liu, Wu and Ahn6). These processes are largely based on systems from the United Kingdom or Australia with a primary focus from payers’ perspective. Local adjustments have been made to accommodate each country’s individual economic, social, geographic, and cultural environments, along with their healthcare system and technological priorities and capacities. Notably, the application of HTA in the APAC region has predominantly taken the form of budget impact analysis or cost-effectiveness analysis.
According to the internationally accepted definition of HTA by the International Network of Agencies for Health Technology Assessment (INAHTA) and Health Technology Assessment International (HTAi), HTA is a multidisciplinary process that uses explicit methods to determine the value of a health technology at different points in its lifecycle. The purpose is to inform decision-making in order to promote an equitable, efficient, and high-quality health system (Reference O’Rourke, Oortwijn, Schuller and Group4). Patient involvement was not originally a core HTA element, but it is now standard, with expectations for HTA bodies to include patient input. Throughout this paper, HTA is used according to the definition provided above, rather than being narrowly defined as solely cost-effectiveness or budget impact.
With the advancement of medicine, direct patient input is increasingly recognized as relevant and even critical to the HTA process, enhancing the understanding of what is significant and most important to patients, especially for diseases that are less understood. The traditional HTA primarily relies on data from randomized controlled trials, as these are considered the gold standard of evidence (Reference Makady, Ham and de Boer7). However, more targeted therapies, such as cell and gene therapies and histology-dependent therapies have been developed as well as therapies for rare diseases or diseases with small patient populations. These new technologies and many “first-in-disease” therapies are meaningful for individuals living with the conditions. However, research and regulatory review face unique challenges, including pragmatic study designs and a lack of validated endpoints to measure clinical outcomes and quality of life. It also created challenges for the subsequent HTA process, requiring additional trials, extended real-world monitoring, and substantial pricing discounts to deal with the inherent uncertainty.
This paper attempts to identify the evolution of patient involvement in HTA processes in various healthcare systems across APAC.
Methods
Search strategy and data sources
A systematic literature review (SLR) was conducted using the meta-ethnographic approach, following the Cochrane Collaboration and Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines (Reference Sattar, Lawton, Panagioti and Johnson8;Reference Higgins, Thomas and Chandler9). A comprehensive literature search was conducted on the OVID platform, including the EMBASE and MEDLINE databases, covering the period up to December 2022. Conference abstracts from the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) and HTAi published between 2018 and 2022 were also included. Additional relevant reference documents such as guides, newsletters, and reports were extracted as part of a grey literature search, which included government websites for HTA and related healthcare websites. This review employed a comprehensive search strategy using key terms such as “patient perspective,” “patient involvement,” “patient-reported outcomes,” “PRO,” “PROM,” “quality of life,” “QoL,” “HRQoL,” and “health technology assessment,” “HTA,” as well as related terms like “value assessment” and “pricing and reimbursement” (Supplementary Tables S1 and S2).
Study selection criteria
Studies were included if they covered patient involvement in HTA, patient engagement in the value assessment framework, and/or the use of patient-reported outcome measures (PROMs) in the APAC healthcare systems. This search specifically focused on Australia, China, Japan, Malaysia, New Zealand, the Philippines, South Korea, Singapore, Taiwan, and Thailand. Studies that were not related to HTA or focused on health promotion purposes and published in languages other than English were excluded. However, government HTA websites and related information in non-English languages were accessed for relevant information.
Two reviewers independently screened all retrieved citations based on pre-defined criteria, with discrepancies resolved by a third reviewer through consensus. Multiple publications from the same study were linked and extracted as a single study.
Thematic coding process and mapping parameters
The thematic coding process was designed to ensure rigorous and transparent categorization of qualitative data, with specific criteria for theme development, coding structure, and validation procedures. The process involved adapting eleven mapping parameters from the National Health Council (NHC) rubric, which were grouped into two thematic categories: “How” and “What.” Full-text publications of all included studies were categorized by their country of origin, and the presented data were systematically analyzed using the adapted framework (Figure 1, Supplementary Table S3) (10;Reference Costantino, Gressler and Highland11). Each study was assessed against the modified mapping parameters, ensuring consistent evaluation across diverse methodologies. To ensure reliability, the coding process was independently reviewed by two reviewers. In case of disagreement, a third reviewer was brought in, enhancing the robustness and transparency of the thematic analysis. The subsequent analysis of these texts included a more in-depth categorization of the presented data using NHC adapted mapping parameters to Capture the Patient Voice” (10). Founded in 1920, the NHC drives patient-centered health policy through its core membership of leading advocacy organizations. Its rubric framework helps patients and healthcare stakeholders evaluate patient-centered attributes and guide meaningful engagement in their activities (10;Reference Costantino, Gressler and Highland11).
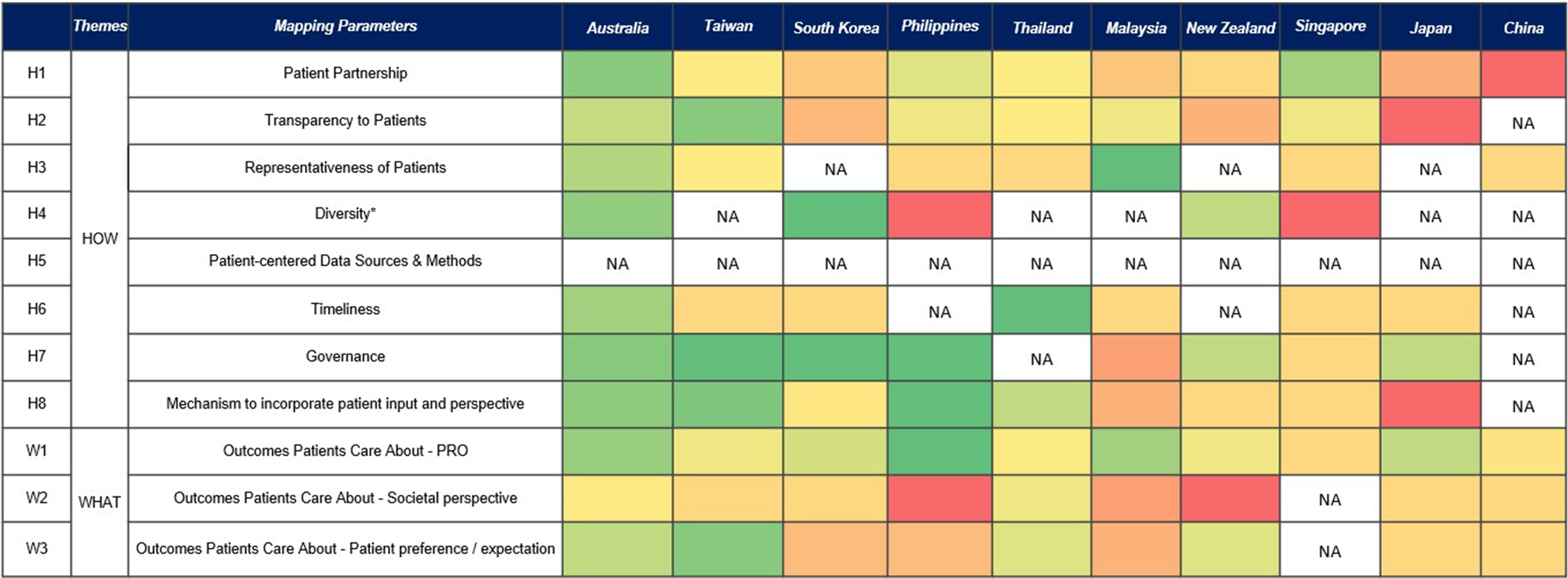
Figure 1. Healthcare system scoring of APAC countries.
NA: Not available, because the parameter is identified as a gap. * Limited information is published around diversity with acknowledgment that diversity consideration factors vary across APAC, making standardization, scoring, and comparison across systems challenging.
Patient engagement is defined as the process of engaging the patient or patient community, relying on patient expertise, and evaluating patient engagement throughout the process (10;Reference Costantino, Gressler and Highland11). The “How” category included eight parameters: patient partnership, transparency to patients, representativeness of patients, diversity, patient-centered data sources and methods (variety of sources of patient-centered data which were collected using both qualitative and quantitative methods), timelines (of soliciting input, such as in case of patient input for a trial, the input should be sought prior to finalizing the protocol), governance, and mechanism to incorporate patient input. Governance and mechanism to incorporate patient input were added to the existing NHC rubric parameters based on the authors’ discussion and alignment, considering the comprehensiveness and relevance to the APAC region. The “What” category includes “outcomes patients care about” from the NHC rubric and further breaks down into three distinct parameters comprising patient-reported outcomes (PROs), societal perspective, and patient preference/expectation to provide clarity on the outcomes that patients care about and types of input from patients (Supplementary Table S3).
PRO is any report of the status of a patient’s health condition that comes directly from the patients. PRO tools are developed to capture important health aspects from patients. Societal perspectives encompass broader public interests, including impact on the welfare of the whole society, for example impact on productivity (10;Reference Costantino, Gressler and Highland11). Patient preference/expectation focus on the lived experiences, specific healthcare needs, and priorities of individuals, highlighting the personal impact of medical decisions and interventions.
Data extraction and scoring of parameters
Each citation was tagged for the relevant parameters for the respective healthcare system. The parameters were further rated on a five-point Likert scale as high (Reference Wong5), medium (Reference Scott and Wale3), or low (Reference Weeks, Polisena and Scott1), based on the rationale as well as the definition of “meaningful and insufficient activities” for each of the parameters from the guide “The NHC Rubric to Capture Patient Voice” (10). Mean value of each parameter’s score was calculated for every healthcare system. Each healthcare system was evaluated based on eleven parameters. For each parameter, the number of times it was tagged was multiplied by its assigned score (5, 3, or 1). Then, the total score for each parameter was calculated by adding up these values. This total score was divided by the total number of studies. Finally, a percentage score was calculated by dividing the total score by 5 (the highest possible score) (Supplementary Table S4). During the data extraction process, parameters were labeled as “not available/NA” for cases where no articles were retrieved. A heat map (Figure 1) was generated using the average values of the parameters from the rubric to illustrate the assessment of the patient centricity of each reimbursement system.
Results
Study characteristics
A total of 159 records were included in the SLR, consisting of fifty-eight full-text articles acquired from OVID databases, twenty-eight abstracts sourced from conference proceedings, and seventy-three records from grey literature search (Figure 2). Among the identified studies, Australia contributed the highest number with a total of 60 studies, followed by Taiwan (n = 25), Thailand (n = 25), Japan (n = 18), South Korea (n = 16), China (n = 12), Malaysia (n = 12), New Zealand (n = 12), the Philippines (n = 12), and Singapore (n = 7). Where studies reported data from multiple healthcare systems, they were counted separately in each relevant healthcare system.
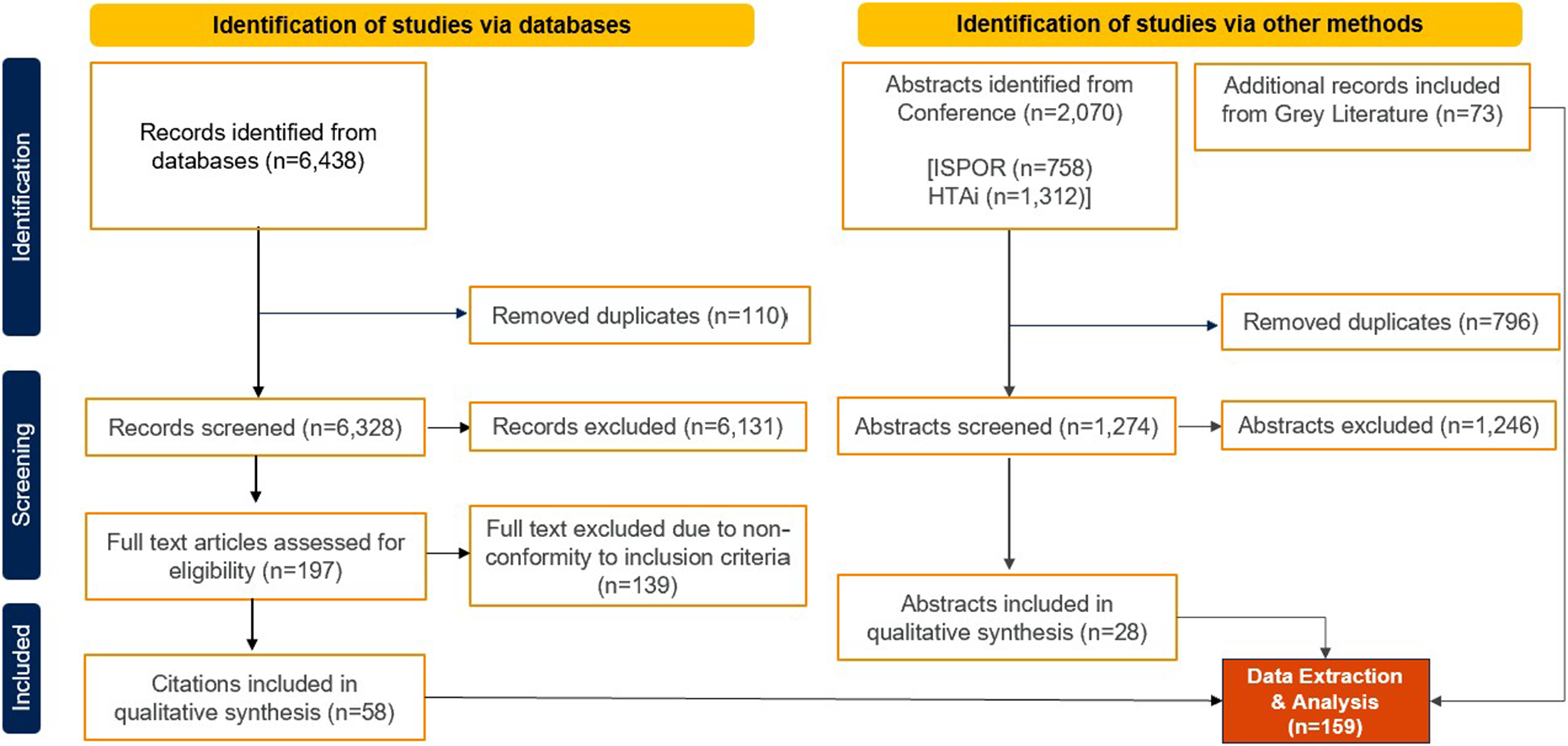
Figure 2. PRISMA flowchart.
Health care system funding decision and patient’s role
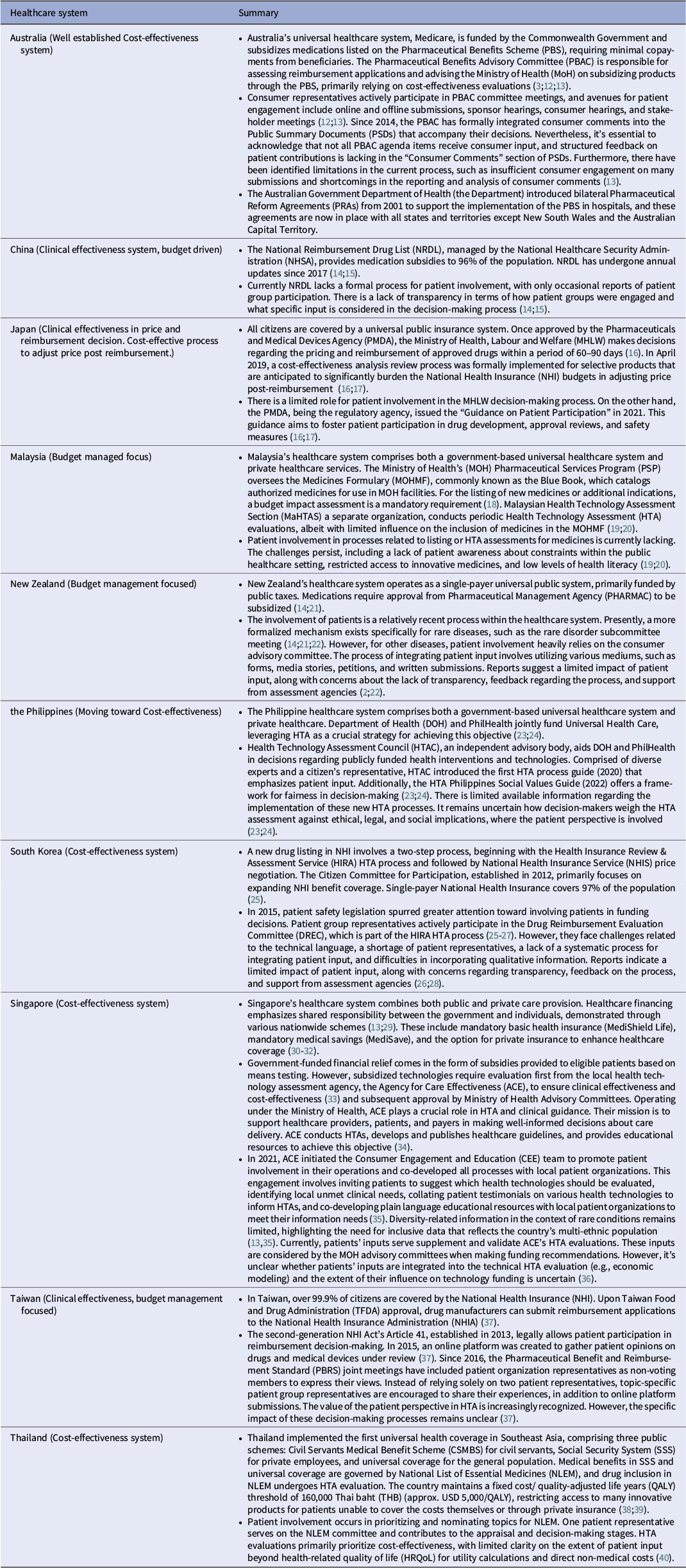
Table 1 summarizes funding decisions and patients’ roles across APAC countries. Beyond patient submissions to the assessment process, the involvement of patients in the appraisal (decision-making) phase has also evolved within HTA bodies over time, albeit with considerable variation. Overall, the tools for collecting patient experience are varied, including “QoL” questionnaires, focus groups, individual interviews, portals for testimonials, and comprehensive surveys. The options for feedback include structured templates, testimonial videos, closed sessions, and public sessions. Only a few systems, such as Australia, South Korea, and Taiwan, have patients participating in the appraisal process as members of recommendation or decision-making committees. For Australia we focused on Pharmaceutical Benefits Advisory Committee (PBAC) process. Thailand, Singapore, and the Philippines have some processes in place for including patient input; however, patients are mostly involved passively as subjects of inquiry, with limited roles in decision-making. In contrast, the formal process involvement of patients in decision-making is still not reported in China and Japan as of the data collection cut-off date.
Table 1. Summary of healthcare system funding decision and patients’ role
Parameter mapping
Through this comprehensive mapping of patient-centric parameters across APAC healthcare systems, key parameters were identified as most frequently mentioned in the literature. These were patient partnership (n = 107), followed by patient-reported outcome (n = 70), mechanism to incorporate patient input (n = 65), transparency (n = 58), patient preference/expectation (n = 41), governance (n = 29), timeliness (n = 22), societal perspective (n = 21), representativeness of patients (n = 21), and diversity (n = 10). There were no references to patient-centered data sources and methods.
Although the frequency of identified parameters does not fully represent the value of patient involvement or its acceptance by decision-makers, the results indicate that Australia, Taiwan, and Thailand report more comprehensively on patient involvement in reimbursement decisions compared to other jurisdictions. Some countries, such as Thailand, Singapore, and the Philippines, have processes for including patient inputs in HTA decision-making; however, further refinement of the process is required to ensure meaningful inclusion of patient participation by decision-makers.
Scoring and heat map
In the heat map (Figure 1), few parameters fall on the extremes of the color spectrum, and most show color gradients amongst different countries. The majority of the parameters for most countries range from light green to dark yellow (i.e., good to moderate). This can be interpreted as varying degrees of efforts being made to improve patient engagement in the respective healthcare systems. The challenges in scoring patient input parameters are especially evident in diverse contexts within the APAC markets. While some healthcare systems like Japan, South Korea, and Taiwan exhibit a more uniform ethnic composition, other APAC markets show greater variability. Parameters such as “societal perspective” had particularly poor representation, with most countries leaning towards the darker yellow and red zones (moderate to poor). Even the otherwise “green” healthcare system in Australia demonstrated gaps in the parameter of incorporating a societal perspective.
Discussion
This SLR provides an in-depth analysis of patient involvement in value assessment frameworks and examines its implications for HTA decision-making in the APAC region with the caveat that patient involvement is sometimes informal, occurring in verbal or group settings, and with limited public record available in written literature. The study findings indicate inconsistencies in what inputs were collected from patients and how patient input is collected and used (qualitatively and quantitatively) by HTA agencies in APAC. Budget impact and economic analysis are the primary focus in decision-making, with patient input and PROs often considered supplementary; however, there is a growing need to shift toward incorporating patient value more prominently in the evaluation process. Especially in healthcare systems that heavily rely upon ICER thresholds for decision-making, there is a need for greater clarity on how decision-makers weigh the patient input versus the output of an economic model.
Most importantly, the lack of transparency regarding how patient input influences decision-making poses a significant challenge. While conducting the SLR, it was unclear how patient input affected the final HTA recommendation or decision regarding access for individual therapies. Across all the reviewed HTA processes and health systems, patient engagement was recognized as critical, yet the absence of transparency on how these inputs were used in decision-making hampers the ability to assess its actual impact. For instance, established practices like informal dialogues between healthcare stakeholders and patient organizations in Australia, which often occur outside the standard framework for patient input, are not captured (Reference Tjeuw and Wonder13). This omission may overlook important but undocumented aspects of patient involvement in decision-making processes. Certain healthcare systems, such as Taiwan, Australia, and Germany, address this issue by involving patients as a part of decision-making bodies, thereby augmenting the patients’ voice and, more importantly, accountability (Reference Weeks, Polisena and Scott1;Reference Tjeuw and Wonder13;Reference Chen, Huang and Gau37;Reference Van Overbeeke, Forrester, Simoens and Huys41-Reference Lou, Kc and Toh43). Including patients in decision-making bodies in these healthcare systems enhance transparency by integrating patient perspectives into formal processes. This fosters inclusivity, provides a direct channel for patient input, and clarifies decision-making. The approach builds trust in healthcare systems and reinforces accountability and commitment to patient-centered care. A recent progress in Singapore (established after the review period of this study) includes the official invitation for patient representatives to contribute to the HTA process by nominating technology for evaluation and providing testimonials to inform HTA and funding decisions. It is reported that 85–89 percent of HTA reviews in 2022 and 2023 included patient input (Reference Ping-Tee and PEARCE44;Reference Jen Hun, PEARCE, Shawn and Ping-Tee45). However, data is not yet available on how many technologies nominated by patients were selected for evaluation by the Singapore decision-making committee, nor is it clear how these patient insights informed funding decisions and weighed up against the quantitative inputs.
This study also highlighted the gap of policymakers making informed and equitable decisions by adopting a societal perspective and considering the impact of healthcare decisions on the larger community. While many existing HTA guidelines in APAC allow for a societal perspective, focus on assessing the societal impact when making decisions is limited (24;35;36).
Our study found that “representativeness” as outlined in the NHC rubric, has not been well-established in the literature, in addition, no literature on “patient-centered data sources or methods.” In NHC Rubric, this was mentioned as having credible sources for effectively incorporating new information while considering the diversity of patient populations and outcomes. No literature using patient-generated health data, such as registries could be identified, as these data sources are often reactive rather than proactively established in APAC.
The modified NHC Rubric framework provides a versatile tool for evaluating key elements of patient-centeredness with adequate specificity into the domains of patient engagement. This adaptation considers various contextual factors, such as cultural differences and local practices, ensuring that the framework is applicable, actionable and relevant in a wide range of settings (10;Reference Costantino, Gressler and Highland11).
Emerging research is expanding the evidence base for patient input, encompassing qualitative evidence and inputs to complement and enhance the QoL data captured in validated multi-attribute utility instruments such as the EQ-5D. These additional inputs may include mechanisms to incorporate patient input and perspective, including patient surveys, testimonials, vignettes, and clinical expert contributions. They offer insights into various dimensions of the patient experience that are not captured in QoL data, thereby increasing the value and relevance of patient engagement in funding decisions. Decision-makers could consider this qualitative evidence in conjunction with quantitative (cost/quality-adjusted life years [QALY]) evidence if this was the current practice.
The study findings did not observe a direct relationship between the level of patient engagement and the overall access to medicines, as evident from metrics like the Global Access to Medicine Index (e.g., Japan surpassing Australia) (46). It is important to note that our implicit assumption is that increased patient access to medicine is the primary goal of patients involvement. However, this may overlook other significant objectives such as equity or improved patient experience. Furthermore, assessing the impact of patient involvement is inherently challenging due to the limited transparency in how patient input is considered along with other evidence, such as clinical benefits and improved quality of life.
This study focuses on highlighting trends and patterns in reported outcomes of patient involvement rather than establishing causal relationships, providing insights into the diverse practices across HTA systems. Nevertheless, this lack of direct correlation can be attributed to several factors. In some healthcare systems, patient engagement or input may have limited impact, as most provided information is condensed into a QALY score. The QALY framework tends to confine patient input to a single quantitative measurement of utility through health-related QoL (HRQoL), potentially not adequately valuing the significance of the patients’ voice. This method, combined with a willingness-to-pay threshold, outweighs the patient input in the decision-making process (Reference Ping-Tee and PEARCE44;46;Reference Wichmann, Adang and Stalmeier47). If the cost/QALY data is preferred without truly considering the patient’s perspective and values, this may potentially result in neither improved outcomes nor better acceptance of the decision.
On the contrary, other systems outside of APAC, such as that of Germany, a clinical effectiveness HTA system, demonstrate alternative methods for valuing and weighing patient input. Entities like the Institute for Quality and Efficiency in Health Care (Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen, IQWiG) and Federal Joint Committee (G-BA) conduct a clinical benefit assessment focusing on “patient-relevant” endpoints—mortality, morbidity, and HRQoL —mandated by German law (Reference Ruof, Flückiger and Andre48). While these endpoints are narrowly defined, meeting them may result in a higher clinical benefit rating, ensuring more consistent and timely patient access outcomes.
With diverse healthcare systems in APAC, there are ample opportunities for patient involvement in HTA decision-making, especially in systems undergoing rapid evolution. Progress is being made by involving more patient input in the decision-making, but the outcomes of decision-making need to be tracked.
Promising trends include the Scottish Medicines Consortium’s (SMC) ultra-orphan drug framework, which enables broader consideration of aspects important to patients beyond the QALY, including joint statements from patients and clinicians that highlight treatment benefits not captured in the QALY or standard economic model (Reference Rothwell49). Examples include summaries about the impact of a condition and the burden of treatments presented by patients. This has provided valuable context for the SMC, aiding appraisal committee members in assessing the true value of a new treatment. This approach is particularly crucial for patient inputs related to rare diseases, where small patient populations and significant variability in disease presentation limit the utility of generic instruments.
Moreover, there is a need for a deeper understanding and acceptance of PRO instruments that solicit feedback on patient experiences with rare diseases. This can be achieved systematically by addressing challenges like data paucity, patient heterogeneity, limited access to comprehensive patient registries, variability in disease progression, and lack of standardized measures across studies and clinical settings.
This study exhibits several strengths. First, we used robust methodology by adapting the existing NHC Rubric and adapting it for suitability, including perspectives from patient advocates, ensuring methodological rigor, and enhancing the study’s comprehensive approach (Reference Perfetto, Oehrlein, Boutin, Reid and Gascho50). Second, the study systematically assessed researched parameters on patient perspectives in APAC’s value assessment frameworks. Third, the study offers valuable insights for policymakers and stakeholders seeking to improve patient outcomes and make informed healthcare decisions in the APAC region. The study highlights the absence of standardized terminology and the challenges posed by diverse stakeholder perspectives in consistently evaluating patient involvement. Definitions of ‘patient partnership’ vary across jurisdictions and stakeholder groups, influenced by cultural, regulatory, and institutional differences.
Despite the approach employed, this study consists of the following limitations. It is not clear why some health systems rank highly in one parameter and not others. We cannot conclude that few or no publications on one parameter mean that the parameter is of less interest or use to the HTA body. Variability in the available English literature creates information gaps, particularly concerning parameters such as patient-centered databases, methods, and diversity. Challenges also exist in assessing the impact of patient input due to a lack of standardized metrics, terminology and transparent decision-making processes across healthcare systems. This study is constrained by the varying levels of maturity in patient involvement among HTA bodies, with more robust findings derived from well-established systems in Australia, Taiwan, and Thailand. Developments in Singapore and the Philippines occurring after the SLR cutoff may not be reflected. Moreover, the study is limited by an overrepresentation of English-speaking countries, whose practices are more commonly documented in journal publications, reducing its ability to fully capture the perspectives of non-English-speaking regions. Additionally, differences in data sources among APAC systems have influenced the scoring matrix, and thus, caution should be adopted while making any cross-system comparisons. Another limitation of this study is the exclusion of specific statewide formulary committee processes for in-patient hospital settings in Australia to maintaining the focus on the evaluation mechanisms for medicines in the public healthcare system. However, these observations do not diminish the significance of patient input; rather, they highlight the importance of continuous improvement to measurably enhance these frameworks. These include enhancing mechanisms for patient involvement in value assessment processes and ensuring robust integration and utilization of patient perspectives in decision-making and providing transparency over how the patient input was used during the decision-making.
Future directions and recommendations
The findings of this study suggest that strategies for patient involvement need to be more effectively aligned with the local context. Each system must consider its unique societal value and healthcare infrastructure when developing and implementing patient engagement frameworks. Moreover, there is a need for more structured methods to integrate patient input, extending beyond the current scope of value assessment frameworks. Additionally, how improved patient involvement relates to outcomes is a potential area for future research.
Based on the study, we propose recommendations below:
-
1. Adopt a broader perspective, considering the impact of healthcare decisions on family and community. Instead of a “one-size-fits-all” solution, a broader perspective should be considered from the viewpoint of what matters to patients with respect to the specific condition under evaluation.
-
2. Enhance patient involvement documentation through written records, ensuring transparency. Encourage publishing patient contributions, not just verbal or group discussions and articulate the impact of patient input in decision-making:
-
• Outline how patients’ input (both quantitative and qualitative) was incorporated into funding decisions via structured feedback in existing public summary documents or through a dedicated patient feedback statement.
-
-
3. Facilitate sharing among systems and patient organizations to learn about the conditions for impactful patient engagement:
-
• Patient advocates with personal experience and knowledge of desired outcomes and conditions for access can have an effective voice in decision-making processes.
-
• Patient advocates with skills and knowledge to influence both the system and decision-makers should participate in policy-making and funding/reimbursement decisions.
-
• Public and community support of the patient voice and support for patient outcomes and access can be improved with increased awareness.
-
• Policymakers need to be supportive of patient engagement, value patient outcomes and educate patient advocates on HTA processes and available scope for contribution.
-
-
4. Identify best practices to guide the integration of both qualitative patient input and quantitative PRO/QoL instruments in funding decision-making.
-
5. Ensure that policies for patient input are tracked over time and correspond to improvement in overall patient access.
Conclusion
Patient perspectives within value assessment frameworks in APAC reveal a heterogenous landscape and healthcare decision-making varies significantly across APAC health systems. The study findings underscore the necessity for a more comprehensive understanding and research of the factors that contribute to enhancing patient access beyond the current scope of value assessment frameworks and the implementation of changes to measurably improve these frameworks.
Our findings emphasize the pressing need for greater incorporation of patient input in decision-making. Encouragingly, various APAC health systems have initiated steps to enhance patient involvement in HTA processes. However, substantial room for improvement remains. Refining the value assessment framework to prioritize a clear reflection of patient value in healthcare decision-making can bring us closer to the goal of achieving a better patient outcome.
Supplementary material
The supplementary material for this article can be found at http://doi.org/10.1017/S0266462325000224.
Data availability statement
All relevant data for this review are present in the manuscript and Supplementary Material. Further data generated during and/or analyzed during the study are available from the corresponding author on reasonable request.
Acknowledgments
Ashish Verma from EVERSANA provided medical writing and publication support and was funded by Johnson & Johnson International (Singapore) Pte. Ltd.
Author contribution
All authors made substantial contributions to study conception, design writing, reviewing and approving the final draft. I.T., J.Y.T., D.W., D.Y.Y., A.K., and A.B. had full access to datasets and contributed to methodology, data curation, data analysis, validation and visualization, project supervision and resource planning and provision of funding.
Funding statement
The study design, conduct, data analysis, medical writing assistance, and publication were funded by Johnson & Johnson International (Singapore) Pte. Ltd.
Competing interest
I.T., J.Y.T., D.W., D.Y.Y., A.K., and A.B. are employees of Johnson & Johnson International (Singapore) Pte. Ltd. and own stock in Johnson & Johnson. The remaining authors declare no competing interests for this work.





