Highlights
-
As systemic and local inflammation are central in CSDH pathophysiology, CRP may be an important component in the development of CSDH.
-
Increased CRP levels at time of second surgery for CSDH may demonstrate a constantly evolving inflammatory process toward the development of recurrence.
-
CRP levels cannot predict CSDH recurrence rates.
Background
Local inflammation plays an important role in the pathophysiology of chronic subdural hematoma (CSDH), as seen by the elevated levels of local inflammatory biomarkers. Reference Edlmann, Giorgi-Coll, Whitfield, Carpenter and Hutchinson1 Biomarkers have previously shown clinical potential as predictors for hematoma recurrence and potential targets for anti-inflammatory treatment. Reference Jensen, Binderup, Olsen, Kjaer and Fugleholm2,Reference Jensen, Olsen, Lelkaitis, Kjaer, Binderup and Fugleholm3 However, relying on experimental biomarkers as predictors for recurrence Reference Jensen, Olsen, Lelkaitis, Kjaer, Binderup and Fugleholm3 is clinically less feasible than using routine markers such as C-reactive protein (CRP).
CRP is an important part of the innate immune system and acts as an acute-phase reactant produced in response to systemic or local inflammation, infection or tissue damage. Reference Black, Kushner and Samols8 CRP is mainly expressed and produced by hepatocytes as a native pentameric CRP (nCRP) that can also dissociate into a monomeric form (mCRP) and has a normal plasma level below 0.3 mg/dL. Reference Nehring, Goyal and Patel9 Both CRP conformations play an important role in systemic inflammation, with nCRP as an important activator of the complement system. Reference Torzewski, Torzewski and Bowyer10 mCRP is intrinsically expressed in brain microvessels, astrocytes and neurons, as well as demonstrating angiogenic properties. Reference Yao, Zhang and Wu11,Reference Di Napoli, Slevin, Popa-Wagner, Singh, Lattanzi and Divani12
CSDH has a recurrence rate of 10%–30%, and research suggests that an ongoing inflammatory process nourishes the hematoma, potentially leading to recurrence. Reference Edlmann, Giorgi-Coll, Whitfield, Carpenter and Hutchinson1,Reference Kolias, Chari, Santarius and Hutchinson13,Reference Weigel, Schmiedek and Krauss14 CRP levels are increased by inflammatory cytokines, particularly interleukin (IL)-6. Reference Wu, Potempa, El Kebir and Filep15 Especially IL-6 has been linked to the pathophysiology of hematoma enlargement in CSDH, and the local level of IL-6 is increased in the hematoma fluid from patients with recurrent CSDH. Reference Pripp and Stanišić7,Reference Frati, Salvati and Mainiero16,Reference Stanisic, Aasen and Pripp17 The role of CRP in CSDH remains poorly understood but seems to be connected to the inflammatory and angiogenetic probabilities of CSDH (18–22). Therefore, CRP may have a role in the development of both primary and recurrent CSDH.
We aimed to explore the role of CRP in patients with CSDH by (1) comparing the systemic and subdural levels of CRP, (2) investigating if CRP can be used as a prognostic predictor for recurrence and death and (3) investigating CRP in the pathophysiology of recurrent CSDH by comparing CRP levels between the first and second operations in patients with recurrent CSDH.
Methods
Study cohort
Adult patients (18 years or older) diagnosed with CSDH by CT orMRI were randomly included in the period between January 2020 and September 2021. Patients with head trauma up to 14 days before surgery were excluded to avoid patients with acute subdural hematomas. Also, patients with previous intracranial surgical treatments were excluded. The following patient variables were registered at the time of admission: sex, age, history of head trauma, use of blood thinning medications, preoperative symptoms and preoperative comorbidity according to the Charlson comorbidity index. Reference Charlson, Carrozzino, Guidi and Patierno23 The radiological variables included midline shift, hematoma volume calculated by the XYZ/2-method, Reference Sucu, Gokmen and Gelal24 localization and radiological subtype. Reference Jensen, Andersen-Ranberg, Poulsen, Bergholt, Hundsholt and Fugleholm25 To investigate if systemic infection affected CRP levels between patients with and without recurrent CSDH, treatment with non-prophylactic antibiotics in the period from the operation to two days postoperative was registered.
Bilateral hematomas were treated as two separate cases, as bilateral hematomas do not necessarily have the same radiological or molecular composition. At 90 days follow-up, recurrent CSDH and death were recorded. The definition of a recurrent CSDH was surgical evacuation of a previously treated same-sided CSDH.
Sample collection
Hematoma fluid samples were obtained through burr hole or craniotomy. To preserve the hematoma membrane and simultaneously prevent contamination of the hematoma blood with blood from surrounding tissue, the dura mater was carefully left untouched during bone removal. Once the bone had been removed, the dura mater was opened. Extraction of hematoma fluid was done with a syringe carrying a blunt needle. The outer hematoma membrane was carefully penetrated, and 10 mL of the hematoma fluid was collected. Patients were excluded if damage to the hematoma membrane caused leakage of subdural fluid prior to sample collection. Immediately after the fluid collection, the samples were transferred to silicone vacuum tubes containing a mix of protamine sulfate and ethylenediaminetetraacetic acid. Systemic venous blood was collected at the time of the operation. The hematoma fluid and systemic blood underwent centrifugation for 10 minutes at 7000 revolutions/minute to isolate the supernatants, which were then stored at −80°C, while the pellets were discarded.
Luminex
The concentrations of CRP in both the hematoma fluid and systemic blood samples were assessed using a Luminex multiplex antibody-based bead kit (Procartaplex, Thermo Fisher Scientific, Denmark), following the manufacturer’s guidelines. Samples were measured and assessed individually (simplex), while standards were measured in duplicates. Samples were initially diluted 2000-fold, and samples lying above the measuring range on the standard curve were further diluted (up to 10,000-fold) for accurate quantification. The concentrations of CRP were measured in pg/mL and recalculated to mg/L, for the values to correspond to clinical CRP values.
Statistics
Data was handled using R version 4.2.1 (R Core Team, Vienna, Austria). Continuous data was presented using median and interquartile range (IQR) due to skewness, while categorical variables were presented using n and percentage. Data was compared using the Wilcoxon signed-rank test for data from the same participant and presented using 95% Hodges–Lehmann confidence intervals (95% HLCI). Due to the severe skewness of the Luminex data, we also performed the Wilcoxon signed-rank test using log-transformed data as a sensitivity analysis. The prognostic ability of CRP was investigated using the area under the receiver operating characteristics curve (AUROC). The AUROC will be interpreted as representing “no better than chance” (∼0.5), low accuracy (0.5–0.7), moderate (0.7–0.9) and high accuracy (>0.9). Reference Schober, Mascha and Vetter26 To account for the censoring for death in the prognostication of recurrence, we performed a sensitivity analysis excluding dead participants. This is an exploratory study, and therefore, an alpha level of 0.05 for all analyses will be interpreted as significant.
Results
Study population
We included 146 hematomas from 111 patients, of whom 25 patients were operated on for recurrent CSDH, and 10 patients died within 90 days. Of the 25 patients with recurrent CSDH, 24 were male (p < 0.001). Recurrence rates were higher in patients with bilateral hematomas (p < 0.001), and patient mortality was higher in patients with a recurrent CSDH (p < 0.001). Full baseline characteristics are presented in Table 1.
Table 1. Patient demographics and comparison of patients with and without recurrent chronic subdural hematoma within 90 days
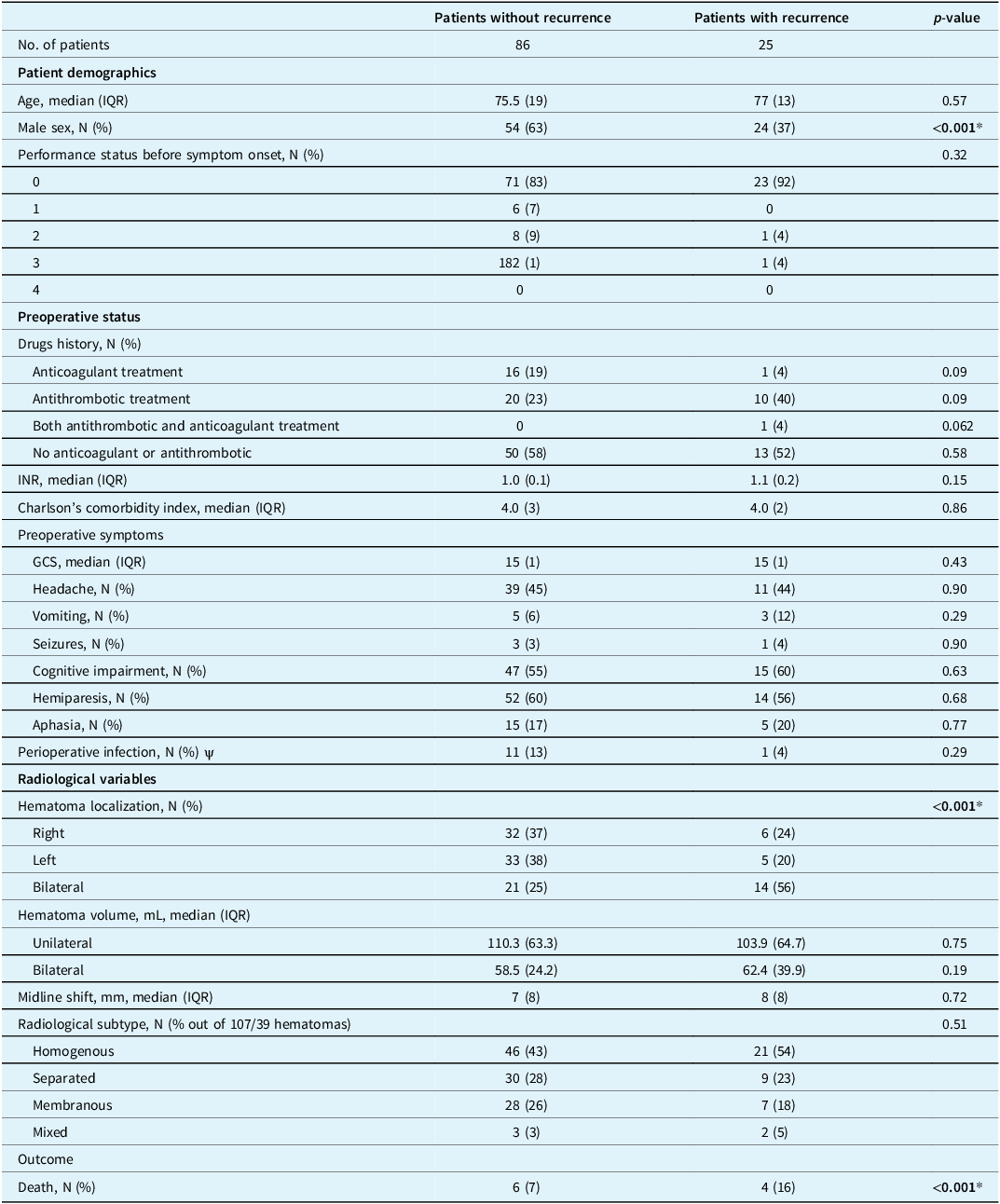
IQR = interquartile range; INR = international normalized ratio; GCS = Glasgow Coma Scale. *Statistical significant (CI 95%), ψ defined as non-prophylactic antibiotic treatment at the time of surgery or started within two days postoperative.
Systemic versus subdural CRP levels
Systemic and subdural CRP levels were 2.54 mg/L (IQR: 1.40–9.75) and 2.09 mg/L (IQR: 0.99–5.22). The median difference between systemic and subdural CRP levels was 1.03 (95% HLCI: 0.50–2.57), which was statistically significant (p < 0.0001). Both the systemic and subdural CRP levels were within the clinically defined normal CRP range in adults below 3 mg/L. Reference Nehring, Goyal and Patel9
CRP as a prognostic predictor for recurrence
Neither systemic nor subdural CRP levels were good predictors of outcome (Figure 1). To solely investigate recurrent CSDH as an outcome, we performed a sensitivity analysis, leaving out mortality. In this analysis, systemic CRP levels in patients without recurrence and with recurrence were 2.81 (1.47–12.38) and 1.76 (0.81–2.64), which predicted recurrence with low accuracy (AUC: 0.66; 95% CI: 0.55–0.76) (Table 2).
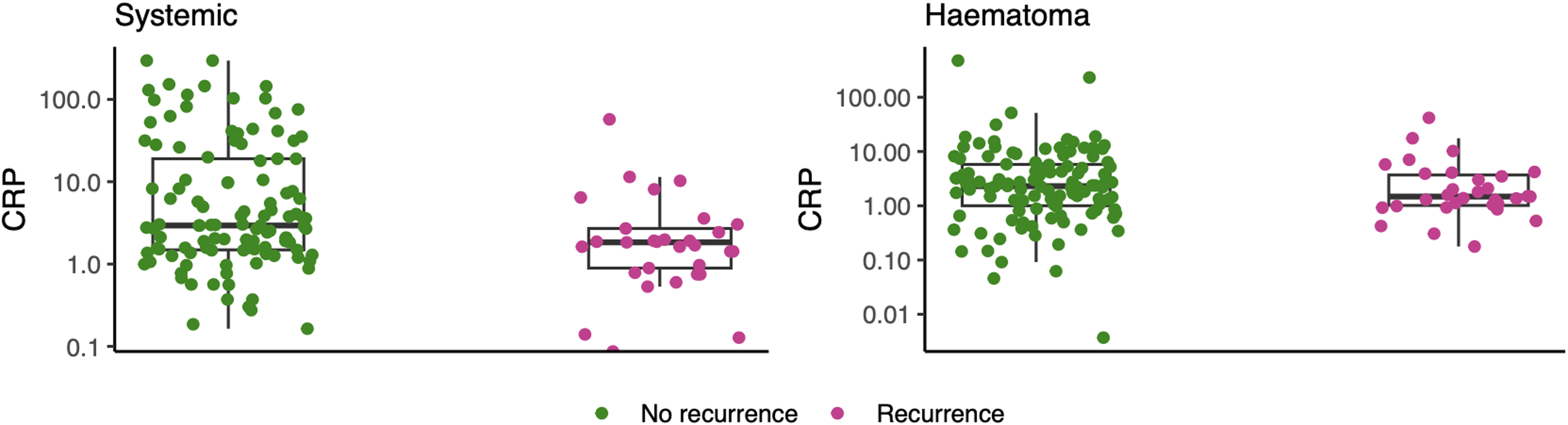
Figure 1. Box plot presenting the systemic (left figure) and subdural (right figure) C-reactive protein (CRP) levels of patients with (green) and without (purple) recurrent chronic subdural hematoma. Both systemic and subdural CRP levels were significantly different (p < 0.0001).
Table 2. This table presents CRP as a prognostic predictor for recurrent CSDH at the time of the primary CSDH evacuation
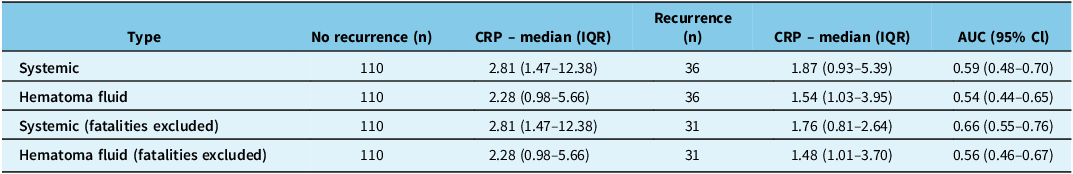
CRP as a predictor is investigated in both systemic circulation and hematoma fluid and in a sensitivity analysis from which fatalities are excluded. CRP = C-reactive protein; CSDH = chronic subdural hematoma; AUC = area under the curve.
CRP levels between the first and second operations in CSDH recurrence
For patients with recurrent CSDH, systemic and subdural CRP levels were 1.87 mg/L (IQR: 1.42–2.71) and 1.50 mg/L (IQR: 1.01–3.70) at the time of the first operation and 5.17 mg/L (IQR: 1.72–19.79) and 5.37 mg/L (IQR: 1.58–15.38) at the time of the second operation. Comparing both systemic and subdural CRP levels between the first and second operations in patients with recurrence showed significantly higher CRP levels at the time of the second surgery (p systemic = 0.004 and p subdural < 0.0001) (Figure 2). The presence of preoperative infection did not suggest any correlation to higher CRP levels (Table 1).
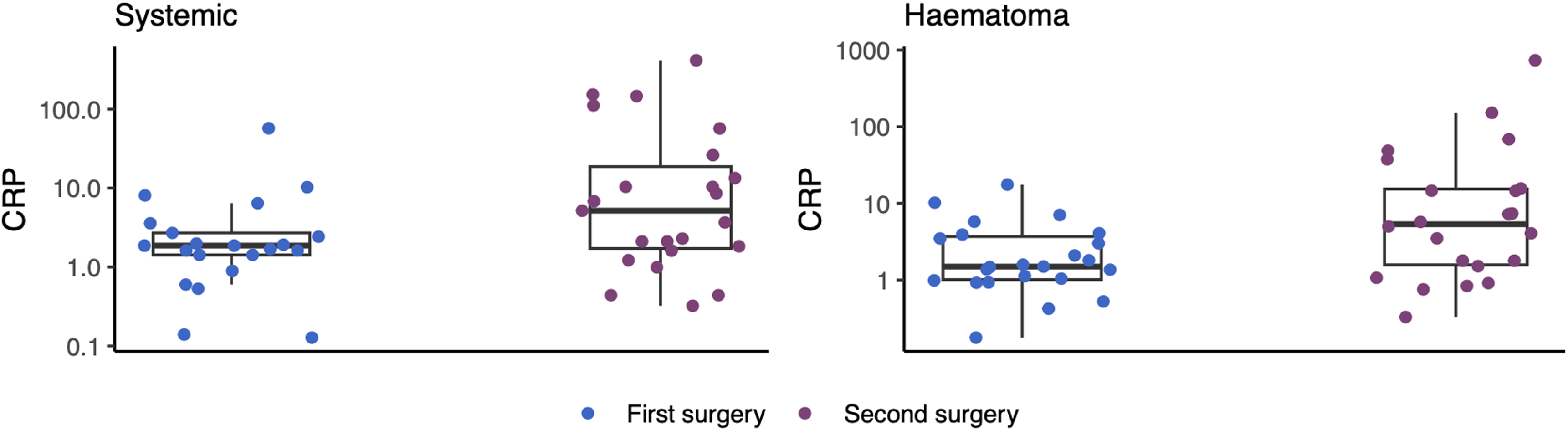
Figure 2. Box plot presenting the systemic (left figure) and subdural (right figure) C-reactive protein (CRP) levels between the first (blue) and second (purple) operations in patients with recurrent chronic subdural hematoma. CRP levels were significantly different between the first and second operations (p systemic = 0.004 and p subdural < 0.0001).
Discussion
In this study, exploring systemic and subdural CRP levels in CSDH patients, systemic CRP levels were higher compared to the subdural CRP levels. Systemic CRP levels could predict recurrence only with low accuracy, albeit the median CRP levels were within the normal range of CRP. CRP levels were significantly higher both systemically and in the subdural fluid at the time of the second operation in patients with recurrent CSDH.
Systemic and subdural CRP in CSDH patients
CRP is mainly synthesized by IL-6-dependent hepatocytes, and following an inflammatory incident, CRP mediates the downstream acute-phase response. Reference Black, Kushner and Samols8 Due to the inflammatory properties of CRP, and as inflammation plays a central part in the pathophysiology of CSDH, it is plausible to suspect an involvement of CRP in the subdural cavity. Contrary to other CSDH-related biomarkers, the systemic CRP levels were higher than the subdural levels. Reference Edlmann, Giorgi-Coll, Whitfield, Carpenter and Hutchinson1,Reference Jensen, Binderup, Olsen, Kjaer and Fugleholm2,Reference Jensen, Olsen, Lelkaitis, Kjaer, Binderup and Fugleholm3,Reference Stanisic, Aasen and Pripp17,Reference Stanisic, Lyngstadaas and Pripp27 As CRP is mainly produced in the liver, it is unlikely that subdural CRP levels should exceed systemic CRP levels, since it is only sparsely produced by leukocytes and nervous tissue. Reference Yao, Zhang and Wu11,Reference Di Napoli, Slevin, Popa-Wagner, Singh, Lattanzi and Divani12 The balance between CRP levels at a pathological site and systemic CRP levels has to some extent been investigated in other diseases. In atherosclerotic disease, CRP binds to damaged cell membranes, contributing to local activation of the complement system. Reference Torzewski, Torzewski and Bowyer10,Reference Kaplan and Volanakis28 In infarcted heart tissue, CRP activates the complement cascades, causing additional heart damage, being only present in the infarcted myocardial cells and not the healthy. Reference Lagrand, Niessen and Wolbink29 Also, it has been shown that CRP is localized in nuclei of synovial cells of patients with rheumatoid arthritis. Reference Gitlin, Gitlin and Gitlin30 Nevertheless, others report on CRP predominantly being localized systemically rather than confined to a specific site of inflamed tissue. Reference Vigushin, Pepys and Hawkins31,Reference Babaei, Javadian and Narimani32 The current understanding of local, tissue-specific higher levels of CRP, compared to systemic levels, is uncertain, and our study points toward CRP as mainly being a systemic biomarker for inflammation driven by CSDH.
CRP is not relevant in predicting CSDH outcome
In the sensitivity analysis, systemic CRP level predicted CSDH recurrence with only low accuracy . Also, the median CRP level of both patients with and without recurrent CSDH was within the normal systemic CRP range below 3 mg/L. Reference Nehring, Goyal and Patel9 Therefore, systemic CRP levels for both patients with and without recurrence would clinically be regarded as normal, resulting in limited clinical value of CRP as a predictor for recurrent CSDH.
Increasing CRP levels point toward increasing inflammation during CSDH recurrence
High IL-6 and IL-8 levels in the subdural fluid are correlated with increased risk of CSDH recurrence. Reference Hong, Kim, Yi, Ko, Oh and Kim6,Reference Frati, Salvati and Mainiero16 As CRP is linked to IL-6, this correlates well with the increasing levels of both subdural and systemic CRP levels in recurrent hematomas found in this study and provides a plausible pathological explanation. Furthermore, an increase in the anti-inflammatory IL-1 receptor antagonist has been demonstrated at the time of the second operation in recurrent CSDH patients, which has been interpreted as a regulatory mechanism against an increased inflammatory processes. Reference Jensen, Binderup, Olsen, Kjaer and Fugleholm2 In a recent analysis integrating several relevant biomarkers, a higher level of anti-inflammatory markers correlated significantly with a lower risk of recurrence. Reference Pripp and Stanišić7 Therefore, the increase in CRP levels between the first and second operations points toward an overall progressive inflammatory process toward a recurrence of a CSDH.
mCRP is expressed in CNS microvessels and by nervous tissue showing angiogenic properties. Reference Di Napoli, Slevin, Popa-Wagner, Singh, Lattanzi and Divani12 This resonates with multiple studies demonstrating a rapid angiogenic process forming fragile and leaky capillaries within the hematoma membrane, nourishing the hematoma and resulting in hematoma expansion, and thereby demonstrates a possible target for future CSDH management, as both inflammation and angiogenesis are important factors in CSDH pathology. Reference Edlmann, Giorgi-Coll, Whitfield, Carpenter and Hutchinson1 Targeting strategies against CRP have been presented with the possibility of lowering circulating CRP levels with both medication-associated decreases in CRP levels induced by statins, bempedoic acids, dipeptidyl peptidase-4 inhibitors and angiotensin-converting enzyme inhibitors Reference Ridker, Danielson and Fonseca33,Reference Cicero, Fogacci, Hernandez and Banach34 but also targeting CRP directly, inhibiting the dissociation of anti-inflammatory nCRP to the proinflammatory mCRP form. Reference Prasad35,Reference Zeller, Cheung Tung Shing and Nero36 Knowledge of local and systemic CRP levels in CSDH patients may therefore assist in an anti-inflammatory, nonsurgical treatment method, the clinical value of which is still uncertain. Reference Hutchinson, Edlmann and Bulters37–Reference Miah, Holl and Blaauw39
Limitations
While most baseline characteristics were similar between patients with and without recurrent CSDH, a few differed. Patients with recurrent CSDH were almost entirely male. To the best of our knowledge, even though no studies demonstrate that male gender is a risk factor for recurrent CSDH, this may be likely, as male gender is known to be a risk factor for developing primary CSDH. Reference Tseng, Tseng, Liu, Lin, Hu and Hsiao40,Reference Andersen-Ranberg, Poulsen, Bergholt, Hundsholt and Fugleholm41 Also, significantly more patients with recurrent CSDH had bilateral hematomas, which may be expected as patients with bilateral CSDH have a higher recurrence rate compared to patients with unilateral CSDH. Reference Andersen-Ranberg, Poulsen, Bergholt, Hundsholt and Fugleholm41 A final limitation is the comparison between systemic versus subdural CRP levels in the first and second operations. As CRP is diffusible over the blood-brain barrier, increased subdural CRP levels at the time of the second operation could well be caused by increased systemic levels, as opposed to being expressed locally.
Conclusion
This study has for the first time correlated CRP levels in systemic blood and in the subdural fluid of patients with both primary and recurrent CSDH. CRP is present in both the subdural fluid and systemic blood and at significantly higher levels in systemic blood; however, both are within the normal range. Clinically, CRP is not suitable for the prediction of CSDH recurrence. However, CRP levels are significantly higher in both systemic blood and subdural fluid at the time of the second surgery in patients with recurrent CSDH, suggesting an evolving inflammatory process toward the development of a recurrent hematoma.
Data availability statement
The datasets generated and/or analyzed during the study are available from the corresponding author upon reasonable request.
Author contributions
JTH drafted the original manuscript with TSRJ, validated and analyzed the results and edited the manuscript with all the co-authors. TTF analyzed and validated the results and edited the original and the final version of the manuscript. MHO managed the statistical analysis, analyzed the results and edited the final version of the manuscript. TB conceptualized the project, supervised and edited the final manuscript and generated, curated and analyzed data. KF conceptualized the project, analyzed and validated the data supervised and edited the final version of the manuscript. TSRJ conceptualized the project, collected the samples, analyzed and validated the data and prepared the first version of the manuscript with JTH. All authors read and approved the final manuscript.
Funding statement
This project received funding from the Aase and Ejnar Danielsens Foundation, Becket Foundation, Grosserer L. F. Foghts Foundation, the Research Foundation of Rigshospitalet and A.P. Moeller Foundation.
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
The study received approval from the Scientific Ethical Committee of the Capital Region of Denmark (Journal no. H-20051073). Either the patient or their next of kin could consent for study participation.
Consent for publication
All authors reviewed and consented publication of the final manuscript.






Target article
Subdural and Systemic C-Reactive Protein in Patients with Chronic Subdural Hematoma Recurrence
Related commentaries (1)
Reviewer Comment on Hansen et al. “Subdural and Systemic C-Reactive Protein in Patients with Chronic Subdural Hematoma Recurrence”