I Introduction
In 2018, the birth of the world’s first ‘CRISPR Babies’ rendered the global community in disbelief. This ‘reckless ethical disaster’ was the catalyst for an international moratorium on Heritable Human Genome Editing (‘HHGE’).Footnote 1 This disaster was said to be caused, in part, by ‘a failure of self-regulation by the scientific community, [due to] a lack of transparency’.Footnote 2 For the first time, the international community was prompted to consider a pathway forward to regulate HHGE, to avoid another CRISPR Babies event.
In light of the evolving maturity of Clustered Regularly Interspaced Short Palindromic Repeats (‘CRISPR’) as a biotechnology,Footnote 3 it is timely to evaluate current legal and regulatory frameworks governing human genome editing in Australia. The development of a regulatory framework requires the examination of unique bioethical and legal issues through a future-oriented lens, to foster a robust, effective mechanism for the application of CRISPR in humans.
The legal and regulatory response to HHGE must carefully balance the need to prevent unethical applications, against the progress of research to improve and refine the technology. Bioethics, particularly in the context of emerging biotechnologies, is arguably the foundation of an effective regulatory regime. It provides a guide to forecast and navigate some of the challenges a regulatory regime will be required to address, manage and possibly resolve. The application of CRISPR technology in human germline cells is a controversial area, confronting society’s well-established norms and expectations concerning the relationship between law, science and ethics.
This article argues Australia’s legislative regime must be reviewed to ensure it has the necessary capabilities to effectively regulate HHGE. The intent of this article is twofold. First, it explores how history has led to Australia’s regulatory approach, by focussing on one aspect: the relationship between law and science. Second, it identifies the regulatory shortcomings of Australia’s current legal governance framework.
This article is confined to a discussion of HHGE and does not consider somatic genome editing or mitochondrial donation.Footnote 4 While significant developments have been made in somatic genome editing,Footnote 5 this article intends to advance a discussion concerning the regulation of HHGE, which remains in need of greater interrogation and consideration. As noted by Giovanni Rubeis and Florian Steger ‘[a]lthough there are no clinical applications of [germline genome editing] available at the moment, it is important to have an intense ethical debate at this early stage in order to be prepared for coming developments’.Footnote 6 While this article does not offer an ethical evaluation of HHGE, this conclusion remains relevant. This article serves to contribute to the literature concerning the regulatory approach to HHGE.Footnote 7
Part II discusses the connection between history and technology, as a means to illustrate the ongoing fraught relationship between the two disciplines. It is through this historical and theoretical lens that we can gain a better understanding regarding the law’s response to emerging technologies. It is argued Australia’s legal approach to HHGE reinforces various theories concerning regulatory discourse in areas involving law and science. This discussion provides context to the development and introduction of Australia’s response to gene editing technology.
Part III provides a policy and legal overview of the relevant federal statutes governing gene editing in Australia, the Gene Technology Act 2000 (Cth) (‘GT Act’), Research Involving Human Embryos Act 2002 (Cth) (‘RIHE Act’) and Prohibition of Human Cloning for Reproduction Act 2002 (Cth) (‘PHCR Act’). The role of the Therapeutic Goods Act 1989 (Cth) (‘TGA Act’) has also been raised to highlight the legislative interaction for gene editing regulation, particularly in a clinical context. This article will solely focus on federal statutes. However, it is acknowledged that each state jurisdiction has introduced similar legislative instruments, which raises considerations in relation to the interaction between state and federal laws. For the purposes of this article, the role of state law falls outside the scope of this discussion, which aims to identify key gaps in Australia’s governing triad of federal statutes.
Following an analysis of these federal statutes, Part IV reveals three regulatory gaps. First, there is no single legislative instrument which explicitly addresses HHGE and its potential uses in a clinical and/or research context. This article does not argue for a single legislative instrument. Rather, the current governance approach produced by multiple interacting statutes contributes to its regulatory complexity. As a result, this complexity threatens the adequate regulation of HHGE as it matures as a biotechnology. Second, legislative ambiguity in the governing legislation raises concerns regarding its practical application for researchers engaged in research involving CRISPR technology. This refers to the interpretation and subsequent enforcement of relevant laws. Third, the appropriateness of each statute as an instrument of HHGE regulation is questionable. In this context, there are two primary considerations: the intention of legislators in crafting the statutes and the legislative objectives identified in the statutes. Together, these regulatory gaps foster a regime that may no longer be fit for purpose.
Part V concludes Australia’s regulatory regime reinforces the notion that the law adopts a reactionary response to advancing technology, which is exacerbated by the lack of communication and understanding between disciplines. Heritable Human Genome Editing challenges current perceptions of the relationship between the law, science and ethics. The legal and regulatory response requires a re-working of this conceptual intersectionality in order to make provision for a robust pathway forward.
II Theoretical Foundation of a Regulatory Response to Emerging Technologies
The concept of a technology has evolved considerably over time. The relationship between technology and history is now regarded as a distinct, flourishing research discipline,Footnote 8 which serves an important educative role in the context of modern, emerging biotechnologies. It is argued that the regulatory response of a biotechnology is informed by a number of factors, including historical perceptions of the sciences and humanities.
The underlying relationship between history and science offers an instructive lens through which we can understand how the law has responded to advancements in technology. This article firstly applies David Hume’s is/ought theory as evidence of the complex relationship between law and science.Footnote 9 Using Hume’s Law as the foundation for regulatory discourse in the context of emerging technology, two additional schools of thought will be discussed: the ‘two cultures’, as identified by CP Snow and Melvin Kranzberg’s laws of technology. It will be argued that Hume’s Law is evidence that reinforces Snow’s two cultures and Kranzberg’s truism.
Further, these theories will be applied to the identified gaps in Australia’s regulatory approach to genome editing. It is argued the very existence of these gaps is evidence of an amalgamation of these theories operating in an environment whereby the relationship between the law and science remains largely disconnected. One of the consequences of this disconnection is the reactionary response adopted by the law when scientific advancements occur.
Hume’s starting proposition notes that value cannot be derived from nature. For example, the way in which CRISPR technology works or its technical limitations represent statements of fact. Hume’s theory argues that a normative conclusion cannot be drawn from statements that report a particular fact.Footnote 10 Consequently, Hume’s Law states ‘one can never derive an ought from an is’.Footnote 11 If this is applied to genome editing, scientific facts/evidence relating to CRISPR technology represent examples of an ‘is’. In contrast, the law and regulation of CRISPR technology may be characterised as enforceable ‘oughts’. Non-compliance with the law enables punitive action to be pursued, given its enforceable authority. Science may identify the technical limitations of the technology, namely its capabilities, but it cannot determine what is an acceptable or unacceptable application or regulatory approach. It is the role of the law to determine these boundaries. This reflects a truism innate within the relationship between law and science — a ‘descriptive-normative divide’.Footnote 12 Science is descriptive, the law is normative. Hume’s Law recognises that a statement of fact may derive authority if this ‘descriptive-normative divide’ is bridged. This firstly acknowledges that science cannot dictate the law; however, it may provide instruction. It also recognises that law and science are separate entities or ‘cultures’ that require syllogism in the context of formulating a regulatory response to emerging technologies. This, in turn, further perpetuates the law’s reactionary stance towards technological advancements, as it relies on science to factually instruct and describe the relevant development requiring regulation.
In the 1959 Rede Lecture, CP Snow, a scientist and writer, argued society divides sciences and humanities into two separate cultures.Footnote 13 The scientists are placed on one pole and literary intellectuals representing the humanities, on the other. This has, in part, been caused by a ‘passion for specialisation’ within an established professional or academic discipline.Footnote 14 For the purposes of this article, humanities and law will be regarded as synonymous, in which law and science may be referred to as two cultures. The gradual development of two separate cultures has created a significant gap, whereby these two disciplines largely operate as independent siloes, in both an academic and practical sense. As a result, Snow argued ‘…the pole of total incomprehension of science radiates its influence on all the rest [of society]’.Footnote 15 In a society that tends to allow social perceptions regarding the relationship between technology and law to crystallise,Footnote 16 this may contribute to the regulatory gap observed in human genome editing.
Snow’s argument held that western society’s separation of science and humanities as two cultures was evident through the disinterest in one another’s culture. Over time, this has led to the polarisation and incomprehension of each other’s discipline and role.Footnote 17 It may also be argued that the two cultures contribute to a reactionary regulatory approach adopted by the law. More generally, Australia’s precautionary response is also indicative of uncertainty relating to scientific development and its potential applications.
Adopting Snow’s school of thought, a legal and regulatory response to HHGE falls within the remit of both cultures. The longstanding establishment of these separate cultures has led to greater rigidityFootnote 18 in public perceptions of science and its relationship to the humanities. If this were to be the starting point for a regulatory response to human genome editing, it is evident that science and law cannot operate in a vacuum. This is especially true in the context of emerging biotechnologies. In an age of technological advancement, a comprehensive understanding of the relationship between science and law will be a highly valued commodity. Snow highlighted the importance of bridging this gap, noting it is a ‘necessity in the most abstract sense, as well as in the most practical. When those two senses have grown apart then no society is going to be able to think with wisdom’.Footnote 19 It is argued this gap is evidence of Hume’s Law, in relation to the innate ‘descriptive-normative divide’ present within the law/science relationship.
Professor Dan Burk expanded Snow’s thesis, arguing the law ought to be characterised as a third culture.Footnote 20 Professor Burk defined the culture of science as ‘outward looking knowledge’ about the world that is ‘predictive, advisory and explanatory’.Footnote 21 In contrast, the humanities involved ‘inward looking knowledge’ pertaining to the human condition.Footnote 22 According to Burk, the law falls between the two, concerned with process and discourse. While the law may be influenced by scientific evidence or objective knowledge, Professor Burk argued that ‘science cannot dictate the law’.Footnote 23 Generally, the presence of a third culture has little impact in relation to Hume’s Law, as science in isolation cannot assume the identity of an enforceable ‘ought’. It requires the law, which for the purposes of this article, has been identified as the enforceable ‘ought’ which carries the authority of compliance.
If Professor Burk’s thesis is applied to HHGE, it would follow that the legal and regulatory response cannot be solely dictated by scientific developments.Footnote 24 It must also be informed by social, moral and ethical factors which require ‘inward looking knowledge’. Consequently, science may complement the law, by identifying the technical limits of the technology’s capabilities, thereby offering instruction in relation to what the law should allow or disallow. As a result, it is argued that both Snow and Burk’s positions merely reinforce Hume’s Law.
In light of the unprecedented potential brought by CRISPR technology, one must consider whether it is time to rely on the ‘external’, ‘outward looking knowledge’ of science,Footnote 25 to guide the legal and regulatory response for HHGE. This reinforces the role of science as a ‘factual instructor’ guiding the law. The uncertainty created by the unforeseen and unknown long-term effects of HHGE may require a new and different approach to legal and regulatory discourse. As a result, it may be an opportune time to re-establish the relationship of law and science to one which is symbiotic. In light of the social, legal and scientific complexity associated with emerging biotechnologies, it is imperative that the law rely on both cultures. This will require greater efforts to bridge the gap between the two cultures, to enable clear communication and understanding of each other’s roles.
Coined the founder of the history and technology discipline, historian Melvin Kranzberg explored the nature, role and impact of technology on society.Footnote 26 Kranzberg’s infamous laws of technology represented a set of ‘truisms’ about the nature of technology.Footnote 27 Most notably, Kranzberg’s first law of technology stated: ‘Technology is neither good nor bad; nor is it neutral’.Footnote 28 This context-specific characterisation of technology remains relevant today. It so follows that societal perception of a technology is dictated by its use or application.Footnote 29 In contrast, ‘defenders of technology’ endorse a consequentialist view,Footnote 30 whereby at its conception, technology is a ‘value neutral’ tool.Footnote 31 This status of neutrality may be subsequently compromised based on its development and use. Therefore, the ‘goodness or badness’ of a technology is contingent upon the ‘goals or ends for which it is used’.Footnote 32 Kranzberg challenged this position, noting ‘to say that technology is not strictly neutral, that it has inherent tendencies or imposes its own values, is merely to recognize the fact that, as a part of our culture, it has an influence on the way in which we behave and grow’. Footnote 33Kranzberg’s characterisation supports the notion that technology has a moral identity capable of shaping societal culture, perception and progress. This also accorded with Snow’s conclusion ‘…there is a moral component right in the grain of science itself, and almost all scientists form their own judgements of the moral life’.Footnote 34
Despite Kranzberg’s opposition to the consequentialist position, his argument retained principles of utilitarianism, as he advocated for the influence of a technology to be ‘directed toward goals worthy of [human]kind’.Footnote 35 This characterisation of a technology is rooted in utilitarianism; the context, which refers to a particular use or application, will determine whether it complies with moral and ethical standards. Further, the context in which a technology is used should retain its utility in promoting desirable outcomes for society.Footnote 36
Ethical theory, as a school of thought, attempts to rationalise our innate convictions of moral rights and wrongs. As Peter Singer noted, an individual lives in accordance with ethical standards ‘if they believe, for some reason, that it is right to do as they are doing’.Footnote 37 Therefore, our ethical judgement or convictions will ‘guide practice’.Footnote 38 If this view is applied specifically to CRISPR technology, our moral convictions of appropriate use/s of HHGE will guide its development and application over time. Moreover, its use to prevent and treat fatal genetic disorders or diseases such as cancer promotes a utilitarian agenda, whereby public health becomes the focus of CRISPR technology. This arguably reinforces Hume’s Law, noting the law remains normative, determining what may be right or wrong, whilst science continues to exist as a factual instructor alongside the law.
In the context of a regulatory pathway forward for HHGE, the theory underpinning the relationship between law and science must be understood and recognised. Hume’s Law merely offers one rationalisation for the observed relationship between law and science. Further, both Snow and Kranzberg’s theses relevantly apply to the legislative regime for HHGE, in that our current laws are largely operating independently of science and technology, which remain an ‘incommunicable art’.Footnote 39 Despite this disconnect between law and science, the prevailing need to retain a utilitarian discourse for the use and regulation of HHGE is reflective of technology’s moral identity. In order to promote a healthier marriage between law and science, a regulatory approach which bridges the ‘descriptive-normative divide’ may be an appropriate way forward.
III The Legislative Regime in Australia
Australia’s current regime creates a highly precautionary and prohibitive approach to the regulation of HHGE in research and clinical applications.Footnote 40 It has been described as a ‘command and control’ legislative framework,Footnote 41 which restricts biological research, especially in the context of emerging biotechnologies.
Currently, HHGE is governed by a triad of federal legislation, composed of the Gene Technology Act 2000 (Cth) (‘GT Act’), Research Involving Human Embryos Act 2002 (Cth) (‘RIHE Act’) and Prohibition of Human Cloning for Reproduction Act 2002 (Cth) (‘PHCR Act’). Together, these statutes prohibit the clinical application of HHGE in viable human embryos and significantly limit research uses. In addition, compliance with the National Health and Medical Research Council’s (‘NHMRC’) National Statement on Ethical Conduct in Human Research and Ethical Guidelines on the Use of Assisted Reproductive Technology in Clinical Practice and Research is required. Due to the current capability of HHGE, its potential clinical application falls under the auspices of an assisted reproductive technology (‘ART’). Consequently, HHGE is also captured by the regulatory and policy ambit of legislation and guidelines governing the use of ART.
Australia has a complex regulatory framework that is difficult to navigate. The interaction between each statute and relevant guidelines further exacerbates this complexity in addressing and dealing with various uses of HHGE. At the inception of these statutes, lawmakers had not foreseen the development of CRISPR technology. More broadly, lawmakers have been confronted with the issue of fostering lawmaking institutions which are responsive to evolving technologies. I argue that Australia’s legislative framework lacks the necessary capabilities to accommodate and regulate future uses of HHGE through the identification of three regulatory gaps.Footnote 42
A discussion of each statute will reveal the gaps in Australia’s current legislative regime, strengthening the argument for a more robust, responsive regulatory discourse. These gaps are also reflective of the disconnect between the law and science, which continue to operate as two cultures.
A Gene Technology Act 2000 (Cth)
This section provides an overview of the regulatory scheme implemented in the Gene Technology Act 2000 (Cth) (‘GT Act’). The underlying catalysts prompting the introduction of this statute are instructive for two reasons. First, it clearly identifies our current regulatory system for gene editing in organisms, which enforces various parameters on acceptable uses of gene editing. This arguably reinforces Hume’s Law, whereby scientific facts and development have instructed the law, by defining the technical limitations of the technology. However, it is the role of the law to determine whether a particular use of the technology is acceptable or unacceptable.Footnote 43 This is reflected in the licensing system implemented by this statute, which acts as the arbiter determining appropriate uses of genome editing techniques. Appointed decision-makers are guided by scientific information, namely the relevant technique and purpose and are vested with authority to approve such uses. Second, and more generally, it reflects Australia’s approach to the regulation of an evolving technology. The development of the statute provides context and justification for our current regulatory regime. This is particularly informative when determining whether this legislation remains fit for purpose, in the context of HHGE.
1 Development and Purpose
Australia was in uncharted regulatory territory, faced with the task of developing a regulatory framework that was independent and transparent and promoted public engagement.Footnote 44 In the 1992 Standing Committee on Industry, Science and Technology’s Report, Genetic Manipulation: The Threat or the Glory?, the Chairman, Michael Lee, identified the challenge created by biotechnology: ‘… nations around the world are grappling with the legal and institutional changes which will be required to cope with the new technology’.Footnote 45 In response to the emerging field of biotechnology, it was determined that Federal Government intervention was necessary to enforce a regulatory regime to oversee the use and advancement of gene technology.Footnote 46 At the time, in the late 1990s, Government interventions were deemed ‘inadequate’ to respond to the rapid growth of gene technology.Footnote 47 Following consultation with the public and relevant stakeholders, the product was a regulatory regime underpinned by a precautionary approach.Footnote 48
The primary focus of the Gene Technology Bill 2000 (Cth) concerned genetic modification within the agriculture and food industry — which is reflected in the ‘plant centric’ language of the legislation.Footnote 49 For example, in its most recent review, the Legislative and Governance Forum on Gene Technology concluded ‘[t]he Scheme was not designed to regulate humans’.Footnote 50 Genetic manipulation of humans, such as cloning or gene therapy, did not fall within the regulatory ambit of the Bill.Footnote 51 This was raised by former Attorney-General Nicola Roxon who noted the ‘legitimate focus’ of the proposed law was narrow in scope, excluding the technology’s potential application in humans.Footnote 52 She argued explicit and precise statutory language was required to ‘ensure the [regulatory] system is appropriate not just for handling and containment of genetically modified organisms in the plant and animal world, but also in the human area’.Footnote 53 Consistent with Michael Lee’s view, Ms Roxon acknowledged in the ‘not too distant future, we will have to grapple with how we legislate or regulate … the treatment of human cells in other areas’.Footnote 54 We are now facing this challenge, as HHGE inevitably continues to mature as a technology.
Although an objective of the Bill was to safeguard public health and the environment, its intention in offering this protection was balanced against risks associated with genetically modified foods.Footnote 55 Whilst the Bill represented Australia’s regulatory response to emerging biotechnologies, the need to future-proof the law to account for further advancements continues to be a recurring issue.
2 Regulation of HHGE
While this regime may have a ‘plant centric’ focus, there is ambiguity in relation to its application in humans, particularly in the context of clinical trials.Footnote 56 Under the current GT Act, a CRISPR-mediated genetic modification is classified as a gene technology and as such,Footnote 57 an organism subject to this modification is deemed a ‘genetically modified organism’ (‘GMO’).Footnote 58 Following a periodic review and amendments to the Gene Technology Regulations,Footnote 59 ‘site-directed nuclease (‘SDN’) techniques’ were introduced as an example of a genetic modification technique captured under the Act.Footnote 60 Techniques including CRISPR-Cas9 systems, zinc finger nucleasesFootnote 61 and transcription activator-like effector nucleases were incorporated into the definition of an SDN.Footnote 62 This change signified the need to update definitions within the GT Act, prompted by developments of scientific techniques.Footnote 63 A distinction in the purpose of undertaking an SDN was also addressed (see Figure 1 below). SDNs which do not incorporate the introduction of a new nucleic acid template do not constitute a GMO.Footnote 64 This is referred to as an ‘SDN-1’ — whereby the organism does not carry new traits resulting from gene technology, indicating a double-stranded break has been induced, without the introduction of a repair DNA guiding template.Footnote 65 This indicates that any genetic manipulation inserting a new DNA sequence, induced through a technique such as CRISPR technology, produces a GMO which is regulated under the GT Act.
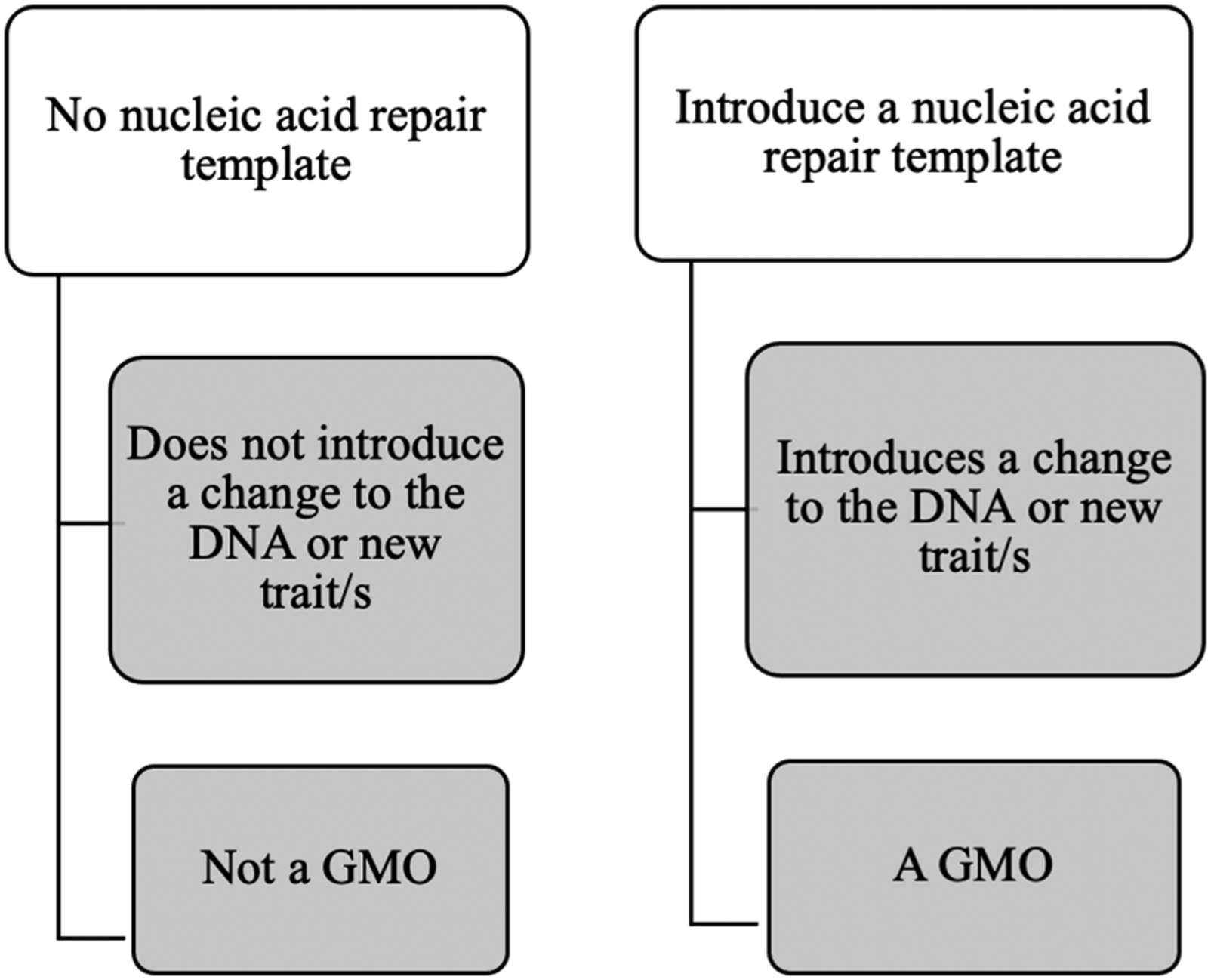
Figure 1. The classification of GMOs according to the purpose of undertaking CRISPR technology in an organism.
3 Governance and Regulatory Framework
The GT Act establishes a governance framework, enforced through the Gene Technology Scheme. The overarching body responsible for the operation of the regulator and the practical enforcement of the GT Act is the Legislative and Governance Forum on Gene Technology (‘LGF’), established by an intergovernmental agreement.Footnote 66 The LGF is a Ministerial Council, composed of Ministers from each state jurisdiction, managing portfolios relevant to gene technology.Footnote 67 The Gene Technology Regulator (‘GTR’) sits below the LGF in the governance framework. The GTR is an independentFootnote 68 statutory body which oversees the use of gene technology and ensures compliance with the regulatory regime.Footnote 69 As a result of the governance structure, the GTR sits within the purview of the LGF’s oversight function.Footnote 70
The GTR is responsible for administering and operating the licensing system enforced by the GT Act.Footnote 71 In addition, when dealings with GMOs serve a therapeutic purpose or a therapeutic good contains a GMO, this requires collaboration between the GTR and the Therapeutic Goods Administration (‘TGA’).Footnote 72 The TGA retains sole responsibility to regulate therapeutic goods, ensuring their safety, quality and efficacy for use in humans.Footnote 73 The interactive effect of the GT Act and Therapeutic Goods Act 1989 (Cth) (‘TGA Act’) raises important considerations relating to the delineation of jurisdiction according to the nature and use of the GMO/product and the appropriateness of the appointed regulator.
Two statutory committees are also established under the GT Act: the Gene Technology Technical Advisory Committee (‘GTTAC’) and the Gene Technology Ethics and Community Consultative Committee (‘GTECCC’).Footnote 74 The GTTAC is composed of members with skills or experience in science, medicine and public health.Footnote 75 Their primary function is to provide scientific or technical advice to the GTR on matters relating to gene technology (including biosafety aspects), GMOs, genetically modified products, applications submitted to the GTR for proposed dealings with GMOs and the development of policy/procedural guidelines, principles and practice.Footnote 76 In contrast, the GTECCC offers diversity in its opinion, with members drawing upon experience or skills in a number of areas, such as law, ethics, religious practices, risk communication, community consultation, business and consumer affairs.Footnote 77 The Committee also provides advice to the GTR on ethical concerns pertaining to gene technology, the development of policy or procedures/guidelines and codes of conduct and community consultation.Footnote 78
Australia’s governance approach aims to centralise day-to-day regulation and enforcement of statutory obligations to one federal regulator, the GTR. Guidance and oversight is facilitated by the LGF and Committees, which serves two purposes. First, it enforces accountability on behalf of the GTR to comply with statutory obligations and justify acceptable dealings with GMOs. Second, the provision of advice is a means to identify different perspectives and opinions in relation to the use of gene technology. This is imperative in the context of law and policy reform in the area of biotechnology.
The primary regulatory mechanism governing the use of gene editing is the enforcement of a licensing system.Footnote 79 This licensing system characterises dealings with a GMO into two groups: an exempt dealing or a notifiable low-risk dealing (‘NLRD’). A licence is required for dealings which fall outside the scope of either group.Footnote 80 In the context of HHGE, preclinical genetic modification of human cells will not be characterised as an exempt dealing.Footnote 81 However, it may be a NLRD, if physical containment is possible.Footnote 82 An Institutional Biosafety Committee (accessed through an accredited organisation) will assess the intended ‘dealing’ to determine whether it is one which falls within the NLRD regulatory scheme.Footnote 83 Criminal sanctions accompany a failure to obtain a licence or comply with conditions of a licence when dealing with GMOs.Footnote 84
It is arguable the GT Act is an embodiment of Hume’s Law and Snow’s two cultures. The role of science as a factual instructor attempts to bridge the descriptive-normative divide. In light of science and law operating as two cultures, the advent of gene technology was the catalyst prompting the law to react to this development by implementing a framework for its regulation in plants. It is arguable that a precautionary approach is merely a reflection of the need for the law to monitor ongoing developments as the technology’s potential remained largely uncertain. Further, the ‘plant centric’ nature of the statute is indicative of a regulatory response that did not adequately apply a future-oriented lens, which was only raised by one member of Parliament, the former Attorney-General Nicola Roxon.Footnote 85
Although the GT Act retains primary responsibility for the regulation of gene technology, its legislative objective did not consider the application of this technology in humans (in particular, for a therapeutic or enhancement purpose). The continued operation of the scheme remains largely ‘plant centric’, as reinforced by the GTR, and, as a result, was not created with the intention to regulate humans as GMOs. Where does this leave Australia regarding the regulation of HHGE? Should HHGE fall within the remit of the TGA Act?Footnote 86
B Research Involving Human Embryos Act 2002 (Cth)
1 Development and Purpose
Advancements in research involving cloning, stem cells and ART prompted the introduction of the Research Involving Human Embryos Bill (‘RIHE Bill’) in 2002. The creation of Dolly the sheep in 1996 and the use of embryos and stem cells within the context of an ART in 1998 represented a new field of research with clinical and therapeutic potential in humans.Footnote 87 These developments raised a number of concerns including the possibility of human cloning, the use of cloning to treat disease and the ethics of embryo research.Footnote 88
In the context of legislating in public policy areas that are emotionally and ethically provocative, such as biotechnology, the role of government was characterised as ‘uncontroversial’Footnote 89 by John Faulkner, Federal Senator and Shadow Minister for Public Administration and Home Affairs. Rather, the ‘regulatory principle embodied in [the RIHE Bill]’ encourages government to play an active role in ‘determining the ethical limits of medical research’.Footnote 90 Much like the introduction of the GT Act, this was a repeat of history. The law was reacting to another scientific advancement, prompting discussions regarding an appropriate national regulatory mechanism to govern and oversee a rapidly advancing field of research.Footnote 91
The Research Involving Human Embryos Act 2002 (Cth) (‘RIHE Act’), in conjunction with the Prohibition of Human Cloning for Reproduction Act 2002 (Cth) (‘PHCR Act’),Footnote 92 creates a ‘complex regulatory and [prohibitive] landscape for research involving genome modification of human embryos’.Footnote 93 At the time of its introduction to Parliament, the RIHE Bill was described as ‘conservative’,Footnote 94 ‘careful’Footnote 95 and ‘strict’.Footnote 96 Despite this strict approach, it was noted the Bill was designed to adopt a ‘very responsible approach to licensing and monitoring’ of this new research field.Footnote 97 The practical effect of the RIHE Act led to the continuation of embryo research in a very limited capacity.
Unlike the GT Act, enquiries and debates concerning the RIHE Act were human centric and dominated by rhetoric surrounding the moral and human status of an embryo.Footnote 98 A primary issue raised in the context of this Bill was the application of research techniques on human embryos and possible uses in ART. Consequently, many stakeholders advocated for a strict and prohibitive regulatory regime.Footnote 99 Despite the presence of two irreconcilable and polarised views pertaining to the ethics and permissibility of embryo research and its uses in ART, the ‘broader duty to society’ was a prevailing factor in favour of allowing research to continue.Footnote 100 The need to proceed with caution and care represented a more accurate reflection of a pluralist society,Footnote 101 which encouraged flexibility in a regulatory approach to account for differences in opinion.Footnote 102 In addition, permissibility of embryo research was informed by an assessment of the potential benefits and risks to human health.
2 Regulation of HHGE
The primary regulatory mechanism governing the use of embryo research is a licensing regime,Footnote 103 enforced by the Embryo Research Licensing Committee (‘ERLC’), a statutory body created under the RIHE Act.Footnote 104 Failure to obtain a licence or adhere to licence conditions incurs criminal penalties, including imprisonment.Footnote 105
The RIHE Act adopts a broad definition of an embryo,Footnote 106 which allows for research and training in ART clinics on egg culture, manipulation and maintenance, provided licence authorisation and approval is obtained. There are limited circumstances in which research involving CRISPR technology may be undertaken. First, pursuant to a licence, the ERLC may authorise the genetic manipulation of embryos to insert genetic material from a third person.Footnote 107 Second, research may be undertaken on embryos not suitable for implantation or excess ART embryos (if appropriately declared by the ERLC).Footnote 108 In order to lawfully use excess ART embryos, statutory requirements arguably impose burdensome practical barriers. For example, section 20 RIHE Act authorises the ERLC to grant a licence for the use of excess ART embryos,Footnote 109 on the proviso that express written authority from the woman and her spouse declares the embryos to be excess ART embryos.Footnote 110 In addition, consent must be obtained from the woman and her spouse and relevant ‘responsible persons’ prior to issuing the licence.Footnote 111
Whilst these circumstances appear to facilitate a lawful avenue for embryo research involving CRISPR technology, the utility of such research is questionable, as an embryo that is unsuitable for implantation is significantly damaged.Footnote 112 The utility of any such research will be dependent on the aim of the experiment.Footnote 113 However, significant damage may limit the validity and utility of the research, as the embryo is not capable of proper functioning to sustain life.
It is clear there are significant barriers limiting CRISPR technology involving viable human embryos for research purposes. The restrictive regulatory approach inherent within the RIHE Act provides limited scope to lawfully create and use embryos for both clinical and basic research purposes.Footnote 114 This may be particularly problematic following the concluding statement of the Organising Committee at the Third International Summit on Human Genome Editing in March 2023. They recognised the progress of germline genome editing, which is not intended for reproduction, in the context of basic research, which uses human embryos or gametes. In light of these developments, it was concluded that basic research in this area should continue.Footnote 115 However, the Committee reiterated that the primary purpose of this research is to improve understanding of ‘aspects of early human development or exploring how the methods might be used to correct gene variants leading to genetic disorders’.Footnote 116 This further strengthens the argument to re-assess Australia’s legal framework in relation to embryo research. Specifically, to reduce the burdensome practical barriers imposed by the current regulatory system.
C Prohibition of Human Cloning for Reproduction Act 2002 (Cth)
1 Development and Purpose
The need for legislation to address cloning was initiated by Dolly the sheep, which marked the success of somatic cell nuclear transfer in an animal model.Footnote 117 Upon its introduction in the House of Representatives, the Prohibition of Human Cloning Bill 2002 (‘PHC Bill’) originally formed part of the RIHE Bill. Due to the plethora of issues raised in the context of embryo research and unanimous support for the outright prohibition of cloning, the proposed Bill was split.Footnote 118 Given the ‘complex ethical and moral judgements’ raised in the context of embryo research and the inevitability of varying views on behalf of elected members, the Government did not oppose splitting the Bill.Footnote 119 As Senator Chris Ellison noted:
Quite appropriately, these bills were separated in order to allow debate on human cloning on the one hand and the merits or otherwise of stem cell research on the other. This afforded those people who had a strong view in relation to stem cell research the opportunity to vote separately on that bill and not have it tied up with the human cloning bill.Footnote 120
Much like the RIHE Bill, the PHC Bill was described as ‘conservative’,Footnote 121 adopting a strict prohibitive approach to address ethical concerns regarding scientific developments within the realm of human reproduction and the use of human embryos.Footnote 122 This BurkeanFootnote 123 approach to reform advocates for change to occur incrementally, by ‘insensible degrees’,Footnote 124 such that it is ‘slow, deliberate, and measured’.Footnote 125 However, this approach must be balanced against the need to further progress research to enable refinement of technology and meet the safety threshold required for human use. This is illustrative of Hume’s Law, whereby the law must rely upon science to instruct its substantive or descriptive content. Once ascertained, the law may respond accordingly, by contextualising the technology and defining its boundaries for appropriate use/s. Snow’s two cultures are also reinforced, as lawmakers are tasked with responsibility to translate scientific developments into enforceable laws.
2 Regulation of HHGE
The clinical application of HHGE directly contravenes the PHCR Act. The legislation prescribes a number of outright prohibitions, which effectively criminalises HHGE.Footnote 126 There are two provisions of particular relevance. First, it is a criminal offence to undertake heritable alterations to the human genome, with a maximum penalty of 15 years imprisonment.Footnote 127 The criminalisation of heritable editing was not the focus of Parliamentary debate, only noted in passing by one Senator.Footnote 128 The effect of this provision bans germline gene therapy, which may be carried out in germ cellsFootnote 129 or the cells of an early embryo.Footnote 130 Despite this ban, in the 2005 review of the RIHE Act and PHCR Act, most commonly known as the Lockhart Review, the Legislation Review Committee recommended that the creation of human embryos (by means other than fertilisation) subjected to HHGE could be undertaken under licence, for research purposes to ‘increase knowledge or treat diseases’.Footnote 131 The practical impact of this recommendation is yet to be known.
Second, it is a criminal offence to place a prohibited embryo into a woman to achieve pregnancy, with a maximum penalty of 15 years imprisonment.Footnote 132 For the purposes of this provision, an embryo subject to HHGE constitutes a ‘prohibited embryo’.Footnote 133 This bans the clinical application of HHGE — reinforcing the illegality of using HHGE as part of ART.
The PHCR Act represents a highly restrictive model of regulating HHGE in human embryos, for both clinical and research purposes. This approach is arguably consistent with Kranzberg’s utilitarian position which reinforces that technology has a moral identity. In the context of the PHCR Act, it is evident that the clinical application of CRISPR for HHGE has been defined as an unacceptable use.Footnote 134 This is reflected in the outright prohibition of clinical uses of HHGE, within the context of ART. Research uses of HHGE appear to be permitted in human embryos created by a means other than fertilisation, pursuant to a licence. However, the statutory language of section 15 PHCR Act creates confusion regarding its applicability to HHGE involving the creation of human embryos for research purposes. The need for clear and unambiguous statutory drafting is vital, given the serious criminal penalties accompanying prohibited uses of HHGE.
D The Role of the Therapeutic Goods Administration
Heritable human genome editing involves the use of a gene technology for therapeutic purposes. As a result, it must be considered whether the TGA Act ought to have a place in the regulation of HHGE.
There are three ‘primary pillars’ for the regulation of therapeutic goods in Australia — quality, safety and effectiveness.Footnote 135 These pillars attempt to enforce adequate regulation, to achieve the ‘correct balance’ between protection of consumers and avoiding undue restrictions on industry.Footnote 136 Therefore, in order to attain this ‘correct balance’, an assessment of the risk-to-benefit ratio becomes an influential part of approval and regulation of therapeutic goods, to ensure the benefits outweigh its risks.
The TGA Act vests the TGA with responsibility for the quality control and assurance of therapeutic goods in Australia.Footnote 137 This ensures the availability and supply of goods that are safe and fit for their intended purpose.Footnote 138 Regulation is facilitated through pre-market assessments, post-market monitoring and adherence to specified standards, licensing of Australian manufacturers and verification of international manufacturer compliance with equivalent/relevant standards.Footnote 139 The primary mechanism underpinning TGA regulation is risk management. This refers to the risk-to-benefit ratio analysis undertaken by the TGA prior to the approval or use of a proposed good/product. The degree of regulatory control is dictated by the classification of the therapeutic good/product and the level of risk attributable to the specific good/product.Footnote 140
1 The Regulation of Genome Editing in Therapeutics
Genetically modified cells, including those subject to CRISPR technology, are classified and regulated as biologicals.Footnote 141 The regulation of biologicals was introduced in 2011 via the implementation of a new regulatory framework under the governing legislation.Footnote 142 The framework was designed and enforced to address the use of emerging technologies.Footnote 143
Biologicals are defined as a distinct group of therapeutic goods which are made from, or contain, human cells and/or tissue.Footnote 144 Consequently, they introduce unique unforeseen risks that are not raised in the context of other therapeutic goods, such as prescribed medicines or medical devices.Footnote 145 Biologicals are regulated in accordance with a risk classification system, whereby class 1 represents ‘very low’ risk and class 4 ‘high’ risk.Footnote 146 On this spectrum, genetically modified cells are characterised as class 4 high risk biologicals.Footnote 147 The Therapeutic Goods Regulations 1990 (Cth) (‘TGA Regulations’) defines class 4 biologicals as products containing human cells or tissues that have been genetically modified to introduce a function that may or may not have been intrinsic to the cells and/or tissues upon retrieval from a donor.Footnote 148 Further, the purpose of the product must be used, or likely to be used, to treat or prevent disease, facilitate a medical diagnosis, inhibit, influence or modify a physiological process, test for susceptibility to a particular disease or replace/modify the anatomy of a person.Footnote 149 Biologicals confined to the parameters of the relevant legislative provisions and regulations can be used in Australia.Footnote 150
A clinical trial involving a biological may proceed in Australia, with approval from the GTR, the TGA and relevant Institutional Human Research Ethics Committees (‘HRECs’).Footnote 151 This reflects the extent of the interaction between the TGA Act and GT Act with respect to the use of genetically modified products in humans. It also highlights the applicability of the GT Act, despite its ‘plant centric’ focus.Footnote 152 The RIHE Act and PHCR Act continue to operate at the periphery, to limit research uses of CRISPR technology and deter improper uses of genetically modified products in humans.
The use of a biological for special or experimental purposes is permitted under the TGA Act, provided the sponsorFootnote 153 adheres to the specified conditions of use imposed by the TGA Regulations.Footnote 154 These conditions may include compliance with specified HREC procedural protocols and guidelines, the National Statement on Ethical Conduct in Research Involving Humans and cessation of use if required by the HREC.Footnote 155
The use of novel biological therapies or gene therapies in humans are subject to the TGA’s Clinical Trial Approval (‘CTA’) Scheme.Footnote 156 The CTA Scheme operates as an evaluative process for high risk or novel treatments, including gene therapy, with limited preclinical scientific data regarding its safety, risks and efficacy.Footnote 157 It involves a comprehensive review of the scientific and ethical issues posed by the novel therapeutic, undertaken by the TGA’s HRECs, prior to the commencement of a clinical trial.Footnote 158 It is the responsibility of the sponsor to submit a formal CTA application to the TGA, with the available data for review.Footnote 159 The sponsor must also ensure compliance with the relevant requirements under the GT Act, to obtain a licence or exemption for the use of a GMO.Footnote 160 In addition, adherence to the Guideline for Good Clinical Practice, the National Statement on Ethical Conduct in Human Research and any relevant protocol approved by the HREC is mandated.Footnote 161
The HREC exercises the primary oversight function, which involves ongoing monitoring of a biological subject to approval for use in humans.Footnote 162 This function involves the review of the benefit-risk ratio, trial results, progress reports, ethical acceptability of its use, amendments to protocols, breaches of conditions and safety information.Footnote 163
2 Contravention of the Therapeutic Goods Act 1989 (Cth)
The TGA Act codifies a number of criminal and civil penalties relating to the import, export, manufacture, supply and use of biologicals.Footnote 164 Criminal penalties are composed of three elements:
-
1. The biological is for use in humans; and
-
2. It is not subject to exclusions or exemptions; and
-
3. The use of the biological:
-
a. Has resulted in, or will result in, or likely to result in harm or injury to a person; or
-
b. If used would result in or likely to result in harm or injury to a person.
-
A breach of the criminal provisions is accompanied by a term of imprisonment.Footnote 165 For example, the importation, exportation and supply of a biological incurs a penalty of 5 years imprisonment.Footnote 166 While the criminalisation of certain conduct acts as a deterrent, it also emphasises the strict regulation of biologicals, due to the inherent risks to human health. The TGA Act also enforces civil penalties.Footnote 167
The potential use of HHGE must also consider the applicability of criminal and civil provisions under the TGA Act.
IV Australia’s Regulatory Approach
‘Regulation is neither static nor staid’.Footnote 168 This is especially the case when regulating novel therapeutics that are subject to ongoing advancements. Australia’s legislative landscape is representative of a piecemeal approach to regulation, which invokes a number of Acts to identify the boundaries of acceptable use for genome editing.
A policy brief of each relevant statute and an examination of its role in the regulation of HHGE identifies three regulatory gaps:
-
1. Regulatory complexity: there is no single legislative instrument which specifically addresses HHGE in clinical and research contexts. Rather, regulation is administered through four statutes. This creates regulatory complexity for those involved in the use of CRISPR technology and raises questions concerning the most appropriate regulator.
-
2. Legislative ambiguity: the lack of prescriptive statutory language creates uncertainty with respect to the practical operation and enforcement of legislative provisions. For example, what if a researcher is charged with a criminal offence under the PHCR Act?Footnote 169 This raises questions regarding the purpose of criminalising HHGE and whether there are any possible uses that may not be captured within the remit of the governing statutes.
-
3. Inconsistent legislative objectives: the governing statutes are not fulfilling their legislative objective, which may indicate they are no longer fit for purpose. It is timely to re-evaluate Australia’s legislative and regulatory frameworks to specifically address the inevitable use of HHGE as a therapeutic good/product.
This article attempts to provide an alternative lens through which Australia’s legislative response to emerging technology may be viewed. It could be argued the piecemeal approach to regulation is a product of the law’s reactionary response to advancing technology and reinforces Snow’s thesis. The disconnection between law and science may further perpetuate the two cultures, compromising their relationship’s ability to achieve synergism. It is further argued the complexity of the two cultures is amplified in areas that are highly politicised and raise moral, social and ethical debate which elicits polarising views. It is in these circumstances that the likelihood of communication between law and science is endangered, creating a larger disconnect between the two cultures.
In the context of emerging technology and specifically CRISPR technology, it is evident that lessons can be drawn from the theses of Hume, Snow and Kranzberg. Most notably, it provides justification for the fraught law/science relationship currently observed in the context of CRISPR technology and its application in humans. It is also integral to note that context is a significant determinative factor influencing the status of the law/science relationship. For example, one may argue the relationship is stronger in the context of climate change and the law.Footnote 170 This may also be the case in the context of genome editing within agriculture.
An interaction of the three theories can be applied to the legal and regulatory response to HHGE. First, Hume’s Law is reinforced. Scientific advancement may be perceived as statements of fact that instruct the law. However, it may not dictate the substantive content of the law. Second, the moral identity of a technology, as raised by Kranzberg, is reflective of its application and defined by law. This identity is informed by ‘inward looking knowledge’ and public debate. It requires an interrogation of topics captured within the auspices of the humanities, such as social, moral, ethical, religious, political and economic issues. Finally, the law’s response to emerging technology is illustrative of Snow’s two cultures, that are separated by Hume’s ‘descriptive-normative divide’. This divide has led to deficiencies in communication between the two. It also highlights the need to encourage collaboration, in order to provide a robust, scientific and ethically informed regulatory discourse.
It is apparent the law is reactive to scientific advancement, adopting a highly precautionary approach. However, it is important to recognise this response is operating against the backdrop of a society that traditionally separates the sciences and humanities into exclusive siloes. The product is a significant disconnect in communication and understanding between the two disciplines. This must be addressed when determining an appropriate pathway forward.
This Part does not seek to provide solutions to these regulatory gaps. Rather, it aims to identify areas requiring further policy development and public engagement to determine an appropriate regulatory discourse.
A Regulatory Complexity
The product of a piecemeal approach to regulation is the absence of a single legislative instrument which solely addresses HHGE in both a clinical and research context. Australia’s approach raises an important question: who are the formal regulators of HHGE in its various applications? The framework provides numerous regulators, including the GTR, TGA and HRECs. Each regulator is tasked with distinct roles and responsibilities, which are further confined to specific applications of CRISPR technology.
Heritable human genome editing is not explicitly addressed in the GT Act or TGA Act but is outrightly prohibited by the PHCR Act.Footnote 171 Figure 2 identifies the relationship between each governing statute, in a research and clinical context. A clinical application of CRISPR technology would invoke the GTR and/or TGA as regulators, whilst the PHCR Act operates to enforce the boundaries of CRISPR technology in humans. Alongside this, the RIHE Act has now been tasked to govern the licensing system for mitochondrial donation in ART for preclinical and clinical use, through ERLC authorisation.Footnote 172 In light of this development, the RIHE Act has traversed into the territory of clinical application. As HHGE progresses towards preclinical use, the role and interaction with the RIHE Act may be raised.
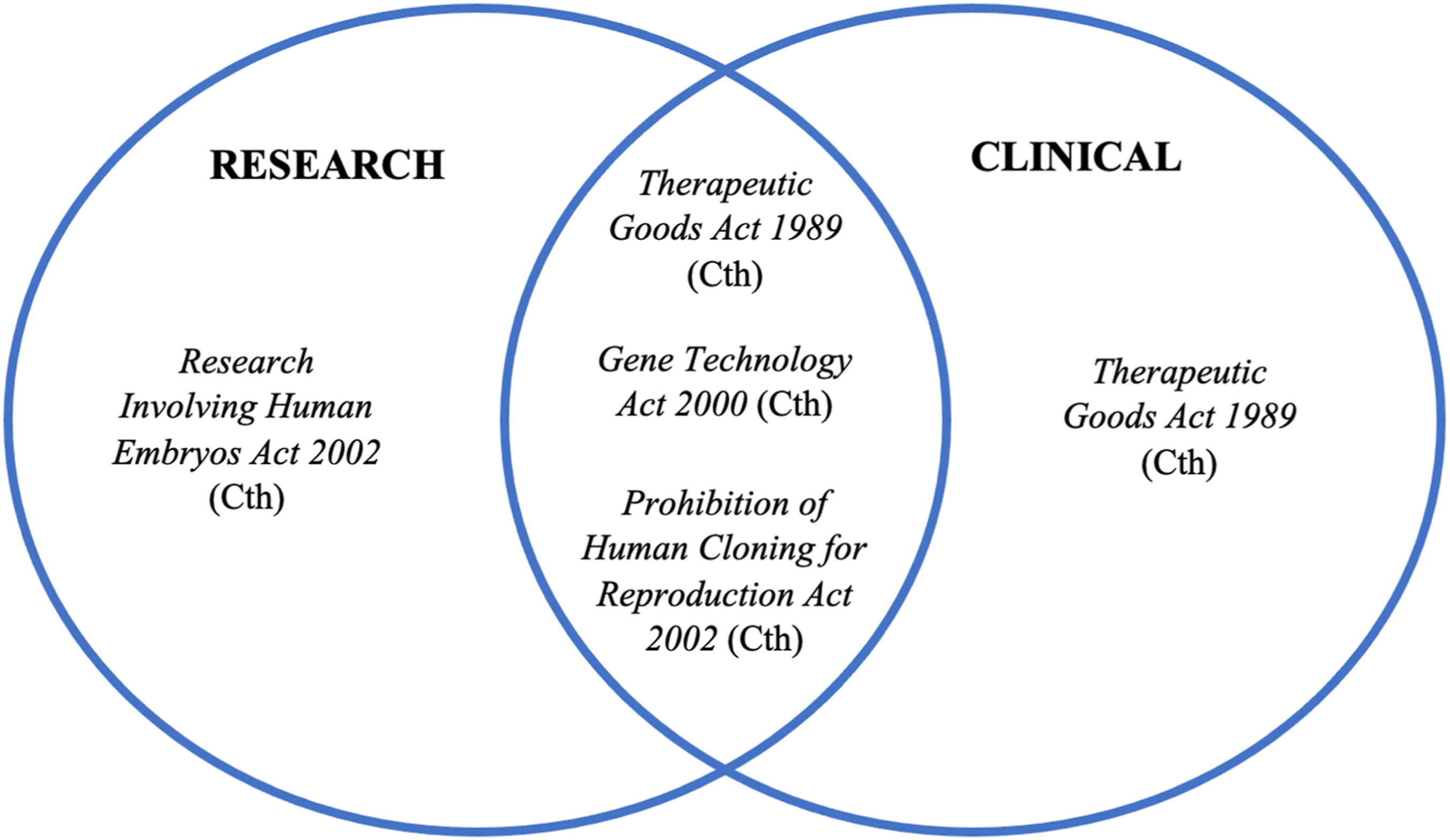
Figure 2. The interaction between four statutes creates a complex regulatory system that is difficult to navigate and adapt to rapidly evolving technologies.
The lack of prescriptive legislative guidance concerning the use of HHGE in clinical and research contexts perpetuates the regulatory complexity observed in Australia. It also strengthens the case for a single statute which identifies the parameters for the use of HHGE at each stage (research, preclinical and clinical) and the relevant regulators in the translational pathway from bench to bedside.
Currently, the GTR retains primary responsibility for the governance of gene technology. However, the GT Scheme was not designed to regulate the application of gene technology in humans. This has been expressly excluded and affirmed in subsequent reviews of the GT Scheme.Footnote 173 Despite this, a sponsor who intends to apply for a clinical trial using a therapeutic product subject to CRISPR technology must obtain a licence or exemption from the GTR. This implies that a human embryo would constitute a GMO for the purposes of the GT Act. In addition, if the application is deemed to be of a therapeutic use, regulation is subsequently shared with the TGA. Within the CTA Scheme, primary responsibility for the ongoing management, monitoring and risk-to-benefit ratio analysis is delegated to the elected institutional HREC/s, as opposed to the TGA.Footnote 174 The capacity for HRECs to undertake this role is a relevant factor to consider when identifying an appropriate regulator.
It is implicit that the use of CRISPR technology, and specifically, HHGE, will fall within the regulatory ambit of both the GT Act and TGA Act. However, this does not preclude the operation and applicability of relevant provisions enumerated in the RIHE Act and PHCR Act. These statutes continue to limit the possible uses of CRISPR technology. The enactment of Maeve’s Law also raises additional considerations which will not be explored in this article, specifically in relation to its impact on the applicability of the PHCR Act.Footnote 175
Australia’s legislative landscape creates an interactive effect between the TGA Act and the triad of governing legislation, leading to a highly complex regulatory system which is difficult to navigate for researchers using CRISPR technology. This is highlighted by the operation of the PHCR Act and RIHE Act. As noted earlier, the clinical application of HHGE is expressly prohibited by the PHCR Act. Most often, HHGE relies on the use of viable human embryos, which are then captured within the regulatory ambit of the RIHE Act. This imposes additional statutory responsibilities and obligations to undertake lawful research. If a researcher intends to undertake CRISPR technology in a human embryo, they must be aware of the constraints and obligations imposed by the triad of governing legislation and the TGA Act, if applicable. For example, a researcher must ensure licence applications are made to the appropriate authority (relevant HREC/s, the GTR and/or the TGA) and understand the statutory limits on acceptable research purposes provided by each governing statute. All the while, the threat of criminal prosecution continues to shadow the researcher.
Complexity may be minimised by clearly identifying a single appropriate regulator for the use of CRISPR technology in humans. However, this requires a comprehensive evaluation of Australia’s current statutes to determine whether this collaborative approach to regulation is most effective. A regime which is designed to regulate the use of CRISPR technology in humans is required to alleviate unnecessary complexity.
B Legislative Ambiguity
Ambiguity is a product of statutes which attempt to dovetail one another. This is evident in the operation of the TGA Act alongside the triad of governing legislation. Each statute governs specific applications of CRISPR technology. For example, the RIHE Act governs research uses of CRISPR technology in embryos. In contrast, the TGA Act governs potential clinical uses of CRISPR technology in therapeutic goods/products. The overall effect of this regulatory approach may create difficulties in reforming each statute as CRISPR technology continues to advance.
The PHCR Act provides a clear example of legislative ambiguity, which raises concerns regarding its practical enforcement. The express prohibition and criminalisation of HHGE is contained within section 15 PHCR Act. It is worthwhile noting the construction of this section:
-
(1) A person commits an offence if:
-
(a) the person alters the genome of a human cell in such a way that the alteration is heritable by descendants of the human whose cell was altered; and
-
(b) in altering the genome, the person intended the alteration to be heritable by descendants of the human whose cell was altered.Footnote 176
-
A plain interpretation of this section consistent with its legislative purposeFootnote 177 indicates this provision applies to both embryos and gametes created by fertilisation or some other means, irrespective of the impact of the edit (whether new genetic material is inserted or genetic material is removed without a replacement sequence).Footnote 178 Therefore, it may also apply to HHGE for research purposes, as the alteration capable of being heritable is inherent within the research project aim and method.Footnote 179 This directly contradicts the position adopted by the Legislative Review Committee in the Lockhart Review, which permitted the use of HHGE for research purposes, provided it increased knowledge or assisted in disease treatment.Footnote 180 For researchers engaged in HHGE research, this raises significant concerns. Clarity with respect to the operation of section 15 PHCR Act in light of the Lockhart Review’s position is warranted.
This legislative uncertainty may lead to a moratorium on research, due to the threat of criminal prosecution and imprisonment. It is logical to presume a researcher would not be inclined to accept this risk.
The fault element contained within section 15 PHCR Act requires that the individual ‘intended the alteration to be heritable’.Footnote 181 The interpretation of this element requires statutory clarification and guidance with respect to its impact on or application to research uses of HHGE. It has been argued the ‘relevant intent’ pertains to the creation of the heritable genetic alteration, as opposed to an intent to pass on a specific genetic alteration to future generations.Footnote 182 The absence of statutory clarity perpetuates confusion about the lawfulness of undertaking HHGE for research purposes (specifically, the methods used to create an embryo) and the operation of the PHCR Act. It is this uncertainty which effectively prohibits research involving HHGE, as researchers will not risk criminal prosecution.
Another example is evident in the most recent Gene Technology Amendment (2019 Measures No 1) Regulations 2019 (Cth) whereby an SDN-1 organism (one which does not contain a repair DNA guiding template and thus does not introduce new traits) does not constitute a GMO.Footnote 183 The practical implications of this amendment in the context of research and clinical uses of CRISPR technology, particularly somatic gene editing, remain ambiguous.Footnote 184 Most recently in 2022, the GTR released guidance pertaining to clinical trials involving GMOs.Footnote 185 The introduction of human genetically modified somatic cells into a person from which they are derived may require a licence from the GTR, unless subject to an exemption.Footnote 186 A licence is not required if the cells cannot secrete or produce infectious agents due to the modification and if modified using a viral vector, they are tested and do not contain other viruses and the viral vector is not present within the cells.Footnote 187 Importantly, once the genetically modified somatic cells are in the person, the GMO is no longer regulated by the GT Act.Footnote 188 It appears that regulation then transfers to the TGA. Despite this guidance, according to the GT Regulations, an organism which carries new traits resulting from CRISPR technology is deemed a GMO and remains within the regulatory ambit of the GT Act. It is uncertain how this interacts with the recent clinical trial guidance in cases where somatic gene editing is used to introduce a new trait or repair a gene in a person. Greater clarity is required with respect to these circumstances, including its operation in relation to HHGE and the regulation of this GMO under the GT Act.
Legislative guidance is necessary to ensure researchers are well informed of the limitations placed on specific uses of CRISPR technology and the relevant regulators. A more streamlined process, which clearly delineates the jurisdiction of each regulator, would help alleviate the uncertainty and ambiguity introduced by the operation of the GT Act.
C Inconsistent Legislative Objectives
The appropriateness of each statute governing CRISPR technology is questionable. In particular, the GT Act, RIHE Act and PHCR Act were not introduced to explicitly address and regulate CRISPR technology. As such, it is argued the regulation of CRISPR technology in humans is inconsistent with the legislative objectives of the triad of governing legislation. Each statute must be examined to determine whether it remains fit for purpose.
Over time, despite its incremental expansion to regulate some uses of CRISPR technology in humans, recent guidance indicates that the GTR ceases to be the regulator once the GMO is introduced into a person. As noted above, the GT Act was not intended to govern the use of CRISPR technology in humans. Section 3 of the GT Act states:
The object of this Act is to protect the health and safety of people, and to protect the environment, by identifying risks posed by or as a result of gene technology, and by managing those risks through regulating certain dealings with GMOs.Footnote 189
Section 4 of the GT Act reinforces this, noting this legislative objective will be met through the establishment of the regulatory framework.Footnote 190 The absence of explicit language pertaining to the regulation of a gene technology in humans is telling and the emphasis on environmental harm is evident:
[The regulatory framework must ensure] … where there are threats of serious or irreversible environmental damage, a lack of full scientific certainty should not be used as a reason for postponing cost-effective measures to prevent environmental degradation …Footnote 191
Subsequent reviews and guidance have merely reinforced the ‘plant centric’ nature of the GT Scheme and supported the collaboration of the TGA in cases involving gene editing in humans for clinical purposes.
Similarly, the RIHE Act responded to advancements in research techniques involving human embryos and was dominated by a moral debate concerning the status of an embryo. It is arguable that the RIHE Act is the most appropriate legislative vehicle to address HHGE, given its potential application in ART.Footnote 192 This is supported by the legislative object of the Act, which states:
The object of this Act is to address concerns, including ethical concerns, about scientific developments in relation to human reproduction and the utilisation of human embryos by regulating activities that involve the use of certain human embryos created by assisted reproductive technology or by other means.Footnote 193
The application of HHGE in ART may fall within the remit of ‘other means’, particularly in cases involving gene editing of a viable human embryo to achieve a pregnancy. The current legislative landscape requires scientists to rely on the RIHE Act to ascertain the boundaries of lawful research involving embryos and navigate the GT and/or TGA Schemes, where applicable.
Finally, the PHCR Act was intended to ‘address concerns, including ethical concerns, about scientific developments in relation to human reproduction and the utilisation of human embryos by prohibiting certain practices’.Footnote 194 Despite this broad statement, Parliamentary debates and policy indicated the primary intent of this Act was to prohibit and criminalise human cloning for reproduction, in reaction to Dolly the sheep. Similarly, the advent of CRISPR technology acted as a recent catalyst prompting a strict ‘law and order’ response from the legislature, which led to the criminalisation of such practices under the PHCR Act. The appropriateness of this response and insertion into the PHCR Act is questionable, as it is not commensurate with its legislative and policy objective.
The evolution of gene technology, and in particular HHGE, had not been foreseen by policy and lawmakers at the time in which each statute was drafted and debated. Rather, as gene technology matured leading to the introduction of CRISPR technology, lawmakers opted to build upon the existing regulatory infrastructure to address these advancements. However, in attempting to do so, this has created a fragmented, complex regime in which the regulation of future uses of CRISPR technology, namely HHGE, may be compromised. Further, the relevant statutes were not drafted nor intended to regulate CRISPR technology in humans and as such do not fulfil their legislative objectives.
Parts II and III of this article attempt to provide context to Australia’s current regulatory approach. The relevant theories applied represent one justification for the law and policymaking approach adopted in the area of emerging technology. Each governing statute in Part III reflects a reactionary response to technological developments, the extent of which is contingent upon the acceptability of its use. This refers to Kranzberg’s notion that a technology’s moral identity will be shaped by societal perceptions of acceptable and unacceptable uses. Further, the underlying theory inherent within these statutes arguably reinforces Hume’s Law, indicating that the law will remain an ‘enforceable ought’, as the arbiter of the ethics accompanying a technology and its moral identity. On the other hand, science will continue to factually instruct the law by offering descriptive evidence of CRISPR technology’s technical capabilities and limitations. Consequently, this instruction will attempt to bridge the ‘descriptive-normative’ divide. An application of these theories also leads to the three regulatory gaps identified.
Given the rapid advances of HHGE and its potential use in a clinical context, such as ART or disease prevention/treatment, it is timely to re-consider the most appropriate approach to regulation and the designated regulator.
V Conclusion
Australia’s current legislative approach is illustrative of Snow’s thesis — the law attempts to communicate with advancements in science, but a large disconnect remains. It also reinforces Burk’s notion that the law may rely on both scientific evidence and accompanying moral/ethical concerns to formulate an appropriate response. Hume’s Law continues to provide an instructive lens to explain the existence of two siloed cultures, which attempt to operate in tandem. However, it is argued the relationship between the two cultures becomes more distant and complex when responding to CRISPR technology and HHGE.
The bridging of these cultures by improving communication, understanding and involvement of science experts as part of policy development and lawmaking is integral. The triad of governing legislation is indicative of technology’s influence on society and its acceptable applications very much inform its moral identity. Whilst an overarching utilitarian purpose is favourable, the outstanding ethical concerns relating to HHGE require public interrogation and consultation.
An examination of each statute reveals a piecemeal and fragmented regulatory approach, reinforcing the disconnect between the two cultures. Despite the arrival of CRISPR technology, it is a repeat of history. The law has reacted in a highly precautionary and prohibitive manner, which may unduly restrict important research required to further refine CRISPR technology. A precautionary approach to avoid another ethical disaster as observed with CRISPR Babies is warranted; however, this must be balanced against a prevailing need to enhance the safety and efficacy of the technology.
An analysis of each statute revealed three gaps in Australia’s legislative landscape: complexity, operational ambiguity and inconsistent legislative objectives. The statutory objectives and policy support the argument that these statutes may no longer be fit for purpose. At the very least, some shifting of the legislative landscape to provide clarity would be welcomed. The interactive relationship between the triad of governing legislation and the TGA Act perpetuates both the complexity of our regulatory system and ambiguity in the enforcement of relevant provisions of law.
It is not within this article’s remit to offer solutions to these gaps. Rather, it serves to identify the nature of these gaps, by examining the governing statutes. Further, the application of theories explored in Part I assisted in applying a contextual lens to the broader relationship between the law and science, as a means to provide one explanation of how Australia arrived at the current legislative landscape.
It is perhaps timely to review each statute to ensure our laws are equipped to effectively regulate HHGE. Past experience in developing policy and law within the field of emerging technology should inform next steps to future-proof Australia’s current regulatory regime.




