A 62-year-old nonsmoking female presented with an eight-month history of a nonproductive cough. Its onset had been sudden, without a prior history of upper respiratory tract infection and had not progressed in severity over time. The cough was constant throughout the day and had a significant impact on the patient’s daily functioning as her speech was interrupted by coughing every few seconds. Past medical history revealed episodes of migraine only. There was no history of asthma, allergies or acid reflux. She had no exposure to angiotensin-converting enzyme inhibitors. The cough was triggered by a sensation described by the patient as an “abnormal tingling at the back of the throat” and was amplified by specific triggers (cold temperature, alcohol consumption and mostly physical effort). She never had cough syncope and denied symptoms of dysphagia or throat pain. A thorough physical examination, including a head and neck, pulmonary and complete neurologic examination, was negative for any relevant findings.
Methacholine challenge test, high-resolution CT of the chest, swallowing study, laryngoscopy and bronchoscopy were all unremarkable except for mild edema of the posterior part of the vocal folds. Skin prick testing revealed dust mite allergy only. Multiple therapeutic trials, including inhaled bronchodilators, proton pump inhibitors, intranasal corticosteroid spray and inhaled and oral corticosteroids, had no effect on her cough.
A neurology consultation was sought one year after the onset of the cough. Right-sided intermittent pharyngeal paresthesia was hypothesized to originate from the ipsilateral glossopharyngeal nerve. A brain MRI showed a right neurovascular conflict from an anterior inferior cerebellar artery (AICA) loop, causing mass effect on the cisternal portions of the glossopharyngeal and vagal nerve, which were posteriorly distorted (Figure 1).
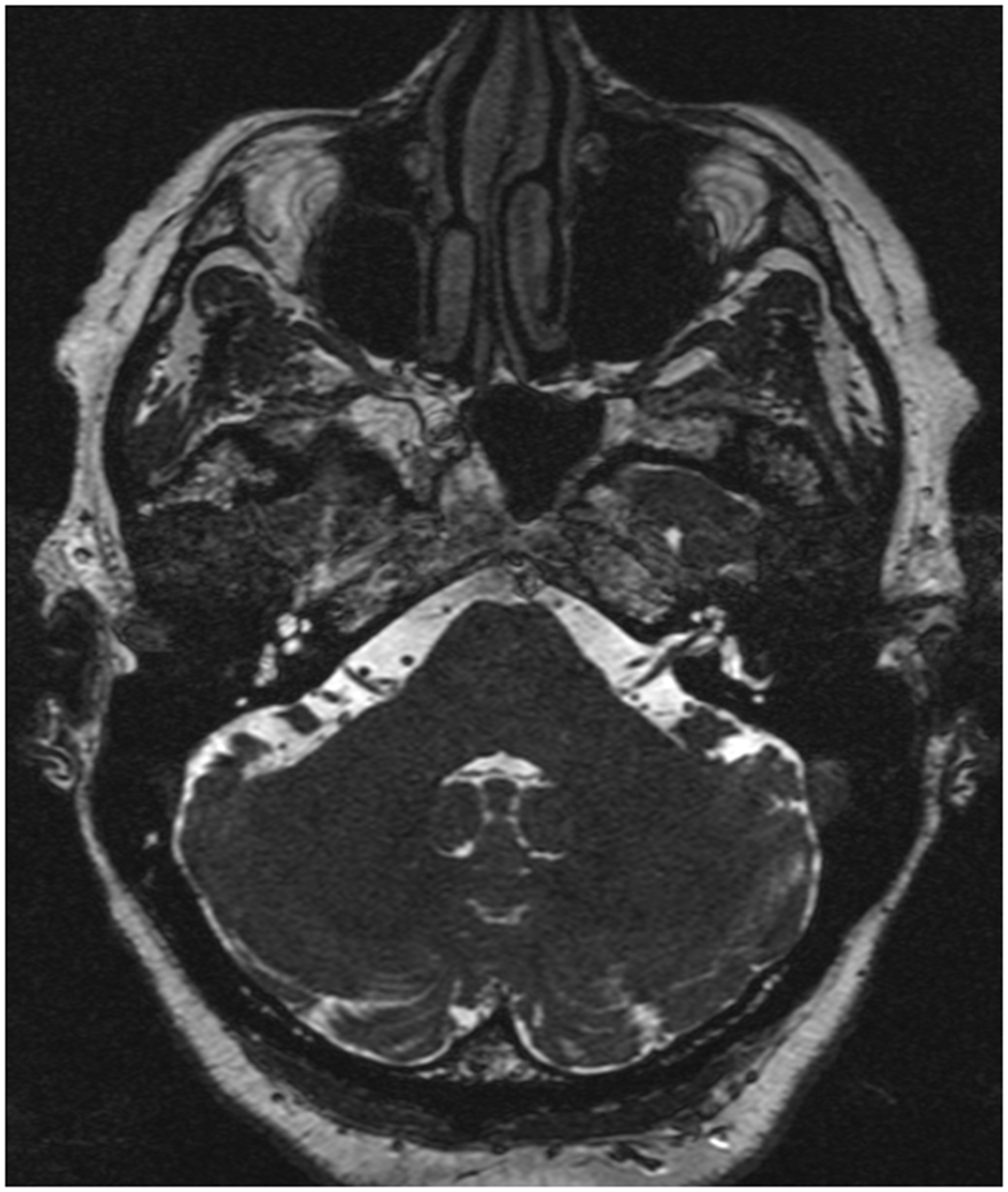
Figure 1. Preoperative axial brain MRI demonstrating right neurovascular conflict, with the anterior inferior cerebellar artery loop making contact on the cisternal portion of the IX–X cranial nerve complex.
A trial of carbamazepine CR 200 mg HS was then initiated with an increase to 400 mg BID over the next four weeks. The patient reported an 80% reduction in the cough frequency and intensity but was not tolerating carbamazepine. Lamotrigine was introduced but was not tolerated either. Carbamazepine was then reintroduced at a lower dose and then switched to oxcarbazepine. A right glossopharyngeal nerve block using 2 mL of 1% lidocaine injected in the right tonsillar fossa was used as a diagnostic and therapeutic procedure and led to a complete resolution of her cough for about 24 hours.
A neurosurgical consultation was requested, and surgical microvascular decompression was discussed. A retrosigmoid craniotomy using neuronavigation confirmed the encroachment of a rootlet of the IX–X cranial nerve complex by a loop of the AICA. A microvascular decompression was performed, moving the AICA away from the glossopharyngeal-vagus complex and securing it with Teflon® and fibrin glue.
The patient had an important decrease in cough frequency postoperatively. She had one episode of self-limited sudden stridor and dyspnea three months after the surgery. Six months after the surgery, she was able to stop oxcarbazepine, and she remains cough-free nine months after the surgery, although she still reports mild pharyngeal postprandial paresthesia.
To our knowledge, glossopharyngeal and vagal neuropathy secondary to an AICA compression presenting as a chronic refractory cough (CRC) with no pain or motor symptoms has not previously been described.
Glossopharyngeal neuralgia (GN) or vago-glossopharyngeal neuralgia (VGPN), which presents as neurogenic cough accompanied by pain or motor symptoms, may respond to anticonvulsants as the first-line therapy. Reference Chen and Sindou1 If refractory to medical treatment, those patients with an MRI suggestive of vascular encroachment of cranial nerve roots could potentially benefit from microvascular decompression. Reference Honey, Krüger, Morrison, Dhaliwal and Hu2
Laryngeal sensory dysfunction (LSD), Reference Novakovic, Sheth and Stewart3 which encompasses disorders of the vagal sensory pathways, was recently described. Common manifestations include CRC and abnormal throat sensation. It is indeed speculated that vagal nerve disorders can manifest as a sudden and exaggerated, but nonpainful, tickling sensation that leads to uncontrollable coughing. Reference Bastian, Vaidya and Delsupehe4 Several phenotypes related to hyperfunctional vagal sensation and sharing similar features have been described, including CRC, laryngospasm and laryngeal sensory neuropathy, but none involving also the IX cranial nerve. Emotional distress, chronic reflux, habitual muscle misuse and post-viral neuralgia were among other hypotheses regarding the etiology of LSD. Reference Novakovic, Sheth and Stewart3
Recently, neurovascular compression of the vagus nerve rootlets by the posterior inferior cerebellar artery (PICA) was presented as a potential cause of vagal neuropathy. Reference Honey, Krüger, Morrison, Dhaliwal and Hu2 Indeed, a recent case report introduced the concept of VANCOUVER syndrome (Vagus Associated Neurogenic Cough Occurring due to Unilateral Vascular Encroachment of Its Root), Reference Honey, Krüger, Morrison, Dhaliwal and Hu2 where vagus neuralgia was reported as a tickling sensation deep to the suprasternal notch that triggers a neurogenic medically refractory cough and responded to antineuralgia medication, similarly to our case. The differences are that the PICA was involved instead of the AICA, that the cough was triggered differently (laughing, exercise, lying down, strong perfume and during episodes of respiratory infection) and that cranial nerve X was the only one involved.
The underlying pathophysiology regarding chronic cough as the presentation of VGPN is described in a recent case series and literature review, Reference Honey, Krüger, Rheaume, Avecillas-Chasin, Morrison and Honey5 where concurrent GN and hemi-laryngopharyngeal spasm (HeLPS) were described, affecting both the motor and sensory pathways of this cranial nerve complex. The hypothesis is that a common cause of GN is a vascular compression of the IX cranial nerve and sometimes the upper rootlets of the X cranial nerve. It is therefore the same mechanism as in HeLPS, which presents as intermittent throat spasms and cough without pain due to compression of the X cranial nerve. Reference Honey, Krüger, Rheaume, Avecillas-Chasin, Morrison and Honey5 According to Honey et al., the sensory fibers of the vagal nerve, which trigger abnormal sensation deep to the suprasternal notch and can lead to coughing, are located more rostrally in the nerve. A vessel compressing the glossopharyngeal nerve (like in our case) is more likely to compress the rostral than the caudal rootlets of the adjacent X cranial nerve, explaining why the associated symptoms are more likely to be coughing than choking. Reference Honey, Krüger, Morrison, Dhaliwal and Hu2
This report may therefore serve as a suggestion to include glossopharyngeal neuropathy in the differential diagnosis of chronic cough, as AICA compression of the cranial nerve complex IX–X can induce this symptomatology.
Acknowledgments
None.
Author contributions
OG: design and conceptualization, literature review, drafting of the manuscript
CB: long-term management of the patient, design and conceptualization, critical revision of the manuscript, OG mentoring
SB: long-term management of the patient, critical revision of the manuscript
PL: long-term management of the patient, critical revision of the manuscript
Funding statement
No funding was received for this manuscript.
Competing interests
The authors have not published, posted or submitted any related manuscripts from the same case. The authors have no relevant conflicts of interest to report. Statistical analysis was not required for the preparation of this manuscript.



