Introduction
There is substantial evidence that a combination of soil water deficit and extreme atmospheric temperatures is the most undesirable condition for crop production (Mittler, Reference Mittler2006; Vile et al., Reference Vile, Pervent, Belluau, Vasseur, Bresson, Muller, Granier and Simonneau2012; Chaniago et al., Reference Chaniago, Syarif and Riviona2017; Khanthavong et al., Reference Khanthavong, Yabuta, Malik, Hossain, Akagi and Sakagami2022). Early establishment and survival of crops in the field is largely dependent on rainfall distribution rather than the total amount in a season (Rizhsky et al., Reference Rizhsky, Liang, Shuman, Shulaev, Davletova and Mittler2004). Similarly, extreme temperatures negatively affect crop growth and development, particularly at the seedling stage. Heat waves are characterized by great variability in intensity, duration, and timing, more often than dry spells (Jha et al., Reference Jha, Bohra and Singh2014; Eggen et al., Reference Eggen, Ozdogan, Zaitchik, Ademe, Foltz and Simane2019a). Prolonged soil water deficit and extremely high atmospheric and soil temperatures that cause damage to physiological functioning in crops are termed drought and heat stresses, respectively (Cairns et al., Reference Cairns, Crossa, Zaidi, Grudloyma, Sanchez, Luis Araus, Thaitad, Makumbi, Magorokosho, Bänziger, Menkir, Hearne and Atlin2013; Cvikrová et al., Reference Cvikrová, Gemperlová, Martincová and Vanková2013; Meseka et al., Reference Meseka, Menkir, Bossey and Mengesha2018). Atmospheric temperatures above 35°C are considered extreme in semi-arid tropics and lead to heat stress in crops (Ahmad et al., Reference Ahmad, Waraich, Zulfiqar, Ullah and Farooq2021). The frequency and magnitude of the drought and heat stresses, separately or in combination, has escalated in recent years due to climate change and variability (Eggen et al., Reference Eggen, Ozdogan, Zaitchik, Ademe, Foltz and Simane2019b; Hassan et al., Reference Hassan, Ahmed and Rashed2022; Lesk et al., Reference Lesk, Anderson, Rigden, Coast, Jägermeyr, McDermid, Davis and Konar2022), which is a great threat to cropping and food production in the semi-arid and arid regions of the world and much of Africa (Hadebe et al., Reference Hadebe, Modi and Mabhaudhi2017; Khalifa and Eltahir, Reference Khalifa and Eltahir2023; Cotrina Cabello et al., Reference Cotrina Cabello, Ruiz Rodriguez, Husnain Gondal, Areche, Flores, Astete, Camayo-Lapa, Yapias, Jabbar, Yovera Saldarriaga, Salas-Contreras and Cruz Nieto2023).
Sorghum bicolor (L.) Moench (sorghum) is a well-documented thermophilic, drought-tolerant, and hardy cereal crop that is grown and serves as a source of livelihood particularly in marginal areas (Pennisi, Reference Pennisi2009; Yahaya et al., Reference Yahaya, Shimelis, Nebié, Mashilo and Pop2023). The crop plays a major role in food security, livestock feed, biofuel, and brewing (Bibi et al., Reference Bibi, Sadaqat, Tahir and Akram2012; Chaniago et al., Reference Chaniago, Syarif and Riviona2017; Khalifa and Eltahir, Reference Khalifa and Eltahir2023). Despite its perceived adaptation to most abiotic stresses (Pavli et al., Reference Pavli, Ghikas, Katsiotis and Skaracis2011; Galicia-Juárez et al., Reference Galicia-Juárez, Sinagawa-García, Gutiérrez-Diez, Héctor, Zavala-García, Villa, Ex, Canada and León2020; Hassan et al., Reference Hassan, Ahmed and Rashed2022), heat and drought stresses occurring separately or in combination affect its productivity negatively (Jagtap et al., Reference Jagtap, Bhargava, Streb and Feierabend1998; Khalifa and Eltahir, Reference Khalifa and Eltahir2023). The situation is likely to worsen in the next few years (Mackay, Reference Mackay2008). Germination, emergence, and seedling stages of any crop are critical growth phases vulnerable to dual abiotic stresses (Howarth et al., Reference Howarth, Pollock and Peacock1997; Yuan et al., Reference Yuan, Qian and Yu2011; Abro et al., Reference Abro, Eisawi, Batyrbek, Sial, Akhtar and Memon2022). Empirical evidence of the detrimental effects of water deficit at seedling establishment and growth in various crops like maize, millets, barley, and sorghum is well-documented (Álvarez-Iglesias et al., Reference Álvarez-Iglesias, Roza-Delgado, Reigosa, Revilla and Pedrol2017; Ahmadi et al., Reference Ahmadi, Pour-Aboughadareh, Fabriki-Ourang, Mehrabi and Siddique2018). Drought stress is known to inhibit cell division and elongation, which impedes the growth of both roots and shoots in seedlings (Craufurd et al., Reference Craufurdtj, Ellis, Summerfield and Menin1996; Hatfield and Prueger, Reference Hatfield and Prueger2015; Queiroz et al., Reference Queiroz, Oliveira, Steiner, Zuffo, Zoz, Vendruscolo, Silva, Mello, Cabral and Menis2019b). On the other hand, heat stress has been shown to destabilize membranes and most temperature-dependent metabolic reactions in plants (Paupière et al., Reference Paupière, van Heusden and Bovy2014; Zhao et al., Reference Zhao, Zhang, Zhao, Wang, Yang, Ta, Li and Hu2016; Bernfur et al., Reference Bernfur, Rutsdottir and Emanuelsson2017). Accordingly, the adverse effects of heat and drought stresses in seedlings are topical, especially to promote sorghum resilience in areas with limited capacity to adapt to climate change and variability (Moshelion, Reference Moshelion2020; Chadalavada et al., Reference Chadalavada, Kumari and Kumar2021). Vast genetic resources are available for sorghum (Chakrabarty et al., Reference Chakrabarty, Mufumbo, Windpassinger, Jordan, Mace, Snowdon and Hathorn2022), which is a starting point for identifying promising genotypes that can cope with heat and drought stresses.
Over the years, research focused on pre- and post-anthesis drought stress for cereals like maize and wheat (Rizhsky et al., Reference Rizhsky, Liang, Shuman, Shulaev, Davletova and Mittler2004; Farooq et al., Reference Farooq, Wahid, Kobayashi and Fujita2009). Recent interest in resilient crops such as sorghum, that thrive in marginal areas characterized by extreme temperature and erratic rainfall, has focused on combined heat and drought stresses rather than isolated stresses (Zegada-Lizarazu and Monti, Reference Zegada-Lizarazu and Monti2013; Rajendra Prasad et al., Reference Rajendra Prasad, Govindaraj, Djanaguiraman, Djalovic, Shailani, Rawat, Singla-Pareek, Pareek and Vara Prasad2021). The alteration of growth by each stressor separately and in combination is certainly unique and diverse among genotypes of a given crop (Rizhsky et al., Reference Rizhsky, Liang and Mittler2002; Mittler, Reference Mittler2006; Yahaya et al., Reference Yahaya, Shimelis, Nebié, Mashilo and Pop2023). Plants’ tolerance to abiotic stress is measured by their ability to establish vigour under limiting environments (Ye et al., Reference Ye, Roorkiwal, Valliyodan, Zhou, Chen and Varshney2018). Fast growth of roots in seedlings is assumed to be associated with early vigour that ultimately determines the final grain and biomass yield (Magalhães et al., Reference Magalhães, Souza, Lavinsky, Pereira, Albuquerque, De and Castro2016; Ahmadi et al., Reference Ahmadi, Pour-Aboughadareh, Fabriki-Ourang, Mehrabi and Siddique2018). This information is very crucial to physiologists and plant breeders, offering great potential in coming up with varieties that cope with abiotic stresses at critical growth stages. Furthermore, a positive correlation has been established between root and coleoptile length in seedlings and grain yield in sorghum (Ali et al., Reference Ali, Abbas, Niaz, Zulkiffal and Ali2009; Ali et al., Reference Ali, Abbas, Awan, Jabran and Gardezi2011). This makes root and coleoptile characters in seedlings critical when evaluating superior germplasm for heat and drought stress.
Laboratory or pot assays reduce cost and labour requirements as compared to field trials (Ahmadi et al., Reference Ahmadi, Pour-Aboughadareh, Fabriki-Ourang, Mehrabi and Siddique2018; Osmolovskaya et al., Reference Osmolovskaya, Shumilina, Kim, Didio, Grishina, Bilova, Keltsieva, Zhukov, Tikhonovich, Tarakhovskaya and Frolov2018; Wasaya et al., Reference Wasaya, Zhang, Fang and Yan2018). The use of polyethylene glycol (PEG) to induce osmotic stress offers a good tool for short-term assessment of seedling response to drought stress (Álvarez-Iglesias et al., Reference Álvarez-Iglesias, Roza-Delgado, Reigosa, Revilla and Pedrol2017; Surbhaiyya et al., Reference Surbhaiyya, Gahukar, Jadhav, Bhagat, Moharil, Potdukhe and Singh2018; Abro et al., Reference Abro, Memon, Abro, Sam, He, Rind, Memon, Solangi, Muhammad, Ali, Ahmed, Dev, Abro, Rajput, Nizamani, Nargis and Kumbhar2020; Abro et al., Reference Abro, Eisawi, Batyrbek, Sial, Akhtar and Memon2022; Rad et al., Reference Rad, Sharifabad, Torabi, Azizinejad, Salemi and Soltanabadi2023). Furthermore, heat stress is easier to induce in controlled environments, where a screening protocol for both heat and drought stresses can be applied (Peacock, Reference Peacock1982). The need for conclusive empirical evidence on combined heat and drought stress, and also their individual effects on sorghum, compels the current study. This study aimed at screening high-potential African sorghum genotypes for heat, drought, and combined stress responses at early and late seedling stages. We hypothesize that sorghum genotypes are distinctly affected by heat and drought stresses induced separately and combined at the seedling stage, taking into account root and shoot growth, which is the most sensitive and easy-to-measure morphological variables that indicate seedling vigour and tolerance to stress.
Materials and methods
Description of the study site
The study was carried out at an experimental plot and the Seed Science Research Laboratory, Faculty of Agricultural Sciences, Lupane State University (18°93’00S, 27°75’93E, and 1016 m above sea level), in Zimbabwe. The study site is situated in the Agro-Ecological Region IV and has a climate characterized by hot summers and cold winters with temperatures ranging from 10 to 41°C. The rainy season usually begins in November and ends in April, and an average of 450 to 650 mm is received annually. The rainy season is usually short as rains begin late and terminate early. During the rainy season, there are early and midseason dry spells. Soils are dominantly Kalahari sands with low water holding capacity and are inherently infertile (Makuvaro et al., Reference Makuvaro, Walker, Munodawafa, Masere, Murewi and Chagonda2014).
Plant material
Fifty sorghum genotypes were sourced from the Genetic Resources and Biotechnology Institute of Zimbabwe and the International Crop Research Institute of the Semi-Arid Tropics (ICRISAT). The majority of the accessions evaluated were landraces of African origin (Table 1), with their selection based on desirable attributes such as high yield, short growth period and plant architecture.
Table 1. Profiles of the 50 selected African sorghum genotypes, their biological status, and origins used in the study
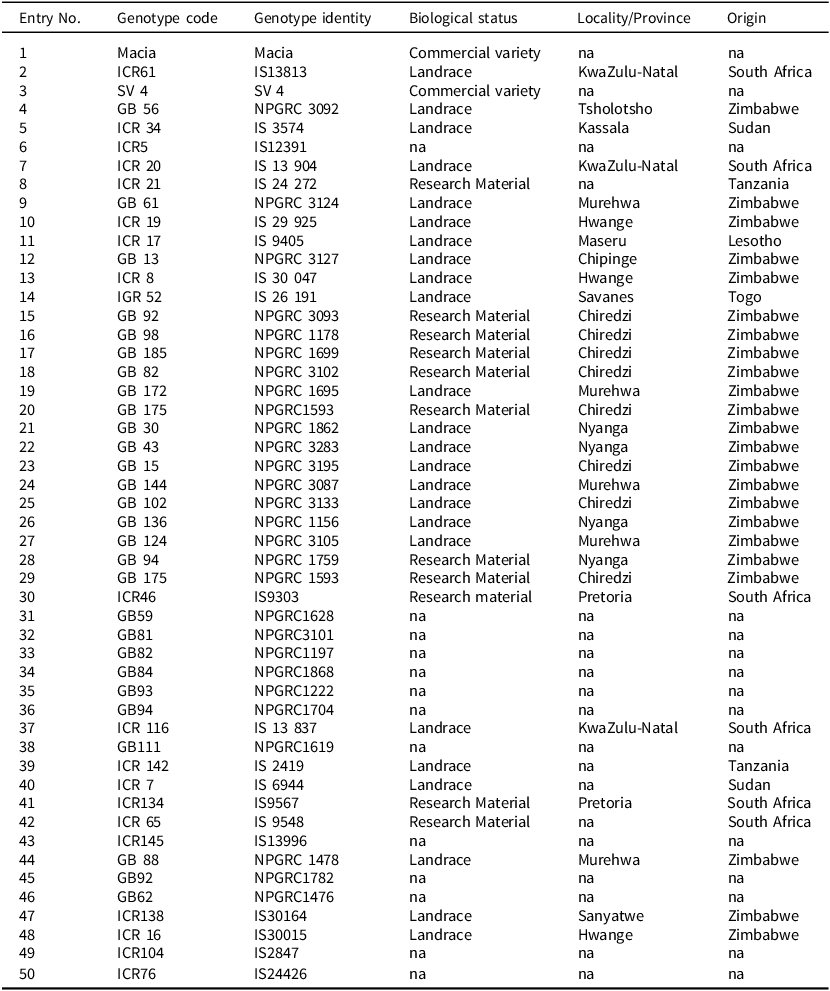
na means that biodata was not obtained or is unknown.
Experiments
The study consisted of two experiments, a laboratory experiment that assessed the effects of stress on 7-day-old seedlings of sorghum genotypes termed the early seedling stage and a pot experiment that assessed the effects of stressors on 21-day-old seedlings. This represents the late seedling stage and was carried out in an open field. Heat stress was induced in modified temperature-controlled walk-in growth chambers (Samsung Split Type air conditioner, HP124CNI model, Harare, Zimbabwe) under artificial light provided by cool white compact fluorescent lamps (Amberlite, Jiangsu, China) providing a light intensity (PAR) of 672 μmol/m2/second with uniform distribution set at room temperatures of 22–25ºC for the non-heat-stressed treatments and 45ºC for the heat-stressed treatments regulated by the forced air-conditioning.
Laboratory experiment: drought and heat stresses at early seedling stage
A two-factorial laboratory experiment was laid out following a split-plot arrangement in a completely randomized design with two replicates. The two factors were stress and genotype. The main factor was stress at four levels: heat stress alone (HS, seedlings placed in distilled water and kept at a controlled atmospheric temperature of 45°C); drought stress alone (DS, 20% PEG solution at 25°C); combined heat and drought stresses (HDS, 20% PEG solution at 45°C); and non-stressed (NS, distilled water at 25°C), as a control. Drought stress was artificially induced by placing seedlings in 20% PEG solution (osmotic potential of –0.85 MPa). Heat stress was induced by placing seedlings in a temperature-controlled growth chamber at 45°C. Combined heat and drought stresses were created by placing seedlings in 20% PEG solution in a controlled room at 45°C atmospheric temperature. Non-stressed environment (control) was induced by placing seedlings in containers containing 100 ml of de-ionized water (osmotic potential of 0 MPa) at 25°C. The second factor was genotype at 50 levels, i.e., 48 sorghum genotypes and two check varieties ‘Macia’ and ‘SV4’ (Table 1). Treatment combinations were as follows: a total of 50 genotypes × four growing conditions, which gave a total of 200 treatment combinations replicated twice. An experimental unit was a 250 ml plastic container with two sorghum seedlings (n = 4 for each treatment) placed in 20 ml of either distilled water or 20% PEG solution. Treatments were induced 7 days after germination and represented the early seedling stage in this study.
Sorghum seeds of similar size for each genotype were sterilized using 1% hypochlorite for 5 min and rinsed thrice using distilled water. Thereafter, 20 seeds for each genotype were germinated in Petri dishes lined with a Whatman No. 2 filter paper moistened with 7 ml of distilled water and placed in an incubator at 25°C. Initial seedling hypocotyl and root length measurements were taken at 7 days after germination before placing seedlings in plastic containers (250 ml) with roots immersed in 20 ml of either 20% PEG solution for drought stress treatments or distilled water for the control and heat stress alone. Seedlings were then left to acclimatize in a growth chamber for 24-h and containers were randomly positioned in a temperature-controlled growth chamber (22–25ºC or 45ºC) for 96 h before taking final measurements.
Pot experiment: drought and heat stresses at late seedling stage
The experiment was laid out as a split plot following a randomized complete block design with three blocks. Treatments were: control, with seedlings placed in a netted shade house with transparent roofs to allow sunlight and optimum atmospheric temperatures (22–25ºC) during the day and 18–20°C at night and watered to soil field capacity; water stress alone, with seedlings placed under optimum atmospheric temperatures and watered to 20% soil field capacity; heat stress alone, with seedlings watered to field capacity and placed under 35–45°C for 1 h between 15h00 and 16h00 every day in growth chambers (Supplementary Material Fig. S1); and combined heat and moisture stresses. Three pairs of kaylite float trays for each treatment were placed randomly in each growing condition for 14 days. Seedlings were assessed at 21 days after emergence, referred to as the ‘late seedling stage’ in this study.
Kaylite float trays (250 cells) were filled with the same amount of Kalahari sand premixed with the recommended fertilizer (7N:14P:7K + 8.5%S) at a rate of 5 g per cell, which is equivalent to 200 kg ha–1. Seedlings of each genotype were germinated in rows of 10 cells in each kaylite tray and two trays placed adjacent to each other were used as an experimental unit. Watering to substrate field capacity (until water started dripping from holes at the bottom of trays) was done using a fine rose head every two days before emergence. After emergence, watering was done when more than 50% of the seedlings began to show signs of drought stress through slight changes in leaf angle (Engelbrecht et al., Reference Engelbrecht, Tyree and Kursar2007). The seedlings were grown in natural sunlight and temperature in an open field for 21 days after emergence (DAE).
Measurements and data collection
In the early seedling stage (laboratory experiment), root and hypocotyl (germinating seedling stem) lengths were measured in two seedlings per replicate, using a digital Vernier caliper (INCCO HDCO01150, Suzhou, China) before and after treatments. Average changes in hypocotyl and root length per day were determined. Measurements before the treatments were subtracted from the measurements after the four days of being exposed to the treatments and divided by the number of days. In the late seedling stage (pot experiment), five seedlings of each genotype were randomly selected from each block after 14 days of treatment. Seedlings were carefully uprooted by pushing through the hole at the bottom of each cell, the soil was washed off, and roots and shoots were separated. Shoot length and root length were then measured with the caliper.
Data analysis
Data for the early and late seedling stages were analysed separately. Data for the early seedling stage were analysed using a two-way analysis of variance (ANOVA) test following a completely randomized design with four stress levels and fifty genotypes as the two main factors resulting in 200 treatment combinations. The experiment was replicated twice, and there were two seedlings per replication. We used the software GenStat 13th Edition (Rothamsted Research, VSN International, UK) to determine any significant differences among means of the main factors of stress, genotypes, and their interactions (Payne et al., Reference Payne, Welham and Harding2012). All the data were tested for normality and homogeneity using Shapiro-Wilk and Bartlett’s tests before analysis. Where significant differences were found at p ≤ 0.05, the means were separated using Bonferroni’s test. In the late seedling stage, data for five seedlings selected in each block for mean daily shoot and root length growth were evaluated for the additive main effects and multiplicative interaction (AMMI) in GenStat 13th edition. AMMI is a multivariate technique that uses the analysis variance and principal component analysis to show the effects of the genotype, environment, and their interactive effects (Gauch, Reference Gauch1992). The AMMI2 model family was used to fit the additive effects of the two components, i.e., fifty genotypes (G), four induced stress conditions (E), and their multiplicative effects for G × E interaction (GEI). AMMI was complemented by the genotype main effects plus Genotype × Environment interaction (GGE2) biplots, and which-won-where graphs (Yan and Tinker, Reference Yan and Tinker2006) which were plotted to qualitatively assess and visualize genotypes performance.
Results
Root and hypocotyl growth of sorghum at early seedling stage
The analysis of variance of average growth of seedlings root and hypocotyl length per day for the 50 assessed sorghum genotypes showed highly significant (p < 0.001) main effects of stress, genotype, and their interaction (Table 2). Stress contributed the greatest variation (51.2%) on shoot length while root length was chiefly influenced by the genotype × stress interaction (30.7%) at the early seedling stage.
Table 2. Two-way analysis of variance of the main effects of in-vitro-induced stress (E), genotypes (G), and their interactions (G × E) on root and hypocotyl length growth per day of 7-day-old sorghum seedlings
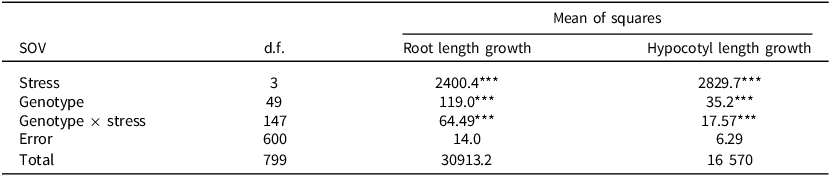
SOV is sources of variation, d.f. is degrees of freedom, *** means significant differences at p < 0.001, n = 4 for each treatment.
Combined drought and heat stresses had the most adverse effect on the mean daily hypocotyl length growth of sorghum seedlings, though it was not significantly different from that of heat stress applied separately (Table 3). Combined stresses reduced hypocotyl length by 77.5%. No significant differences were observed among the three stressful conditions assessed, i.e., heat stress alone, drought stress alone, and combined stresses, on root length growth per day at an ‘early seedling stage’, though seedlings that were exposed to combined stresses had the lowest mean daily length elongation (Table 3).
Table 3. Mean hypocotyl and root length growth per day along the experiment in 7-day-old seedlings of fifty sorghum genotypes assessed in four in vitro-induced environments
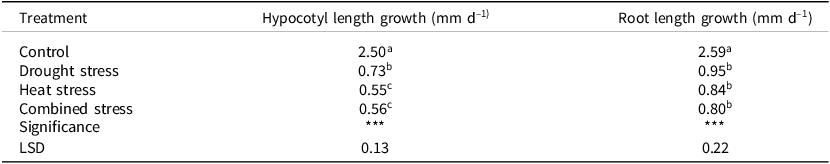
Means superscripted with different letters in the same column were significantly different at p < 0.05, *** significant at p < 0.001, n = 4 for each treatment.
The main effects of the genotype significantly influenced hypocotyl and root length growth in sorghum seedlings at the early seedling stage. Genotype IS13904 significantly outperformed 90% of the genotypes assessed in hypocotyl length growth per day while genotype IS3574 had a mean daily hypocotyl length growth significantly higher than 84% of genotypes (Table 4). Other genotypes that exhibited dominance in hypocotyl growth were NPGRC3092 and IS24272. Genotypes IS13904 and IS3574 also showed dominance in root growth at early seedling stage assessed on 7-day-old seedlings, outperforming 74% of their counterparts. The two check varieties ‘Macia’ and ‘SV4’ were amongst the lowest performing genotypes in hypocotyl and root elongation per day and were outperformed by 16% of the genotypes under study. The other poorest-performing genotypes included IS26191, IS12391, and NPGRC1782 (Table 4). Other genotypes that exhibited poor performance in root length growth at early seedling were NPGRC1592, NPGRC1178, and IS26191.
Table 4. Root and hypocotyl growth per day along the experiment in 7-day-old seedlings of 50 sorghum genotypes
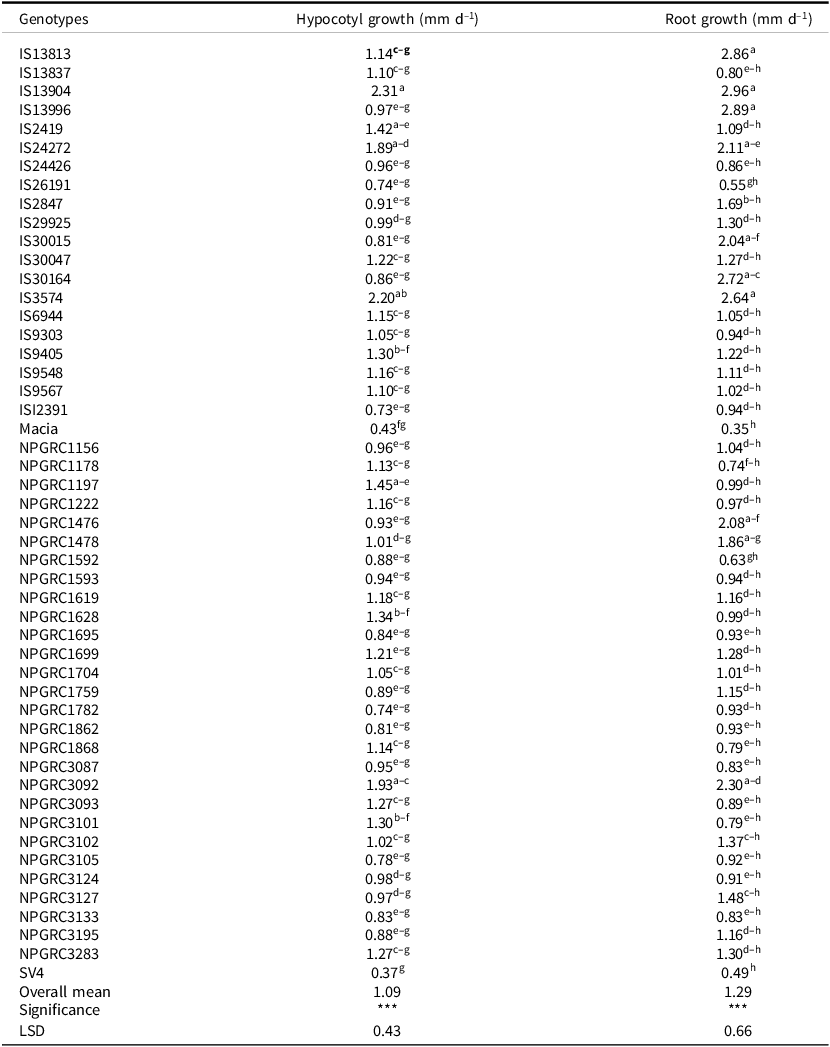
Means superscripted with different letters in the same column were significantly different at p < 0.05, *** significant at p < 0.001, n = 4 for each treatment combination.
Following the observed highly significant interaction of the genotype and environment on both root and hypocotyl growth in sorghum seedlings assessed on 7-day-old seedlings, most genotypes exhibited higher mean daily hypocotyl and root length growth under non-stressed conditions. However, it is noteworthy that genotypes IS13904, IS3574, IS9405, and NPGRC3124 showed no significant differences in hypocotyl length under heat stress when compared with the non-stressed treatment (Table 5). Genotypes IS3574, NPGRC1699, IS30047, NPGRC3097, and NPGRC1197 also showed resilience in root length growth under drought stress applied separately (Table 5). Genotypes NPGRC1476 and NPGRC1478 also exhibited dominance to combined stresses in terms of root growth (Table 5). Genotype IS3574 exhibited the highest daily hypocotyl length changes in all three stressed environments. Genotype IS13813 showed superiority in root length growth under heat stress and drought stress and genotype NPGRC1476 showed superiority under combined stresses at the early seedling stage. The two check varieties ‘SV4’ and ‘Macia’ together with genotypes IS29925, IS12391, and IS26191 showed poor performance in both hypocotyl and root length growth under the three stress treatments, and even their non-stressed seedlings were outperformed by most genotypes under stressful conditions (Table 5).
Table 5. Mean daily root and hypocotyl length growth for 7-day-old sorghum seedlings as influenced by stressful conditions (heat stress, HS; drought stress, DS; combined stresses, HDS) and genotypes. As a reference, plants were not stressed (NS)
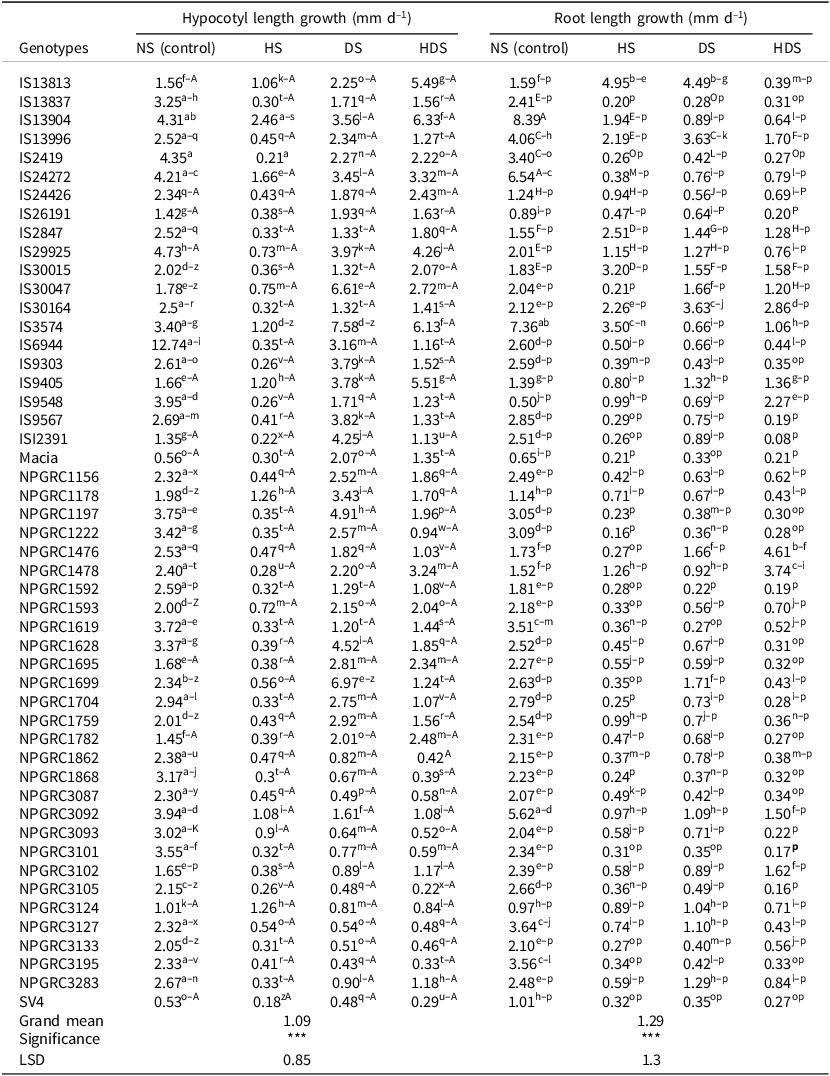
Genotype means with similar superscripted letters in the same column were not significantly different at p < 0.05, *** means significant difference at p < 0.001, n = 4 for each treatment.
Following the observed significant interaction of genotypes and stress conditions particularly in hypocotyl length of sorghum seedlings at the early seedling stage (Table 5), data were further plotted into a which-won-where graphical display of the GGE biplot (Figure 1) which visually displays the responsiveness and performance of genotypes in the tested stress conditions for hypocotyl length at early seedling stage. The first two principal components accounted for 89% of the total variation of the G × E interaction for hypocotyl length. The which-won-where polygon view of the GGE biplot shows that for daily hypocotyl length changes, genotypes IS3574 and IS13904 highlighted in yellow fell on the furthest vertex of the polygon. The two genotypes fell inside the sector highlighted in blue containing two stress conditions, i.e., heat stress and drought stress applied separately (circled in blue in Figure 1), this explains their higher responsiveness and performance in hypocotyl daily length growth under these two conditions. Most genotypes were positioned within the polygon and close to the origin of the biplot, indicating that these genotypes had the least performance in any of the stress environments tested.
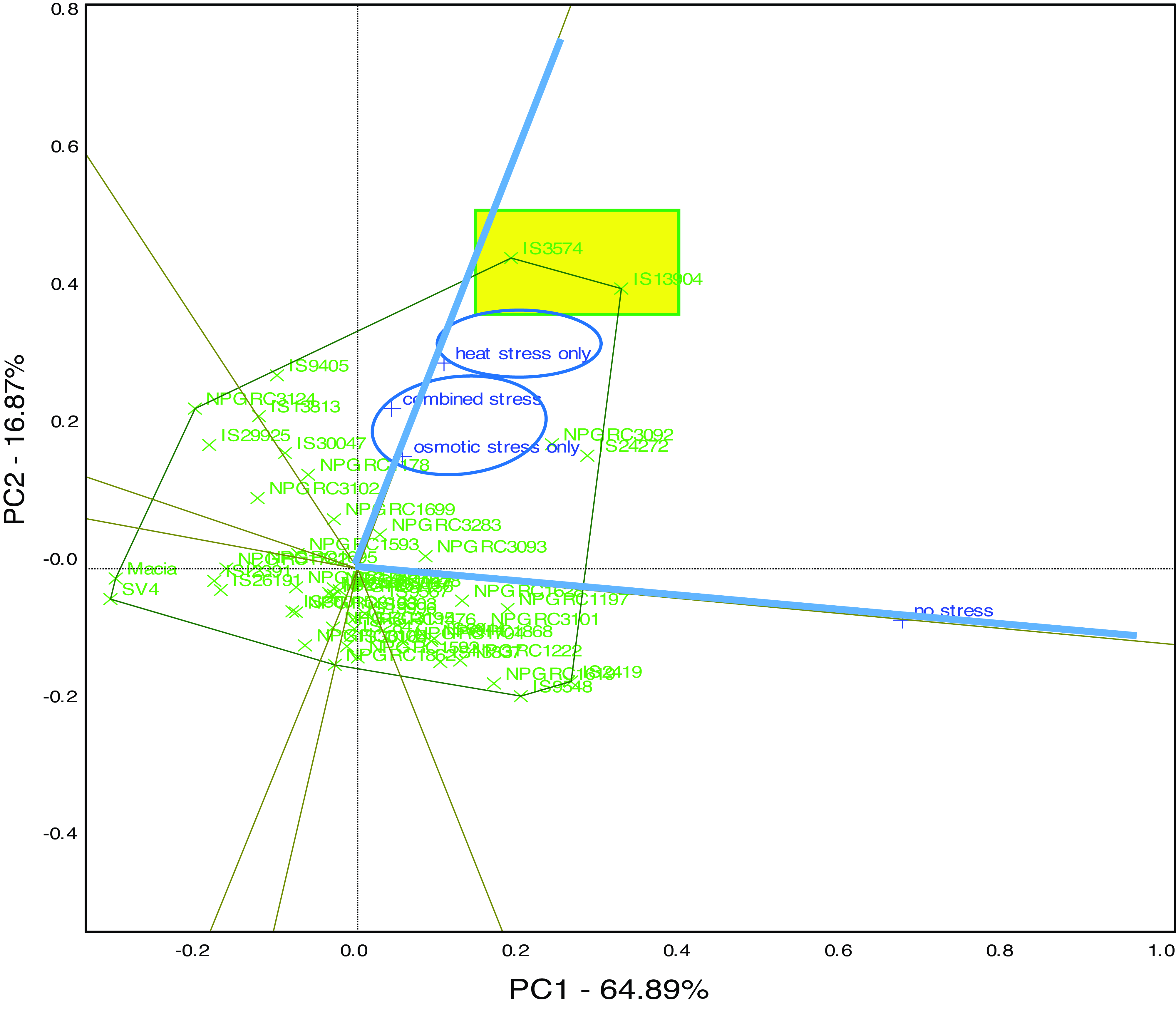
Figure 1. The GGE biplot and which-won-where polygon view for hypocotyl length growth per day of 50 sorghum genotypes at the early seedling stage and subjected to heat stress, drought stress, combined stresses, and no stress conditions. The experiment was replicated twice, and there were four seedlings for each treatment. Genotypes highlighted in yellow at the vertex of the polygon had an outstanding performance under the two stress conditions circled in blue within the blue sector containing that particular vertex.
In a similar manner, a GGE biplot (Figure 2) which visually displays the genotypes that performed exceptionally well in tested conditions for root length growth per day at the early seedling stage was plotted. The first two principal components accounted for 77% of the total variation of the G × E interaction for root length. The which-won-where polygon view of the GGE biplot shows that for root length the vertex genotype was IS9303 (highlighted in yellow). The genotype fell inside the blue sector of the polygon containing combined stresses and no stress (circled in blue) signifying its higher responsiveness and performance in terms of daily root length growth under the two conditions at the late seedling stage. Genotype IS24272 highlighted in orange fell at a similar furthest vertex from the origin but in a sector indicated in red that contains no test condition placing it as the most undesirable genotype for the tested conditions in terms of root growth at the early seedling stage.
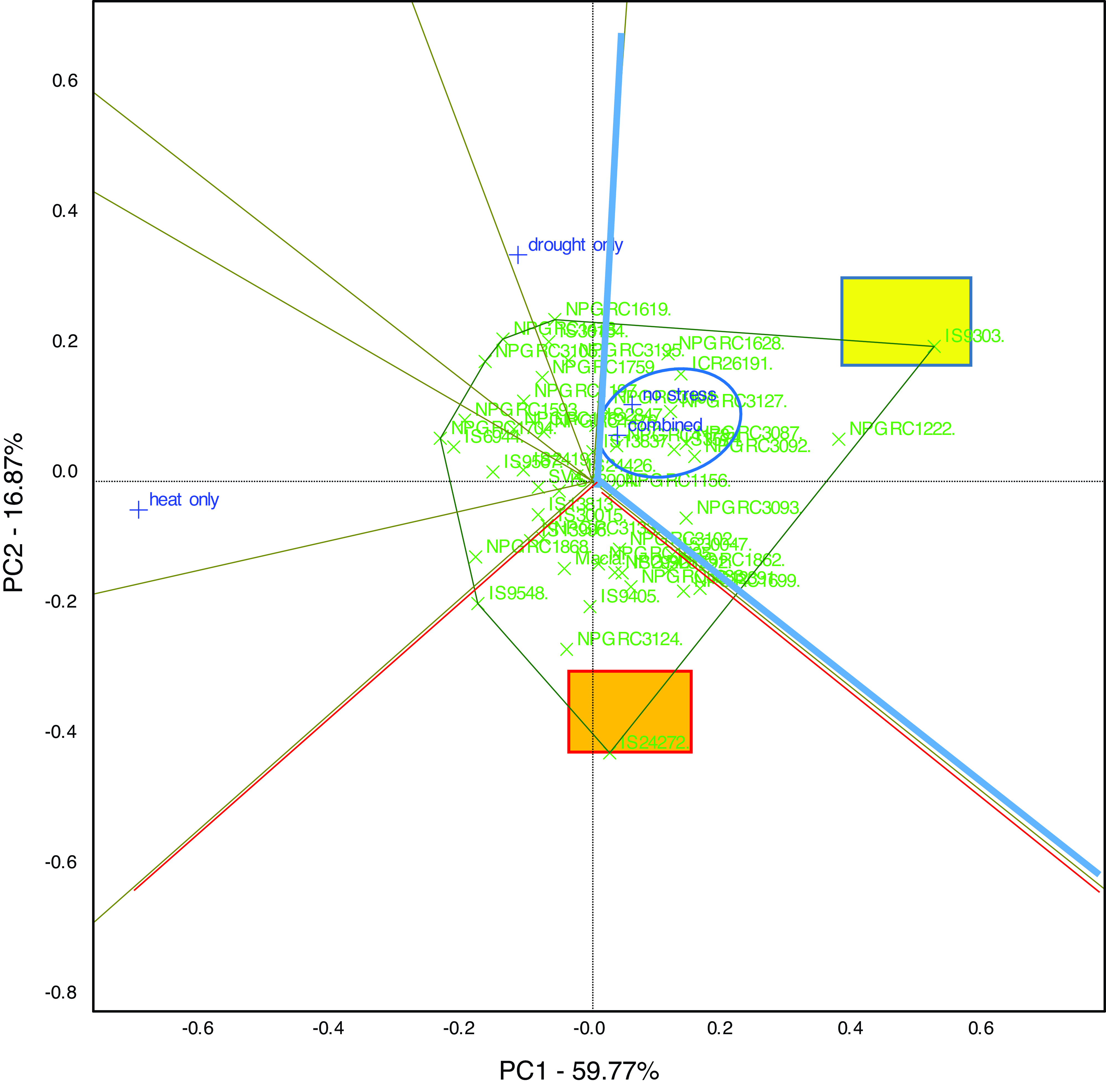
Figure 2. The GGE biplot and which-won-where polygon view for root length growth per day of 50 sorghum genotypes at the early seedling stage and subjected to heat stress, drought stress, combined stresses, and no stress conditions. The genotype highlighted in yellow at the vertex contained within the blue sector of the polygon had an outstanding performance under the conditions circled in blue within the sector containing that particular vertex. The genotype highlighted in orange within the red sector had the lowest performance for the tested conditions.
Root and shoot growth of sorghum at late seedling stage
AMMI revealed that the effects of genotypes, artificial stress conditions, and G × E interaction were all highly significant (p < 0.001) for root and shoot length growth per day at the late seedling stage (Table 6). AMMI analysis indicated the highest proportion of total sum of squares attributed to the induced stress levels at the late seedling stage was on shoot length (25.6%), with 6.5% attributed to genotypic effects, and 13.5% to the G × E interaction effects (Table 6). A weaker contribution of the genotype and environmental effects was noticed on root length, i.e., 5.9% and 2.3% respectively, while the interaction of the two combined stresses was higher (17.1%).
Table 6. AMMI analysis for root and shoot length growth per day at the late seedling stage of 50 sorghum accessions evaluated in four simulated conditions
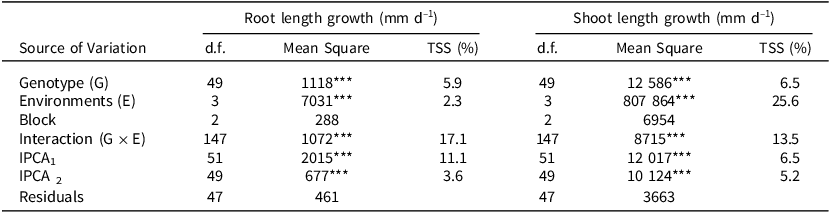
d.f. = degrees of freedom, *** = significant at p < 0.001, TSS = Total sum of squares explained, IPCA = Interaction principal component axes, n = 15 for each treatment.
Four models for the two parameters of the AMMI model family indicated the best-performing sorghum genotypes under drought, heat, and combined stresses (Table 7). Seedlings exposed to combined heat and drought stresses had the lowest shoot length growth (7.71 mm per day), followed by the drought stress (visualized in Supplementary Material Fig. S2). Mean root length growth for seedlings exposed to drought stress alone was 4.47 mm per day, which was close to heat stress and combined stresses (Table 7). Mean shoot length growth showed a higher variability than the root length growth. The best performers under drought, in terms of root length growth shown in bold text, were IS6944 and NPGRC1478 (Table 7). While genotypes IS9527, IS30164, and IS30015 exhibited resilience across the three stress conditions in terms of shoot length growth, while IS6944 and NPGRC1478 were amongst the best performers in root length growth in at least two stressed environments that were tested (Table 7).
Table 7. Best performing sorghum genotypes under four conditions (heat stress, HS; drought stress, DS; combined stresses, HDS; and non-stressed conditions, NS) based on seedling root (RLG) and shoot (SLG) length growth at late seedling stage according to the AMMI2 model family
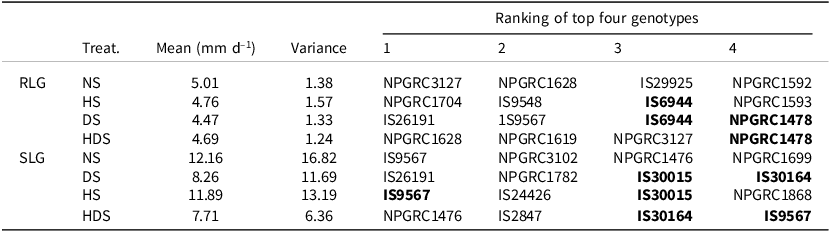
Genotypes in bold appeared as the top four performers in more than one stress condition. Fifty genotypes were assessed under four growing conditions, being replicated three times with five seedlings (n = 15).
Genotype IS30164 showed superior performance in both shoot and root length growth under heat stress alone and combined stresses. Genotypes NPGRC1476, IS2847, IS30164, and IS9567 were the best performers for shoot length growth under combined stresses (Table 7). Under drought stress, which was observed as the second most suppressing test environment for shoot length growth (Fig. S2), the best-performing genotypes were IS26191, NPGRC1782, IS30015, and IS30164. Good performers for shoot growth were not necessarily the best for root growth, except for IS26191 under drought stress.
Discussion
The adverse effects of heat stress and combined stresses on shoot length growth of sorghum were more intense than that of drought stress. However, heat stress alone, drought stress alone, and these two stresses combined had a similar suppressive effect on root length growth, though singly applied drought stress caused less injury than heat stress and combined stresses. ANOVA and AMMI revealed a highly significant influence of genotype, stress, and their interactions on root and shoot length growth at the late seedling stage (Table 7). In most studies, drought stress was found to be less detrimental to seedling root growth, probably because plants tend to prioritize water uptake and invest more resources in roots than in shoots at early growth stages (Ehdaie et al., Reference Ehdaie, Layne and Waines2012; Ahmadi et al., Reference Ahmadi, Pour-Aboughadareh, Fabriki-Ourang, Mehrabi and Siddique2018). In this regard, our findings are in agreement with other studies on sorghum (Craufurd and Peacock, Reference Craufurd and Peacock1993; Jagtap et al., Reference Jagtap, Bhargava, Streb and Feierabend1998), wheat (Machado and Paulsen, Reference Machado and Paulsen2001; Keles and Oncel, Reference Keles and Oncel2002), pearl millet (Cairns et al., Reference Cairns, Crossa, Zaidi, Grudloyma, Sanchez, Luis Araus, Thaitad, Makumbi, Magorokosho, Bänziger, Menkir, Hearne and Atlin2013; Yadav et al., Reference Yadav, Arya, Singh, Kumar and Panchta2016), and maize (Meseka et al., Reference Meseka, Menkir, Bossey and Mengesha2018; Tandzi et al., Reference Tandzi, Bradley and Mutengwa2018; Shivhare and Lata, Reference Shivhare and Lata2019; Wipf et al., Reference Wipf, Bùi and Coleman-Derr2021; Khalifa and Eltahir, Reference Khalifa and Eltahir2023) that reported more injurious effects of combined heat and drought stresses on shoot growth. The adverse effects of heat stress on shoots are possibly attributed to increased levels of reactive oxygen species that damage biomolecules and affect mostly the foliage in delicate seedlings (Gosavi et al., Reference Gosavi, Jadhav, Kale, Gadakh, Pawar and Chimote2014). The significant influence of genotype, stress conditions, and their interactions on root and hypocotyl (shoot) length at both early and late seedling stages is also in line with several reports for various crops, in which seedling root and shoot growth are regulated by genetics and environmental effects (Bibi et al., Reference Bibi, Sadaqat, Tahir and Akram2012; Ehdaie et al., Reference Ehdaie, Layne and Waines2012; Ye et al., Reference Ye, Roorkiwal, Valliyodan, Zhou, Chen and Varshney2018; Abro et al., Reference Abro, Memon, Abro, Sam, He, Rind, Memon, Solangi, Muhammad, Ali, Ahmed, Dev, Abro, Rajput, Nizamani, Nargis and Kumbhar2020). The shoot is more sensitive to stresses like drought and heat because several essential metabolic reactions occur in leaves (Pyngrope et al., Reference Pyngrope, Bhoomika and Dubey2013; Tripathi et al., Reference Tripathi, Nam, Oldenburg and Bendich2020).
Roots are the first organs to detect drought stress in the soil, and defence mechanisms are signalled to enable adjustments of metabolic and physiological processes that conserve water and promote root length at the expense of shoot growth, hence offering roots some level of tolerance to mild water deficit more than the aboveground parts (Zahra, Reference Zahra2012). Water uptake during drought stress is maintained through osmotic adjustment in plants (Patanè et al., Reference Patané, Saita and Tubeileh2012; Devnarain et al., Reference Devnarain, Crampton, Chikwamba, Becker and O’Kennedy2016; Suzuki et al., Reference Suzuki, Rivero, Shulaev, Blumwald and Mittler2014; dos Santos et al., Reference dos Santos, Ribas, de Souza, Budzinski and Domingues2022), and increased root length under limited soil water conditions enables the plant to reach deeper soil levels to absorb water (Ye et al., Reference Ye, Roorkiwal, Valliyodan, Zhou, Chen and Varshney2018; Osmolovskaya et al., Reference Osmolovskaya, Shumilina, Kim, Didio, Grishina, Bilova, Keltsieva, Zhukov, Tikhonovich, Tarakhovskaya and Frolov2018). Thus, root length has been proposed as a good indicator of drought tolerance, an important trait for seedling establishment under mild drought conditions (Queiroz et al., Reference Queiroz, Oliveira, Steiner, Zuffo, Zoz, Vendruscolo, Silva, Mello, Cabral and Menis2019a; Rajendra Prasad et al., Reference Rajendra Prasad, Govindaraj, Djanaguiraman, Djalovic, Shailani, Rawat, Singla-Pareek, Pareek and Vara Prasad2021). It is common for certain traits to respond to one stress and not the other (Vile et al., Reference Vile, Pervent, Belluau, Vasseur, Bresson, Muller, Granier and Simonneau2012), and the differences in the response of root and shoot morphological traits to heat, drought, and combined stresses in sorghum seedlings observed herein are in agreement with Machado and Paulsen (Reference Machado and Paulsen2001), Tsuji et al., (Reference Tsuji, Inanaga, Araki, Morita, An and Sonobe2005), Magalhães et al., (Reference Magalhães, Souza, Lavinsky, Pereira, Albuquerque, De and Castro2016), Wasaya et al., (Reference Wasaya, Zhang, Fang and Yan2018), Ahmadi et al. (Reference Ahmadi, Pour-Aboughadareh, Fabriki-Ourang, Mehrabi and Siddique2018), Rajendra Prasad et al. (Reference Rajendra Prasad, Govindaraj, Djanaguiraman, Djalovic, Shailani, Rawat, Singla-Pareek, Pareek and Vara Prasad2021).
Unlike at the early seedling stage, drought stress suppressed root growth at the late seedling stage more than combined stresses and heat stress applied individually. These results are contrary to most research findings where root length has been identified as less sensitive to drought stress applied separately (Ehdaie et al., Reference Ehdaie, Layne and Waines2012; Chaniago et al., Reference Chaniago, Syarif and Riviona2017; Abro et al., Reference Abro, Memon, Abro, Sam, He, Rind, Memon, Solangi, Muhammad, Ali, Ahmed, Dev, Abro, Rajput, Nizamani, Nargis and Kumbhar2020). Perhaps the induced drought stress levels in the potted experiment were too severe given the limited amount of soil present in cells of kaylite trays, which led to rapid cellular dehydration reducing cell division, expansion and elongation, eventually reducing the growth of seedlings (Keskin et al., Reference Keskin, Tumer and Birinci2010; Tsago et al., Reference Tsago, Andargie and Takele2014). The observed differences in the response of roots and shoots to stressful conditions at the two assessed seedling stages could be suggestive of independent shoot and root physiological responses to heat and drought stress, which is stage-dependent in as much as it is genotype-dependent. Differences in stress detection, signal transduction, and gene expression in response to stress by sorghum genotypes are the bases of the differential response among genotypes when dealing with stress levels (Rizhsky et al., Reference Rizhsky, Liang and Mittler2002; Conde et al., Reference Conde, Chaves and Gerós2011). Thus, the tested conditions were effective in segregating the genotypes in terms of shoot and root length growth. This implies that elite genotypes can be selected at the seedling stage based on their specific and broad adaptations to a given environment (De Vita et al., Reference De Vita, Mastrangelo, Matteu, Mazzucotelli, Virzì, Palumbo, Storto, Rizza and Cattivelli2010).
Genotype IS30164 showed tolerance to drought stress alone and to combined drought and heat stresses but not to heat stress applied separately at both seedling stages. From the GGE biplot for shoot length at early growth stage (Figure 1), it can be inferred that the genotype also exhibited dynamic stability and specific adaptability which means consistently high performance above the mean to specific environments (De Vita et al., Reference De Vita, Mastrangelo, Matteu, Mazzucotelli, Virzì, Palumbo, Storto, Rizza and Cattivelli2010). Such cross-tolerance in sorghum genotypes means that exposure to one of these two stressors stimulates the tolerance to the other (Christine et al., Reference Christine, Brwa, Jack and Robert2016; Woldesemayat et al., Reference Woldesemayat, Modise, Gemeildien, Ndimba and Christoffels2018; Rajendra Prasad et al., Reference Rajendra Prasad, Govindaraj, Djanaguiraman, Djalovic, Shailani, Rawat, Singla-Pareek, Pareek and Vara Prasad2021; Wipf et al., Reference Wipf, Bùi and Coleman-Derr2021; Hassan et al., Reference Hassan, Ahmed and Rashed2022). The observations also point to the existence of synergistic effects of drought and heat stresses and existence of overlapping defence mechanisms to both stresses in sorghum (Suzuki et al., Reference Suzuki, Rivero, Shulaev, Blumwald and Mittler2014). NPGRC1476 with high performance to combined stresses when considering shoot length (Table 3) and NPGRC3105 tolerant to heat stress based on root length (Table 4) were among the dynamic stable genotypes to note. Such genotypes are responsive to favourable environmental conditions (Setimela et al., Reference Setimela, Lunduka, Zaman-Allah, Ndoro and Cairns2017).
The GGE biplots displayed specific adaptation of genotypes to the tested conditions and showed potential adaptation of IS3574 and IS13904 to drought and heat stresses applied to shoots at early seedling stage (Figure 1), while genotype IS9303 displayed tolerance to combined stresses applied to roots (Figure 2) at early seedling stage. This implies that the genotypes sustained higher shoot or root growth of seedlings in the tested conditions, showing less variability in performance between the stressed and non-stressed conditions (De Vita et al., Reference De Vita, Mastrangelo, Matteu, Mazzucotelli, Virzì, Palumbo, Storto, Rizza and Cattivelli2010). ‘Macia’ and ‘SV4’ used as check varieties in this study were bred for their biological stability using locally adapted genotypes to improve their adaptability to suit smallholder farmers with minimum resources in marginal areas; hence, they exhibit minimal fluctuations in their phenotypic expression regardless of changes in the surrounding environment. Genotypes like IS9567 exhibited such traits of high performance in all the environments, and of course, some poor performing and erratic cases like genotypes IS24272 and NPGRC3093 showed contrary performance which may render them less desirable.
In general, the tolerance of sorghum genotypes to heat and drought stresses is attributed to their origins (Enyew et al., Reference Enyew, Carlsson, Geleta, Tesfaye, Hammenhag, Seyoum and Feyissa2022). A significant number of genotypes like NPGRC1478, IS3574, and IS13904 that exhibited tolerance to combined drought and heat are local landraces of Zimbabwe and from regional areas in the sub-Saharan region of Africa such as KwaZulu-Natal Province, a typically dry and hot area in neighbouring South Africa (Table 1). Dugas et al. (Reference Dugas, Monaco, Olsen, Klein, Kumari, Ware and Klein2011) explained the development of heritable morphological characteristics like the rooting system, which enhances tolerance to drought and heat stresses. Continuous exposure to an environmental stressor alters biological functioning such as time for sensing external stimuli and activation of defence mechanisms rendering differences in tolerance to abiotic stress among genotypes (Gong et al., Reference Gong, Chen, Li and Guo2001; dos Santos et al., Reference dos Santos, Ribas, de Souza, Budzinski and Domingues2022; Guihur et al., Reference Guihur, Rebeaud and Goloubinoff2022). Given the diversity of agro-ecological regions where the assessed germplasm was collected from, such varied responses to the stresses were expected.
Conclusions
The study investigated the effects of drought, heat, and combined stresses on root and shoot length of 50 sorghum genotypes at the early and late seedling stages. Combined heat and drought stresses were found to significantly suppress shoot length at both seedling stages. Root length growth was equally suppressed by all the stress treatments at the early seedling stage while drought stress alone was found to be the most adverse condition impairing root growth at the late seedling stage of sorghum. The highest mean performance in shoot length and specific adaptation to drought and heat stress applied separately was exhibited in IS3574 and IS13904 but not under combined stresses at the early seedling stage. In root length at the early seedling stage, IS13813 showed superior performance in all three stressed conditions while genotype IS9303 showed specific adaptability to combined stresses. At late seedling stage genotypes NPGRC1478, NPGRC1619, and NPGRC3105 were identified as the best performers in more than one type of stress for either root or shoot growth. For both shoot and root lengths at both seedling stages, IS30164 showed the most tolerance to drought stress alone and in combined stresses. We recommend NPGRC1478 and IS13904 for breeding programmes that seek to improve sorghum tolerance to combined stresses at the seedling stage for cropping in marginal areas of the arid and semi-arid tropics. There is empirical evidence of a positive correlation between final grain yield and sorghum root and shoot development at seedling stages. Further evaluation of these two genotypes’ tolerance to combined stresses at pre- and post-anthesis stages is needed under field conditions.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0014479725100082
Data availability statement
The raw data that support the findings will be available in [Mendeley] at DOI:10.17632/dkff5gcyns.1 following a [6 month] embargo from 16 November 2023 to allow publication of the article first.
Acknowledgements
Genetic Resources and Biotechnology Institute for the landraces, International Crop Resources Institute for Semi-Arid Tropics, Bulawayo, Zimbabwe for a generous donation of germplasm.
Author contributions
Conceptualization (EN), acquisition of research materials (EN; MM), data collection (EN), data analysis (EN, MM), writing of original draft (EN, MM, JVS). All authors read and approved the final manuscript.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.












