Introduction
Globally, the multidrug-resistant (MDR) bacteria-caused deaths exceeded 4.71 million in 2021, the number of which was estimated at 10 million annually by 2050 (Naghavi et al. Reference Naghavi, Vollset and Ikuta2024) As the biggest antibiotics consumer in food animal industry since 2010, China has banned the antibiotics usage as growth promoter in 2020 to alleviate the antimicrobial selection pressure, contributing to less spread of MDR clones (Chen et al. Reference Chen, Huang and An2024). However, the prevention against pathogens including Salmonella, Escherichia coli O:78 and Clostridium perfringens was weaker after the ban due to its high reliance on the antibiotics (Chen et al. Reference Chen, Huang and An2024; Smith et al. Reference Smith, Fernando and King2024; Sun and Chen Reference Sun and Chen2024). Probiotics, defined as live microorganisms which can benefit hosts when ingested sufficiently, is the most widely used, natural and safe substitutes to enhance health and growth in poultry production, among which LR, a hetero-lactic acid bacteria with superior probiotic characteristics, can regulate immune response, modulate gut microbiota, produce beneficial metabolites, restore impaired intestinal barrier function by reducing intestinal epithelial cells apoptosis via PKC-Nrf-2/HO-1 pathway activation and NF-κB pathway inhibition, and induce intestinal stem cell differentiation to Paneth cells by activating Wnt-β-catenin pathway, thus leading to better growth performance (Ding et al. Reference Ding, Tang and Zhao2024; Xu et al. Reference Xu, Li and Ding2023; Zhou et al. Reference Zhou, Wu and Chen2022). In previous studies, we screened an LR strain from broiler gut microbiota with significant bacteriostatic and anti-inflammatory effects owing to its reuterin producing ability (Ding et al. Reference Ding, Tang and Zhao2024; Xu et al. Reference Xu, Ding and Wang2022a, Reference Xu, Li and Ding2023, Reference Xu, Wang and Ding2022b).
Prebiotics refer to substrates selectively utilized by the gut microbiota bestowing a positive impact on the host health, which can promote the proliferation and/or activity of beneficial bacteria, as well as increase the generation of advantageous metabolites such as short-chain fatty acids, mostly non-digestible carbohydrates, especially short-chain oligosaccharides (Salminen et al. Reference Salminen, Collado and Endo2021; Sanders et al. Reference Sanders, Merenstein and Reid2019; Zeng et al. Reference Zeng, van Pijkeren and Pan2023). Gluco-oligosaccharides (GlcOS) were reported to show prebiotic potentials, including antimicrobial effect, anti-inflammation, antioxidation and providing selective substrates for certain probiotics (Chaari et al. Reference Chaari, Belghith-Fendri and Zaouri-Ellouzi2015; Hu et al. Reference Hu, Heyer and Wang2020). It is reported that GlcOS can activate intraepithelial natural killer cells and increase the relative abundance of commensal lactic acid bacteria in young broiler chickens, contributing to improved intestinal health and growth performance in broilers (Meijerink et al. Reference Meijerink, de Oliveira and van Haarlem2021).
Synbiotics can facilitate the colonization and proliferation of advantageous gut microorganisms, typically consisting of Lactobacillus, Bifidobacterium and Streptococcus as the probiotic ingredient, and oligosaccharides, fiber or inulin as the prebiotic ingredient (Krumbeck et al. Reference Krumbeck, Maldonado-Gomez and Ramer-Tait2016). Synbiotics were effective in improving antioxidant capacity, immune function, intestinal morphology and microecological homeostasis, whose dual synergistic role in broilers was increasingly evident (Chen et al. Reference Chen, Wen and Zhou2018; Kridtayopas et al. Reference Kridtayopas, Rakangtong and Bunchasak2019; Mounir et al. Reference Mounir, Ibijbijen and Farih2022; Tanner et al. Reference Tanner, Lacroix and Del’Homme2015). Notably, LR can utilize some prebiotics like GOS as a carbon resource, providing the foundation for symbiotic development.
Therefore, we aimed to investigate the effects of LR in combination with GlcOS on growth performance and gut health of broilers, and to preliminarily explore its underlying mechanism by analyzing the intestinal mucosal morphology, gut microbiota and metabolome to lay a theoretical foundation for practical application in production.
Materials and methods
Ethics statement
The experimental procedures complied with the animal welfare guidelines established by China and were approved by the Animal Care and Welfare Committee as well as the Scientific Ethical Committee of Zhejiang University (No. ZJU20220310, Hangzhou, China).
Experimental design and diets
A total of 900 1-day-old male yellow-feathered broilers were randomly distributed into three groups with six replicates of 50 each. The strain used for study is a single Lactobacillus reuteri strain (LR 21), previously isolated from broiler cecum contents kept in laboratory stock (Xu et al. Reference Xu, Li and Ding2023). Experimental treatments were as follows: basal diet (CON group), basal diet supplemented with 2500 g/t microencapsulated Lactobacillus reuteri (LR) (1 × 107 CFU/g feed, LR group) and basal diet supplemented 2500 g/t microencapsulated LR (1 × 107 CFU/g feed) and 900 g/t GlcOS (RG group). The total of 56 day-experiment period was divided into two phases, namely early growth phase (1–21 days) and the later growth phase (22–56 days) (Bhogoju et al. Reference Bhogoju, Khwatenge and Taylor-Bowden2021; Chai et al. Reference Chai, Guo and Mohamed2023). All broilers were subjected to routine feeding management and immunization schedules. Feed and water were provided ad libitum. Basal diet was formulated in accordance with the guidelines of the nutritional requirements of broiler chickens as recommended in NY/T 3645-2020, with its composition and nutrient levels detailed in Table 1.
Table 1. Composition and nutrient levels of the basal diets
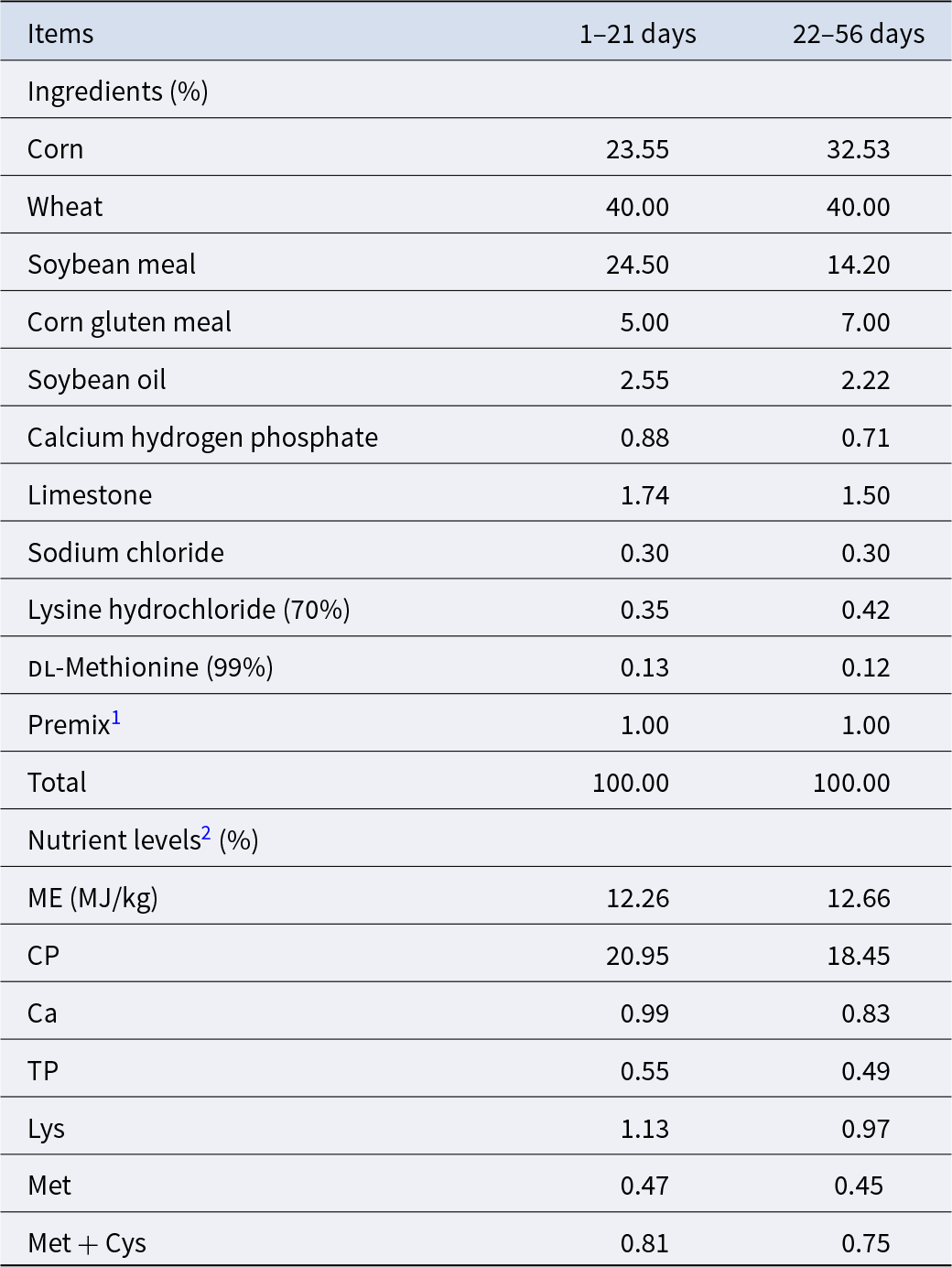
1 Supplied per kilogram of diet: vitamin A, 9,600 IU; vitamin D3, 2,700 IU; vitamin E, 36 mg; vitamin K3, 3.0 mg; vitamin B1, 3.0 mg; vitamin B2, 10.5 mg; vitamin B6, 4.2 mg; vitamin B12, 0.03 mg; folic acid, 1.5 mg; nicotinamide, 60 mg; d-calcium pantothenate, 18 mg; biotin, 0.225 mg; choline chloride, 1,000 mg; Fe, 80 mg; Cu, 8.0 mg; Mn, 80 mg; Zn, 60 mg; I, 0.35 mg; Se, 0.15 mg.
2 Metabolizable energy (ME) is a calculated value; other nutrient levels are the measured values.
All ingredients were provided from the Zhejiang xinxin Feed Co., Ltd. The feed preparation process as follow: First, corn flour as the adsorption carrier was put into the mixer, where it was thoroughly mixed with microencapsulated LR (prepared by the Feed Science Institution of Zhejiang University) and/or GlcOS (provided by DSM (Shanghai) Co. Ltd). Subsequently, other feed ingredients listed in Table 1 were added to the mixer and blended uniformly. Finally, the mixture undergone low-temperature conditioning (steam temperature ≤65°C) to produce either powdered feed or pelleted feed, which was then rapidly cooled to room temperature by a cold air blower for subsequent experimental. The feed used in the early growth phase is powder form while in the late growth stage, it is pelleted.
Growth performance
Body weight and feed intake for each replicate (six broilers) were recorded at the conclusion of each period, the average daily gain (ADG), average daily feed intake (ADFI) and feed-to-gain ratio (F/G) were calculated during the early growth phase (1–21 days), the later growth phase (22–56 days) and the overall period (1–56 days).
Sample collection
After the feeding trial, two broilers with body weights close to the average were randomly selected from each group per replicate for 10 mL of blood samples collection from the wing vein, ultimately sacrifice and sampling, totaling 36 broilers. One-centimeter samples of duodenum, jejunum and ileum were collected and fixed in 4% paraformaldehyde solution. Another 1 cm segment of jejunum was collected and fixed in 2.5% glutaraldehyde solution. The cecum was separated, and the cecal content was gathered and preserved at −80℃ for subsequent analysis of gut microbiota and metabolites.
Intestinal morphology
The intestinal segments of the duodenum, jejunum and ileum fixed in 4% formaldehyde solution were dehydrated with alcohol, transparent with xylene, embedded in paraffin. These segments cut into 5 µm thickness, and stained with hematoxylin–eosin (HE) and periodic acid–Schiff’s (PAS) staining for analysis, respectively. The HE-stained and PAS-stained sections were observed using an optical microscope (Nikon, Tokyo, Japan) to capture and documented images. Image-Pro software (MediaCybernetics, Rockville, MD) was utilized to measure villus height (VH), crypt depth (CD) and the number of goblet cells was counted per 1 mm jejunum.
The jejunal samples were fixed in 2.5% glutaraldehyde at 4°C overnight and rinsed three times with 0.1 M phosphate buffered saline (PBS). Subsequently, the samples were treated with 1% osmium for 1 h. After rinsing in PBS three times, the samples were dehydrated with Gradient ethanol, embedded in epoxy resin, sectioned by using a LEICA EM UC7 ultratome (Leica Microsystems, Wetzlar, Germany). Finally, the sections were observed with a Hitachi H-760 TEM (Hitachi, Tokyo, Japan).
Measurements of intestinal permeability and digestive enzyme activity
Following the manufacturer’s manuals, the serum concentration of d-lactic acid and diamine oxidase (DAO) was determined using enzyme-linked immunosorbent assay (ELISA) (Jiangsu Enzyme Free Industrial Co., LTD., Yancheng, China).
Pre-preparation of duodenal, jejunal and ileal tissue homogenates. First, the thawed duodenum, jejunum and ileum were quickly dissected, and the contents were rinsed with PBS solution. Then the intestinal mucosa was scraped from 0.1 to 0.15 g with a small blade, and the corresponding PBS solution was added in the ratio of 1:9 between the weight of the tissue (g) and the volume of PBS solution (mL). It was homogenized with a tissue homogenizer, and then centrifuged at 3,500 rpm, 10 min at 4℃; and 10% of the tissue homogenate was obtained by taking the supernatant. The activities of amylase (AMS), lipase (LPS) and trypsin (TPS) in duodenum, jejunum and ileum were detected with the kit instructions (Nanjing Institute of Jiancheng Bioengineering, Nanjing, China).
Extraction of RNA and RT-qPCR analysis
The RNA of jejunal tissues was extracted using the Trizol kit (Nanjing Novozymes Bioscience and Technology Co., Ltd., Nanjing, China) following the provided manuals. The RNA obtained was then transcribed reversely into complementary DNA with the HiScript II Q RT SuperMix Reverse Transcription Kit (Novozymes Bioscience and Technology), and subsequently stored at −20°C. The primer sequences presented in Table 2 and were synthesized by Beijing DynaPro Biotechnology (China). RT-qPCR was conducted using ChamQ Universal SYBR qPCR Master Mix (Novozymes Biotechnology) in a CFX96 Real-Time PCR System (Bio-Rad, Hercules, CA) The relative expression of each target gene was calculated by 2-ΔΔCt method with relative to β-actin.
Table 2. Primer sequences for RT-qPCR

The genes encoding for PepT1, EATT3, B0AT, CAT1, SGLT1, GLUT2 and FATP4 are designated as SLC15A1, SLC1A1, SLC6A19, SLC7A1, SLC5A1, SLC2A2 and SLC27A4, respectively.
16s rRNA-based microbiota analysis
The cecum microbiota DNA was extracted using TIANGEN kit following the manufacturer’s manuals. Primers 341F (5ʹ-CCTAYGGGRBGCASCAG-3ʹ) and 806R (5ʹ-GGACTACNNGGGGTATCTAAT-3ʹ) were synthesized for V3-V4 regions amplification, then purified, quantified and homogenized to construct a sequencing library, which was subjected to high-throughput sequencing on Illumina platform. The α- and β-diversity analyses were performed, and the relative abundance and differential flora were analyzed.
LC-MS cecal metabolomics analysis
Metabolites extraction and liquid chromatography–tandem mass spectrometry LC-MS/MS analysis of cecal contents were as previously described (Yi et al. Reference Yi, Yu and Shi2022). Briefly, after separation of the sample on an HSS T3 column, the mass spectrometric data were acquired in the Data-Dependent Acquisition mode. The liquid chromatography–mass spectrometry (LC-MS) raw data were imported into the Progenesis QI (Waters Corporation, Milford, USA) software for preprocessing. The metabolites were identified by searching through databases including Human Metabolome Database (http://www.hmdb.ca/) and Metlin (https://metlin.scripps.edu/). The R package “ropls” (Version 1.6.2) was used to conduct principal component analysis (PCA) and orthogonal least partial squares discriminant analysis (OPLS-DA). The significantly different metabolites were identified based on the variable importance in the projection (VIP) scores from the OPLS-DA model and P-value derived from the t-test, t with criteria set at VIP > 1 and P < 0.05. Finally, metabolic pathway annotation of these significant differential metabolites was performed by Kyoto Encyclopedia of Genes and Genomes (KEGG) database (https://www.kegg.jp/kegg/pathway.html) to identify the differential metabolites implicated in major pathways, with a significance threshold of P < 0.05.
Statistical analysis
The experimental data were expressed as mean ± standard deviation (Mean ± SD), and then one-way analysis of variance (ANOVA) was carried out using SPSS 20.0 statistical software, with Duncan’s multiple range test employed for multiple comparisons. P < 0.05 was considered indicative of a significant difference. Significance was shown as *P < 0.05, **P < 0.01 and ***P < 0.001. Figures were plotted by GraphPad Prism 7.0 software, while comparison without significance was not shown for better presentation.
Results
Growth performance
The effects of LR and its combination with GlcOS on growth performance of broilers across the early growth phase, the later growth phase and the overall period are shown in Table 3. During the early growth phase, no significant differences were observed in all performance indicators among all groups (P > 0.05). During the later growth phase, compared with the CON group, the effect of LR and RG dietary supplementation was significant in ADG and F/G (P < 0.01), and RG supplementation significantly increased FW (P < 0.05), whereas there was no significant difference in ADFI among all groups (P > 0.05). During the overall period, the LR group exhibited significant decrease in F/G (P < 0.05), while RG supplementation both decreased F/G (P < 0.05) and increased ADG (P < 0.05), with no significance in ADFI (P > 0.05) difference among groups, which strongly testify the synergistic benefits between LR and GlcOS during the later growth phase and overall period.
Table 3. Effects of LR and its combination with GlcOS on growth performance of broilers

IW, initial weight; FW, final weight.
Values were presented as mean ± SD of six replicates.
a, b Values in a same row with different superscript letters are significantly different.
Intestinal morphology and barrier function of intestine
The intestinal morphology and barrier function of the CON, LR and RG groups are shown in Fig. 1. In comparison to the CON group, dietary LR and RG significantly increased VH (P < 0.05) and decreased CD (P < 0.001), leading to an increased VH/CR ratio (VH:CD) (P < 0.001) (Fig. 1A–C), and displayed more compact intestinal structure (Fig. 1D). The microvilli in jejunum of the LR and RG groups were better-arranged (Fig. 1E), and the number of goblet cells in the LR and RG groups significantly more than the CON group (P < 0.001) (Fig. 1F, G).

Figure 1. The effects of LR and its combination with GlcOS on intestinal morphology and barrier function of intestine. VH (A), CD (B), VH: CD (C), morphological microstructure (D) of duodenum, jejunum and ileum (40×). (E) Jejunum microvillus microstructure under transmission electron microscope (TEM). (F) PAS staining of jejunum (100×). (G) The number of goblet cells in the jejunum. Serum level of DAO (H) and d-lactic acid (I). (J) mRNA expression of jejunal tight junction genes. Data were presented as mean with SD (n = 6). Significance was shown as *P < 0.05, **P < 0.01 and ***P < 0.001.
In addition, dietary LR and RG significantly reduced the serum concentrations of DAO (Fig. 1H) and d-lactic acid (Fig. 1I), while the mRNA levels of Occludin and Mucin 2 (MUC2) in the jejunum were notably increased in the RG group (P < 0.05) (Fig. 1J). These results suggested that the synergistic effect of LR and GlcOS could improve the intestinal morphological structure and enhance the intestinal barrier function.
Intestinal digestion and absorption
Figure 2 shows the activity of intestinal digestive enzymes and expression of jejunal transporter genes. In terms of small intestinal digestive function, the activity of LPS and TPS was significantly improved by both LR and RG additives, in addition to AMY in the RG group (P < 0.05). Meanwhile, the activity of TPS and AMS in the ileum in the RG group was notably higher than the CON group (P < 0.05).
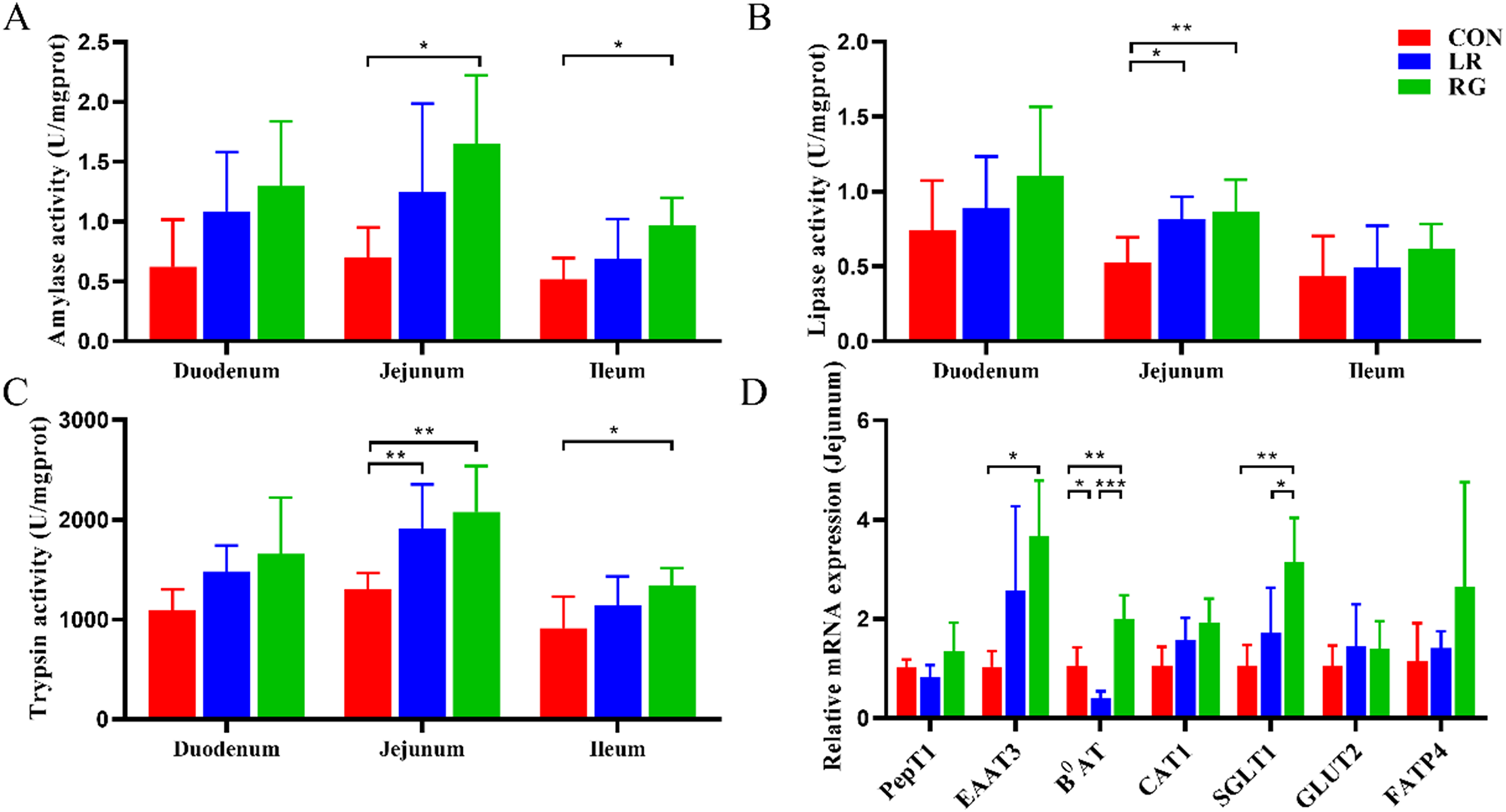
Figure 2. The effects of LR and its combination with GlcOS on intestinal digestion and absorption function. AMY (A), LPS (B) and TPS activity (C) of duodenum, jejunum and ileum. (D) mRNA expression of jejunal transporter genes. Data were mean with SD (n = 6). Significance was shown as *P < 0.05, **P < 0.01 and ***P < 0.001.
In terms of small intestinal absorption function, the mRNA expression of EAAT3, B0AT and SGLT1 significantly increased in the RG group, revealing that the digestive and absorptive functions of the small intestine could be enhanced by simultaneous supplementation of LR and GlcOS as growth promoter.
Cecum microbiota analysis
The Venn diagrams showed that all groups shared 640 operational taxonomic units (OUTs), and there were 232, 229 and 175 unique OUTs in the CON, LR and RG group, respectively (Fig. 3A). In regard to α-diversity, the Chao, Shannon and observed species indices were not notably different among groups, but community richness measured by the Simpson index of the RG group was significantly lower than the CON group (P < 0.05), indicating higher microbial diversity of the RG group (Fig. 3B). Beta-diversity, conducted through the principal coordinate analysis (PCoA), revealed that the bacterial communities of groups formed distinct clusters, with the microbiota structure in both LR and RG groups deviating from the CON group (Fig. 3C), which is also indicated by analysis of similarities (ANOISM) (Fig. 3D).
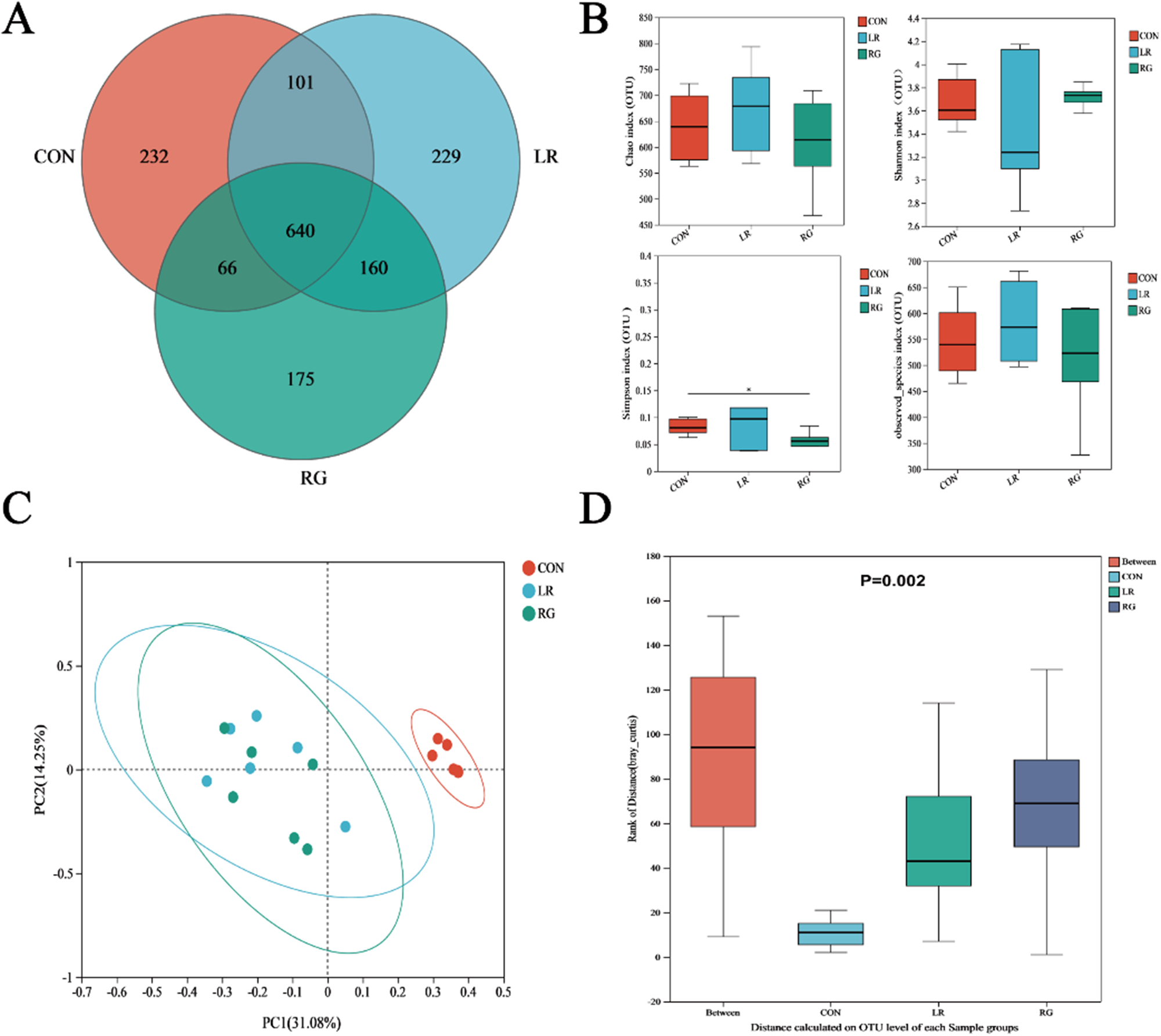
Figure 3. The effects of LR and its combination with GlcOS on structure of the cecum microbiota. (A) Venn diagram of otus. (B) Alpha-diversity analysis based on indices of the Chao, Shannon, Simpson and observed species indices. (C) Beta-diversity analysis based on PCoA. (D) Beta-diversity analysis based on ANOISM. Data were mean with SD (n = 6). Significance was shown as *P < 0.05.
As shown in Fig. 4, the microbial community composition of the three groups was different at the phylum, family and genus levels. Bacteroidota and Firmicutes were the top 2 most abundant phyla in each group, accounting for more than 80% of all microorganisms (Fig. 4A). The relative abundance of Desulfobacterota increased significantly (Fig. 4B) (P < 0.01). Meanwhile, the ratio of Bacteroidetes to Firmicutes was higher in the LR and RG groups, though without significance, still indicating the evident benefits of LR and RG on gut microbiota. At the family level, the cecum microbiota of the CON group was dominated by Ruminococcaceae, Barnesiellaceae, Rikenellaceae and Lachnospiraceae. Bacteroidaceae, Ruminococcaceae, while Rikenellaceae and Lachnospiraceae were majority in the LR and RG groups (Fig. 5A). The higher relative abundances of Bacteroidaceae (P < 0.05), Prevotellaceae (P < 0.05) and Desulfovibrionaceae (P < 0.01) were observed in the LR and RG groups (Fig. 5C, E, G), while the relative abundances of Barnesiellaceae (P < 0.001) and norank_o_Clostridia_UGG-014 (P < 0.01) showed a significant decrease (Fig. 5B, F). At the genus level, Barnesiella, Alistipes and norank_f_norank_o_Clostridia_UCG-014 dominated the microbiota in the CON group; Bacteroides, Faecalibacterium, Alistipes and norank_f_Muribaculaceae dominated in the LR group; and Bacteroides, Alistipes, Prevotellaceae_UCG-001 and norank_f_Muribaculaceae dominated in the RG group (Fig. 6A). Compared with the CON group, the relative abundances of Bacteroides (P < 0.05) and Prevotellaceae_UCG-001 (P < 0.05) in the LR and RG groups showed significant increase (Fig. 6B, E), while the relative abundances of Barnesiella (P < 0.001) and norank_f_norank_o_Clostridia_UCG-014 (P < 0.01) showed a significant decrease (Fig. 6D, F). In addition, the relative abundance of Ruminococcus_torques_group (P < 0.05) in the RG group was significantly higher compared to the LR group (Fig. 6G).
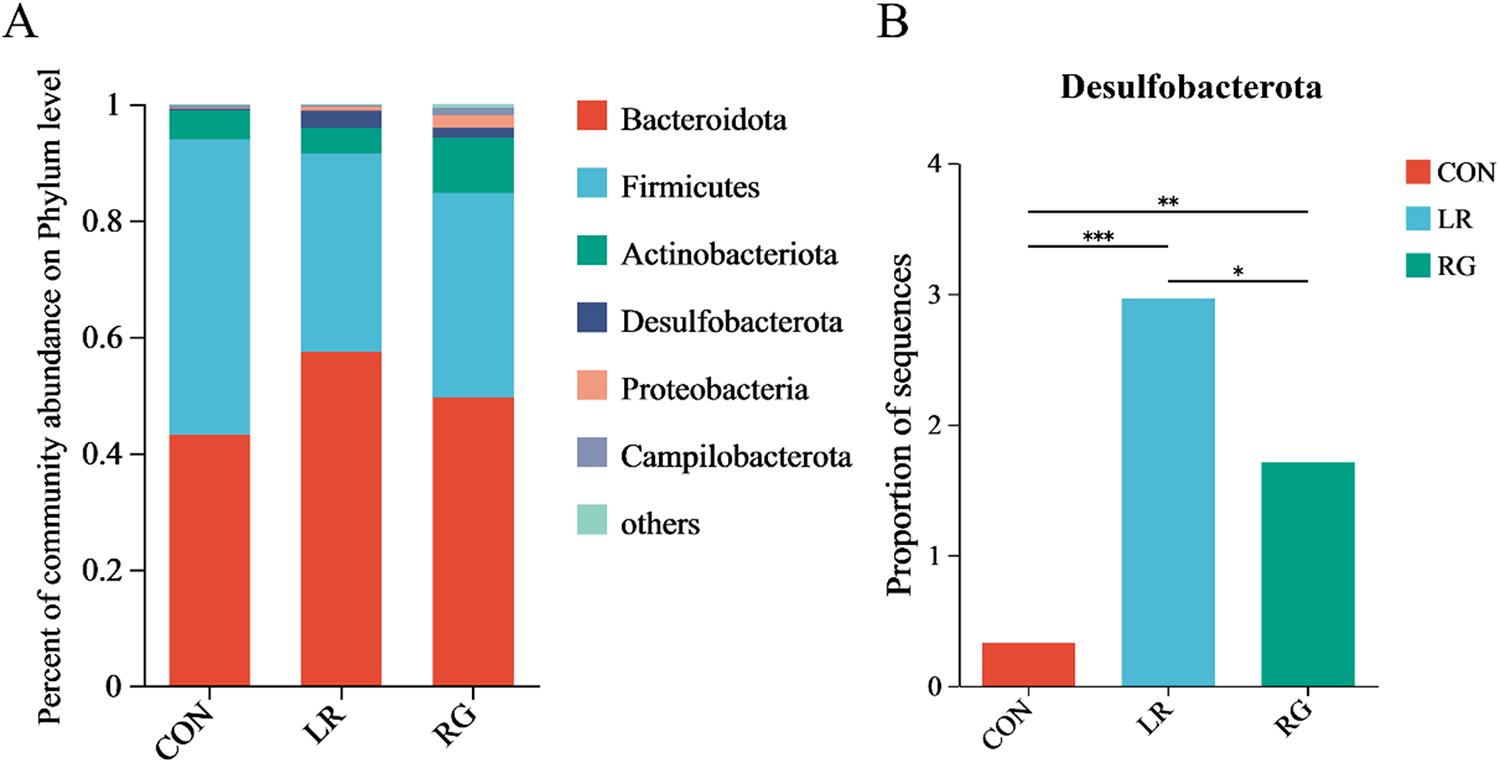
Figure 4. The effects of LR and its combination with GlcOS on composition of the cecum microbiota at the phylum level. (A) Relative abundance of microbiota at the phylum level. (B) The relative abundances of Desulfobacterota in all groups. Significance was shown as *P < 0.05, **P < 0.01 and ***P < 0.001.
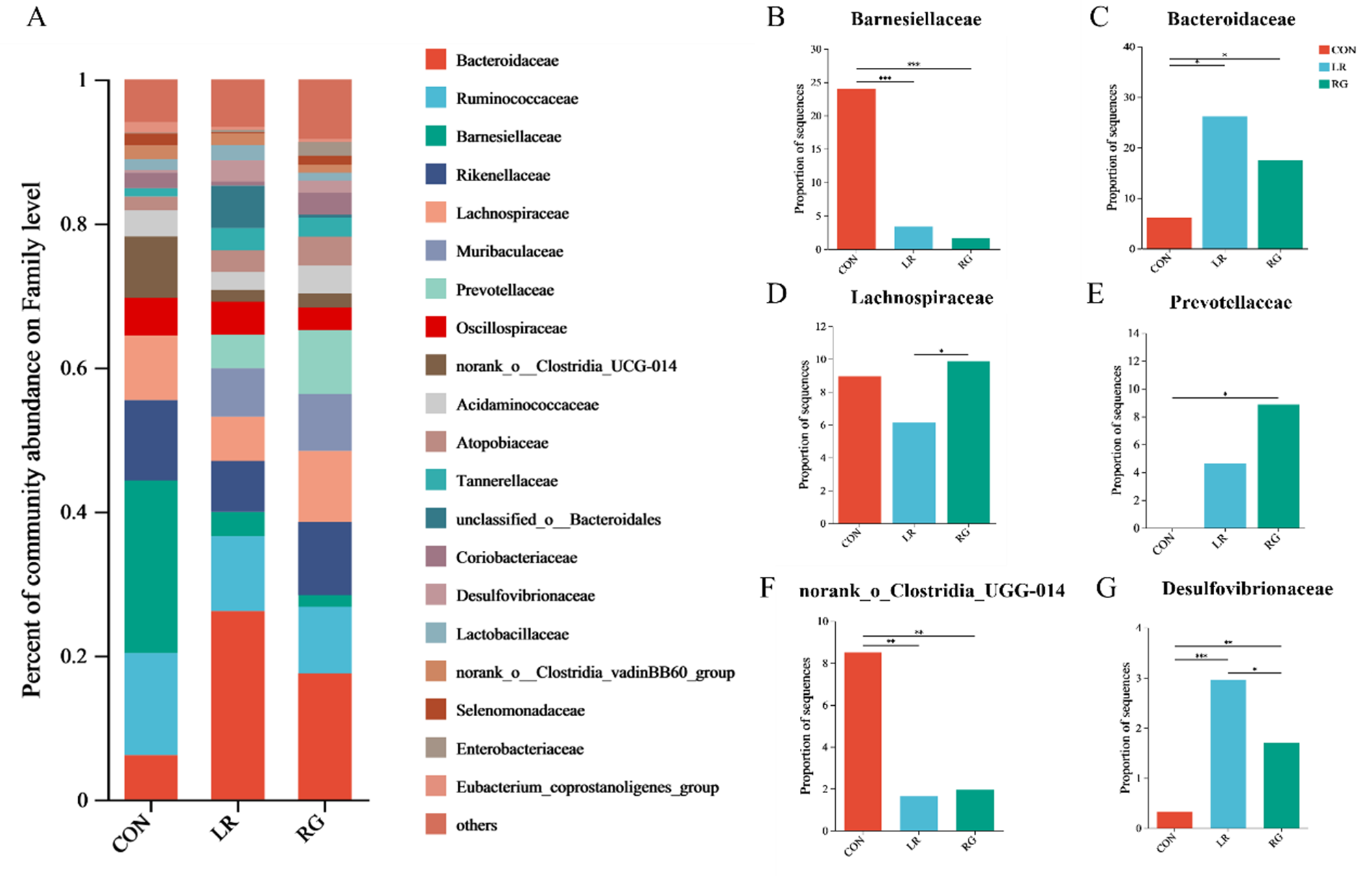
Figure 5. The effects of LR and its combination with GlcOS on composition of the cecum microbiota at the family level. (A) Aabundance of the microbial composition at the family level. The relative abundances of Barnesiellaceae (B), Bacteroidaceae (C), Lachnospiraceae (D), Prevotellaceae (E), Norank_o_clostridia_ugg-014 (F) and Desulfovibrionaceae (G) in all groups. Significance was shown as *P < 0.05, **P < 0.01 and ***P < 0.001.

Figure 6. The effects of LR and its combination with GlcOS on composition of the cecum microbiota at the genus level. (A) Abundance of the microbial composition at the genus level. The relative abundances of bacteroides (B), Alistipes (C), Barnesiella (D), Prevotellaceae_ucg-001 (E), Norank_f_norank_o_clostridia_ucg-014 (F) and Ruminococcus_torques_group (G) in all groups. Significance was shown as *P < 0.05, **P < 0.01 and ***P < 0.001.
The deviations of microbial communities among groups were screened based on LEfSe analysis (P < 0.05 and linear discriminant analysis score, LDA score > 4). It was found that genera Bacteroides, norank_f__Clostridium_methylpentosum_group and Desulfovibrio were enriched in the LR group, and Prevotellaceae_UCG-001, norank_f_Muribaculaceae and Ruminococcus_torques_group were enriched in the RG group, but not in the CON group (Fig. 7).

Figure 7. LEfSe multilevel species discriminant analysis of cecum microbiota. Bacterial taxa with LDA score >4 are selected as biomarker taxa.
Cecum metabolomic analysis
To investigate the effect of LR and its combination with RG supplementation on cecal microbiota, the cecal metabolite concentrations in the three groups were examined by LC-MS. Multivariate analysis including PCA and OPLS-DA was conducted between groups, before which the OPLS-DA, permutation test (n = 200) was applied to assure the good robustness of the model (Fig. 8A). The PCA score plot showed that the positive and negative ion differential metabolites in the LR and RG groups were significantly separated from the CON group (Fig. 8B). Further analysis using OPLS-DA revealed specific differences among the three groups. The results demonstrated that the positive and negative ion metabolites were separated obviously between the CON group and experimental groups, with their respective clustering into groups without overlap (Fig. 8B), suggesting that significantly different metabolites in cecum.

Figure 8. (A) OPLS-DA score plot and permutation test of broiler cecum metabolites. OPLS-DA plot and permutation test of the CON and LR groups in positive ion mode (A1) and in negative ion mode (A2). OPLS-DA plot and permutation test of the CON and RG groups in positive ion mode (A3) and in negative ion mode (A4). OPLS-DA plot and permutation test of the LR and RG groups in positive ion mode (A5) and in negative ion mode (A6). PCA score plot of broiler cecum metabolites in positive ion mode (B1) and in negative ion mode (B2).
P-values were statistically analyzed by t-test, and VIP values were statistically analyzed by OPLS-DA to screen the differential metabolites between the CON, LR and RG groups. The criteria for determining differential metabolism are P < 0.05 and VIP > 1, and up/downregulation difference multiples > 1. According to the results, there were 539 identified differential metabolites in the LR group (337 upregulated and 202 downregulated) (Fig. 9A) and 637 differential metabolites in the RG group (350 upregulated and 287 downregulated) (Fig. 9B). Annotation of these differential metabolites between groups was mainly lipids and lipid-like molecules, organic acids and organic oxygen compounds (Fig. 9C-D). Furthermore, the differential metabolites were imported into the KEGG database to screen the top 30 significantly enriched metabolic pathways. Different metabolites between the CON and LR groups were mainly enriched in choline metabolism in cancer, axon regeneration, GnRH secretion and dopaminergic synapse (Fig. 10A). While biosynthesis of cofactors, alanine, aspartate, glutamate, glycerophospholipid, tyrosine and tryptophan (Trp) metabolism pathways were most enriched in the differences between the CON and RG groups (Fig. 10B).

Figure 9. Volcano plot and classification of different metabolites between groups. Differential volcano plot between the CON and LR groups (A) and between the CON and RG groups (B). Classification of differential metabolites between the CON and LR groups (C) and between the CON and RG groups (D).

Figure 10. Top 30 most enrichment pathways based on metabolites difference between the CON and LR groups (A) and between the CON and RG groups (B). The size of the bubbles in the figure represents the number of the pathway enriched, and the color of the bubbles indicate the magnitude of enrichment significance based on P-values.
In order to visualize the differences in metabolite patterns more comprehensively, hierarchical clustering analysis was performed based on the top 50 different metabolites screened in the LR and CON groups, and the RG and CON groups, and the results are shown in Fig. 11. Tryptophanamide, alanyllysine, 11-hydroxy-9-tridecadienoic acid, dodecanedioic acid and 6-hydroxynicotinic acid were significantly upregulated in the LR group compared with the CON group, while lysophospholipids were significantly downregulated. Substances such as indole-3-carboxylic acid, dodecanedioic acid, 11-hydroxy-9-tridecenoic acid and tryptophanamide were significantly upregulated in the RG group compared with those in the CON group, while lysophospholipids, oxoglutaric acid and acetophenone were significantly downregulated.

Figure 11. (A) Hierachical clustering analysis of the differential metabolite in the LR and CON groups. (B) Hierachical clustering analysis of the differential metabolite in the RG and CON groups.
Correlation analysis between cecum microbiota and metabolomics
To further investigate the relationships between cecal microbiota and metabolites, Spearman correlation analysis was performed in the three groups (the CON and LR groups, the CON and RG groups). The relative abundance of Bacteroides was positively correlated with tryptophanamide, alanyllysine, 11-hydroxy-9-tridecadienoic acid, dodecanedioic acid, 6-hydroxynicotinic acid, and negatively correlated with lyso-phosphatidylcholine and lyso-phosphatidylethanolamine (Fig. 12A). Meanwhile, the relative abundance of Bacteroides, Prevotellaceae_UCG-001, norank_f__Muribaculaceae and Ruminococcus_torques_group was positively correlated with indole-3-carboxylic acid, dodecanedioic acid, 11-hydroxy-9-tridecenoic acid and Trp amide (Fig. 12B). In addition, the relative abundance of Barnesiella and norank__o___Clostridia_UCG-014 was positively correlated with the common differential metabolites lysophospholipids, oxoglutaric acid and acetophenone (Fig. 12B).
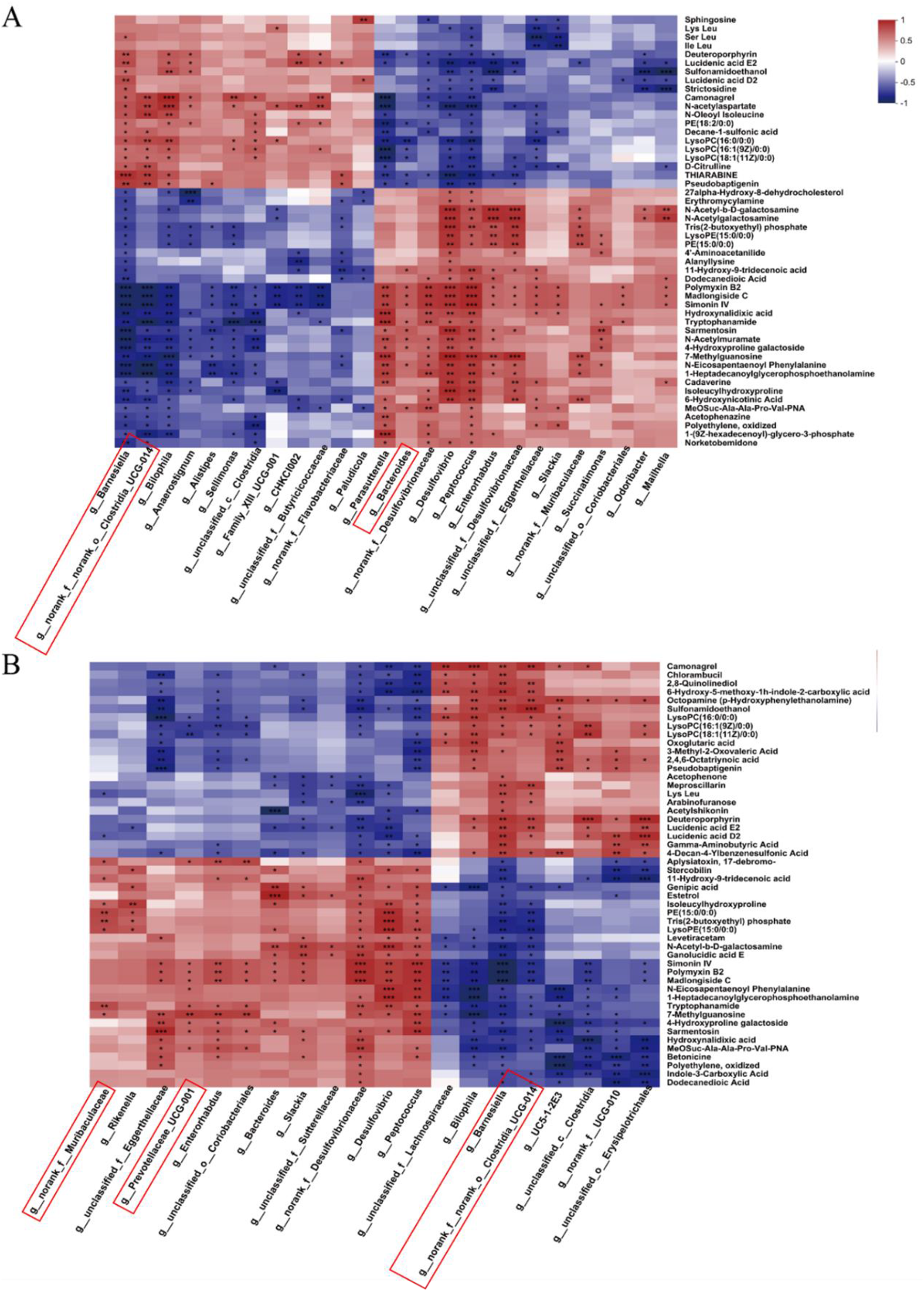
Figure 12. Correlations between significantly altered cecal microbiota and metabolite between the CON and LR groups (A), and between the CON and RG groups (B). Significance was shown as *P < 0.05, **P < 0.01 and ***P < 0.001.
Discussion
After the ban on antibiotics as feed additives, synbiotics is ideal alternatives for the promoting growth performance. Previous research has shown that LR and its combination of oligofructose could improve the growth performance in poultry, owing to LR abilities to produce bacteriocins and organic acids, and GlcOS catabolized and metabolized to provide suitable substrates for probiotics, inhibiting the adhesion of pathogenic bacteria on the intestine, as shown the in analogous effect of Bacillus subtilis, xylo-oligosaccharide and mannan oligosaccharides combination on broilers (Bhogoju et al. Reference Bhogoju, Khwatenge and Taylor-Bowden2021; Min et al. Reference Min, Yang and Xu2016; Quigley Reference Quigley2019; Song et al. Reference Song, Li and Wang2022; Sureshkumar et al. Reference Sureshkumar, Lee and Lee2022; Xu et al. Reference Xu, Wang and Ding2022b). Our investigation demonstrated that a dietary LR and GlcOS could significantly improve the ADG and decrease the F/G of broilers, the combination of which is even more efficient than solitary LR, aligning with the findings of our previous studies, showing an analogous effect (Ding et al. Reference Ding, Tang and Zhao2024; Xu et al. Reference Xu, Ding and Wang2022a, Reference Xu, Li and Ding2023, Reference Xu, Wang and Ding2022b).
One of the underlying mechanism of probiotics as growth promoters can be attributed to the improved intestinal morphology and barrier function, meaning enhancement of gut health and nutrients absorption (Paiva et al. Reference Paiva, Walk and McElroy2014; Wang et al. Reference Wang, Xu and Xu2021b). In this study, dietary LR and RG improved the mucosal morphology of the small intestine, showing as higher VH, VH:CD and better-arranged microvilli, with RG demonstrating even superior effect than LR, assembling the effect of synbiotics containing LR and fructo-oligosaccharides in broilers (Jiang et al. Reference Jiang, Mohammed and Jacobs2020). Besides, our findings revealed that dietary LR and RG significantly increased the number of goblet cells in the jejunum of broilers, contributing to enhancing the intestinal mucosal barrier integrity and function, in accordance to previous studies (Figueroa-Lozano et al. Reference Figueroa-Lozano, Ren and Yin2020; Pelaseyed et al. Reference Pelaseyed, Bergström and Gustafsson2014). In terms of barrier function, indigestible metabolite d-lactic acid, fermented by gut microbiota, and DAO, an intestinal intracellular enzyme, are normally precluded by intact mucosal barrier from serum, serving as markers of intestinal epithelium permeability, the serum level of which are significantly reduced by LR and RG. Tight junction proteins, such as ZO-1, Occludin and Claudin-1, and mucus barrier protein MUC2 constitute a crucial element of the intestinal in resistance to pathogens intrusion (Chelakkot et al. Reference Chelakkot, Ghim and Ryu2018; Cornick et al. Reference Cornick, Kumar and Moreau2019; Murray et al. Reference Murray, Barbose and Cobb1993; Tsunooka et al. Reference Tsunooka, Maeyama and Nakagawa2006; Van Itallie and Anderson Reference Van Itallie and Anderson2014). In our research, LR and RG supplementation markedly increased the mRNA expression of Occludin and MUC2, suggesting that LR and RG supplementation provided a solid intestinal barrier of tight junction and mucin secretion by goblet cells.
Probiotics promoting growth attribute not only to the improved intestinal morphology and barrier but also more active digestive enzymes and higher nutrient transportation efficiency (Zhang et al. Reference Zhang, Wang and Zhang2022). It can be concluded from our results that probiotics applied can directly secrete digestive enzymes based on the fact that RG feed additives increased the activities of AMS, LPS and TPS in the jejunum, as well as the TPS and AMS activities in the ileum. Moreover, functional oligosaccharides can provide energy for the intestinal tract continuously, reduce the loss of digestive enzymes and indirectly improve the activity of digestive enzymes (Zhang et al. Reference Zhang, Zhang and Zhan2016). Nutrients transportation efficiency via transporter carriers is crucial for absorption and utilization. Glucose absorption is predominantly mediated by the Na+-dependent glucose transporter carrier-1 (SGLT1) (encoded by SLC5A1) (Breves et al. Reference Breves, Kock and Schröder2007). PepT1 (encoded by SLC15A1) is primarily responsible for the transport of small peptides, the primary digestion products of proteins, while amino acids are absorbed and transported mainly through EAAT3 (encoded by SLC1A1) and B0AT (encoded by SLC6A19) (Bröer Reference Bröer2009; Daniel Reference Daniel2004; Kanai and Hediger Reference Kanai and Hediger2004; Mann et al. Reference Mann, Yudilevich and Sobrevia2003). The RG group showed significantly increased mRNA levels of SGLT1, EAAT3, B0AT and in the jejunum of broilers, which indicated that RG could promote the absorption of amino acids and glucose and thus improve the growth performance of broiler chickens.
The gut microbiota plays a pivotal role in broilers gastrointestinal health, the dysbiosis of which can be relieved by feed supplementation of synbiotics to maintain balanced structure (Graham and Xavier Reference Graham and Xavier2020; Peng et al. Reference Peng, Tang and Huang2021; Schirmer et al. Reference Schirmer, Garner and Vlamakis2019; Zheng et al. Reference Zheng, Liwinski and Elinav2020). The RG group exhibited significant efficacy in augmenting the α-diversity of the intestinal microbiota in broilers, leading to more diverse gut microbiota and stable microbial equilibrium. Our results showed LR and RG induced higher relative abundance in immunity-regulating Ruminococcaceae and Lachnospiraceae, short-chain fatty acid producing, Rikenellaceae, Inhibiting the production of proinflammatory lipid peroxidation metabolites Desulfobacterota, butyrate-producing Bacteroidaceae, carbohydrates and proteins-degrading Prevotellaceae, but lower abundance of pro-inflammatory and intestinal barrier impairing Barnesiella and norank_f_norank_o_Clostridia_UCG-014 showed a significant decrease in the LR and RG groups (Guo et al. Reference Guo, Xiang and Mao2021; Li et al. Reference Li, Zhang and Zhang2022; Liu et al. Reference Liu, Wu and Chen2022; Shang et al. Reference Shang, Shan and Cai2018; Song et al. Reference Song, He and Pan2023; Sun et al. Reference Sun, Ji and Ye2024; Walker et al. Reference Walker, McEwan and Wallace2003; Wang et al. Reference Wang, Nan and Zhao2021a; Zhang et al. Reference Zhang, Lv and Zhong2023; Zhao et al. Reference Zhao, Zhang and Ma2017). In summary, our results indicate that dietary LR and RG can promote the probiotics colonization and suppressing the propagation of harmful bacteria.
The alterations in the intestinal microbiota structure would inevitably modulate intestinal metabolism and regulate host health. In this study, a cecal metabolomics analysis of broilers revealed significant changes in enrichment in cofactor biosynthesis, amino acid metabolism, glycerophospholipid and choline metabolism, suggesting their potential as targets for the health-promoting effects of LR and RG on broilers. Cofactor biosynthesis plays a significant role in a variety of life activities of the organism such as energy metabolism, DNA and protein synthesis, and immune function (Magnúsdóttir et al. Reference Magnúsdóttir, Ravcheev and de Crécy-lagard2015; Peterson et al. Reference Peterson, Rodionov and Osterman2020). The findings above demonstrated a marked elevation in the levels of the cofactor metabolites flavin adenine dinucleotide (FAD) and 4-hydroxybenzoic acid in the RG group. As the active form of riboflavin in vivo, FAD orchestrates the regulation of redox reactions, the tricarboxylic acid cycle and reactive oxygen species, thereby mitigating oxidative stress (Powers Reference Powers2003). Moreover, 4-hydroxybenzoic acid stimulates Nrf2 signaling to curtail apoptosis and mitigate inflammation associated with intestinal injury (Han et al. Reference Han, Guo and Bu2021). In contrast, RG supplementation significantly decreased tyramine levels, attributing to alleviated intestinal stress (Jacobs et al. Reference Jacobs, Lagishetty and Hauer2023). Moreover, alanine, aspartate and glutamate metabolism are integral to the biosynthesis of the endogenous antioxidant glutathione. Aspartate undergoes citric acid cycle transforming to glutamate, an essential amino acid constituent of glutathione (Brand and Chappell Reference Brand and Chappell1974). Research indicates that these metabolites contribute to energy providing in the intestinal tract, protecting intestinal mucosa, mitigate mucosal injury induced by bacterial endotoxins, delay lymphocyte apoptosis and facilitate cellular proliferation (Wang et al. Reference Wang, Liu and Li2015). In the cecum of the RG group, the levels of alanine, aspartate and glutamate were significantly higher than the CON group. Recent studies have uncovered the critical roles of microbial Trp metabolites in mediating colonization resistance against significant fungal and bacterial enteric pathogens. In general agreement with the results of the former studies, dietary RG elevated the levels of the Trp metabolite, indole-3-carboxylic acid, which activate the AhR signaling pathway and enhance T cell production of interleukin-17 (IL-17) to promote antimicrobial peptide production and enhance barrier function (Fang et al. Reference Fang, Pan and Wang2022; Montgomery et al. Reference Montgomery, Eckstrom and Lile2022). A microbial Trp metabolite indole-3-aldehyde contributes to hydrocarbon receptor (AhR)-dependent-IL-22 transcription that helps to protect against Candida albicans infection (Zelante et al. Reference Zelante, Iannitti Rossana and Cunha2013). Prior research indicated that Trp metabolite (including indole-3-aldehyde, indole-3-pyruvate and indole-3-ethanol) provide resistance against enterohemorrhagic E. coli and Citrobacter rodentium, further confirming the intimate association between altered cecal microbiota and corresponding metabolite profiles due to the dietary inclusion of RG (Scott et al. Reference Scott, Fu and Chang2024). In our research, the relative abundance of advantageous bacteria in the LR and RG groups positively associated with a significant increase in metabolites; however, it was negatively correlated with the increase in metabolites such as lysophospholipids, oxoglutaric acid and acetophenone. The robust correlation between gut microflora and metabolites suggests that significant changes may improve the structure of the intestinal microbiota and modulate intestinal metabolism, and ultimately enhance the growth performance of broilers.
Conclusions
In summary, dietary supplementation with LR and RG significantly enhances the intestine integrity, digestion and absorption, microbiota structure and metabolic activity of broiler chickens, thereby facilitating intestine health and growth performance, providing RG as a potential antibiotics alternative in poultry industry. However, the experiment was carried out under controlled conditions, the evidence supporting the field application of which is still lacking. Moreover, synergistic mechanisms between LR and GlcOS in immune regulation remained unexplored.
Acknowledgements
This work was supported by the Key R & D program of Zhejiang Province (project: 2023C02026, 2022C02043), National Nature Science Foundation of China (project: 32372892), Nature Science Foundation Key Program of Zhejiang Province (project: Z25C170005), the Hangzhou Agricultural and Social Development Key Research and Development (project: 202203A10), China Agriculture Research System of MOF and MARA (project: CARS-41), and Postdoctoral Fellowship Program of CPSF.
Author Contributions
Conceptualization, X.Z. and A.F.; Methodology, R.T., J.C. and S.L.; Software, R.T., S.L. and J.C.; Validation, R.T., J.Z. and B.C; Formal analysis, X.D. and Y.X.; Data curation, R.T. and S.L.; Writing-original draft, R.T.; Writing -review &editing, R.T. and J.C. Visualization, R.T., J.C. and S.L.; Supervision, X.Z.; Project administration, X.Z. and A.F.; Funding acquisition, X.Z. A.F. and Y.X. All authors have read and agreed to the published version of the paper.
Conflicts of Interest
The authors declare no competing interests.
















