Introduction
About half of the children in the US will experience at least one type of adversity, which can include exposure to abuse, neglect, violence, separation from caregivers, or household substance use (Giano et al., Reference Giano, Wheeler and Hubach2020). These experiences in childhood have lasting effects on development into adulthood, as evidence indicates that multiple early adverse experiences increase risk for both negative mental and physical health outcomes (Hughes et al., Reference Hughes, Bellis, Hardcastle, Sethi, Butchart, Mikton, Jones and Dunne2017). Studies have found that early adversity and maltreatment are associated with risk for depression and early initiation of substance use (Andersen & Teicher, Reference Andersen and Teicher2009; Pagliaccio & Barch, Reference Pagliaccio and Barch2016). The development of cognitive control is particularly salient during adolescence given that cortical areas typically undergo protracted development during this period (Casey & Jones, Reference Casey and Jones2010), and neurodevelopmental trajectories of these brain regions can be influenced by early adversity (Duffy et al., Reference Duffy, Mclaughlin and Green2018). Identifying mechanisms of adversity related to brain development that may increase risk for negative outcomes is important to advance prevention and intervention efforts that promote positive development and resilience. This study focuses on addressing how different characteristics of maltreatment in childhood and adolescence – such as the timing, chronicity, and subtype – influence neurodevelopment during cognitive control and mental health outcomes during young adulthood.
Maltreatment type and timing associations with cognitive control
Adolescence is a particularly vulnerable neurodevelopmental period which includes neural maturation in regions related to both higher-order cognitive control and reward processing. Current developmental neuroscience models propose that heighted reward seeking coupled with more slowly maturing cognitive control processes may lead to risky decision making and other poor mental health outcomes (Casey et al., Reference Casey, Getz and Galvan2008). Extant neuroimaging studies identify adverse experiences such as child maltreatment as critical risk factors of suboptimal brain development. First, a few structural neuroimaging studies have examined individual differences in brain structure varying by maltreatment experiences in brain regions known to be involved in cognitive control. For example, adults with emotional maltreatment before the age of 16 exhibited reduced dorsal medial prefrontal (dmPFC) cortex volume compared to those with no history of childhood maltreatment (Van Harmelen et al., Reference Van Harmelen, Van Tol, Van Der Wee, Veltman, Aleman, Spinhoven, Van Buchem, Zitman, Penninx and Elzinga2010). Similarly, young adults with a history of childhood maltreatment (combined experiences of abuse and neglect) showed lower gray matter volume in prefrontal regions (Kirsch et al., Reference Kirsch, Tretyak, Radpour, Weber, Nemeroff, Fromme, Strakowski and Lippard2021).
Next, evidence from functional neuroimaging research using cognitive control tasks supports that cumulative adversity and experiences of abuse and neglect are associated with altered activation and resting-state connectivity in regions involved in cognitive control. Specifically, a cross-sectional study found associations between maltreatment history (combined experiences of abuse and neglect) and heightened activation in the dorsomedial frontal regions during an inhibitory control task among adolescents (Lim et al., Reference Lim, Hart, Mehta, Simmons, Mirza and Rubia2015). Further, in a longitudinal study of adolescents, abuse (ages 1–17) was associated with a steeper developmental decrease of frontoparietal activation during a cognitive control task during adolescence (ages 14–17), suggesting that abuse is related to accelerated maturation of regions involved in cognitive control (Kim-Spoon et al., Reference Kim-Spoon, Herd, Brieant, Peviani, Deater-Deckard, Lauharatanahirun, Lee and King-Casas2021). Similarly, in a sample from the Adolescent Brain Cognitive Development (ABCD) study, greater number of negative life events (e.g., death of family member, victim of crime/violence/assault) assessed at 10–11 years was associated with greater negative cortico-limbic resting-state functional connectivity over a two-year period, suggesting an accelerated neurobiological maturation (Brieant et al., Reference Brieant, Sisk and Gee2021).
Alternatively, there is evidence suggesting that cumulative childhood maltreatment (combined experiences of abuse and neglect) was associated with an increase in resting-state functional connectivity between default mode and frontoparietal networks at age 16 to age 19 (Rakesh et al., Reference Rakesh, Kelly, Vijayakumar, Zalesky, Allen and Whittle2021b). This finding, in contrast to previous evidence of accelerated maturation, appears to suggest a developmental delay associated with maltreatment, as maltreatment was positively related to between-network resting-state functional connectivity, which decreased with age (Rakesh et al., Reference Rakesh, Kelly, Vijayakumar, Zalesky, Allen and Whittle2021b). The discrepancy between the two resting-state functional connectivity studies (Brieant et al., Reference Brieant, Sisk and Gee2021; Rakesh et al., Reference Rakesh, Kelly, Vijayakumar, Zalesky, Allen and Whittle2021b) may be in part due to the fact that data were collected at different times in ontogeny. That is, early maltreatment may be linked with an accelerated maturation during adolescence (or earlier), whereas it is linked with a developmental delay during late adolescence and in adulthood, as the control system approaches its full maturation. Lastly, in a sample of adolescents ages 11–19 years, neglect, not abuse, was associated with negative amygdala resting-state connectivity with the dorsolateral prefrontal cortex (dlPFC) and positive amygdala resting-state connectivity with the supplementary motor area (SMA), both regions involved in higher-order cognitive processing (Cheng et al., Reference Cheng, Mills, Miranda Dominguez, Zeithamova, Perrone, Sturgeon, Feldstein Ewing, Fisher, Pfeifer, Fair and Mackiewicz Seghete2021). Although the authors did not discuss whether these results were consistent with accelerated or delayed maturation, these findings highlight the distinct effects of neglect versus abuse on resting-state connectivity within cognitive processing regions.
Collectively, prior research has focused on brain structure, local activation, or resting-state connectivity (i.e., intrinsic connectivity) and suggest that maltreatment may influence neurodevelopment of cognitive control systems. Importantly, despite the theoretical proposal suggesting accelerated brain maturation following early life adversity (Callaghan & Tottenham, Reference Callaghan and Tottenham2016; Herzberg et al., Reference Herzberg, McKenzie, Hodel, Hunt, Mueller, Gunnar and Thomas2021), there is a lack of longitudinal research that is necessary to understand the developmental trajectories of task-based functional connectivity (as opposed to resting-state connectivity) during cognitive control following childhood maltreatment. One critical way to enhance our understanding of the effects of maltreatment on brain development is to examine how the timing, subtype, and chronicity of maltreatment experiences may differentially influence developmental trajectories of neural connectivity within these cognitive control systems across adolescence during which neurodevelopmental plasticity is heightened (Fuhrmann et al., Reference Fuhrmann, Knoll and Blackemore2015; Gee, Reference Gee2021). The dimensional model of adversity and psychopathology proposes that threat and deprivation are separate dimensions that distinctly influence brain development (McLaughlin et al., Reference McLaughlin, Sheridan, Humphreys, Belsky and Ellis2021). Using a developmental psychopathology perspective, this model assumes maltreatment reflects early experiences that involve a lack of expected inputs from the environment (i.e., neglect) or experiences that involve harm or threat (i.e., abuse). This approach supports that experiences of threat are related to changes in emotional development, whereas experiences of deprivation are most related to changes in cognitive development (McLaughlin et al., Reference McLaughlin, Sheridan, Humphreys, Belsky and Ellis2021). The current study will incorporate this perspective by examining abuse and neglect effects on neurocognitive functioning within multiple developmental periods. Specifically, we will focus on regions of the salience network (e.g., dorsal anterior cingulate cortex, dACC, and insula) as prior work has consistently linked experiences of maltreatment with reduced connectivity between these two key regions of the salience network (Gerin et al., Reference Gerin, Viding, Herringa, Russell and McCrory2023).
Neural cognitive control as a mechanism linking maltreatment and mental health
In line with developmental neuroscience models proposing the critical role of neural cognitive control in the likelihood of risky decision making (Casey et al., Reference Casey, Getz and Galvan2008), evidence suggests that altered cognitive control processes are associated with increased substance use, particularly during adolescence and the transition into young adulthood. For instance, adolescents who were more likely to use cannabis and alcohol showed lower behavioral inhibitory control (Morin et al., Reference Morin, Afzali, Bourque, Stewart, Séguin, O’Leary-Barrett and Conrod2019). Similarly, altered neural activation during inhibitory control predicted the initiation of substance use in adolescence (Norman et al., Reference Norman, Pulido, Squeglia, Spadoni, Paulus and Tapert2011). Further, adolescents completed a working memory task and altered neural activation in frontoparietal and executive planning regions predicted future initiation of cannabis use (Tervo-Clemmens et al., Reference Tervo-Clemmens, Simmonds, Calabro, Montez, Lekht, Day, Richardson and Luna2018). Findings suggest that there may be a neurocognitive vulnerability associated with risk for early and severe substance use. Indeed, prior research shows that the insula and dACC are associated with drug addiction (Naqvi et al., Reference Naqvi, Gaznick, Tranel and Bechara2014; Paulus & Stewart, Reference Paulus and Stewart2014) and self-reported substance use during adolescence (Lauharatanahirun et al., Reference Lauharatanahirun, Maciejewski, Kim-Spoon and King-Casas2023). These two regions of the salience network are invovled in multiple cogntive control processes such as conflict monitoring, motor planning, and inhibition (Botvinick et al., Reference Botvinick, Cohen and Carter2004; Bush et al., Reference Bush, Shin, Holmes, Rosen and Vogt2003; Molnar-Szakacs & Uddin, Reference Molnar-Szakacs and Uddin2022). Therefore, the current study aimed to focus on dACC–insula connectivity within the salience network during cognitive control.
Alternatively, cognitive theories of depression highlight low cognitive control among individuals with depression (Gotlib & Joormann, Reference Gotlib and Joormann2010). Recent cognitive neuroscience models further posit that understanding how these neurocognitive processes confer risk for the onset and maintenance of depression could inform the development of targeted interventions and cognitive control training programs (Grahek et al., Reference Grahek, Everaert, Krebs and Koster2018). Indeed, there is evidence of altered resting-state connectivity within cognitive networks in high-risk adolescents whose parents have a history of depression (Clasen et al., Reference Clasen, Beevers, Mumford and Schnyer2014). Additionally, another study found that greater recruitment of regions associated with cognitive control (i.e., insula and dmPFC) during reappraisal of sad images was associated with enhanced emotion regulation strategies in youth at-risk for depression (Elsayed et al., Reference Elsayed, Rappaport, Luby and Barch2021). Thus, the finding indicates that neural cognitive control processes may play a role in emotion regulation, which has implications for depression risk during adolescence. Prior research has highlighted the importance of disrupted anterior cingulate cortex connectivity in the development of depression due to its critical role in cognitive and affective processing that guides self-regulation (Lichenstein et al., Reference Lichenstein, Verstynen and Forbes2016). Similarly, a resting-state connectivity study found that increases in within salience network connectivity (e.g., insula, ACC) mediated the association between maltreatment and higher depressive symptoms in an adolescent sample (Rakesh et al., Reference Rakesh, Allen and Whittle2021a). Together, studies highlight the importance of the dACC and insula as mechanisms linking maltreatment and psychopathology. Thus, the current study will investigate how dACC and insula connectivity during cognitive control may mediate dimensions of maltreatment (e.g., abuse and neglect) and future depressive symptoms.
As such, review of prior research provides reason to believe that understanding how maltreatment influences neurocognitive vulnerability that increases risk for substance use and depression is important for developing preventative intervention strategies, as improving neurocognitive functioning may mitigate the effects of adversity on negative mental health outcomes. However, little work has been done, and available research thus far focused only on resting-state connectivity, yielding mixed results: For example, one study examining adolescent resting-state connectivity within the salience network found that both abuse and neglect predicted stronger within-network connectivity, which predicted lower problematic substance use but higher depressive symptoms (Rakesh et al., Reference Rakesh, Allen and Whittle2021a). Clearly, more research is needed to gain a mechanistic understanding of how different characteristics of maltreatment may serve as risk factors for substance use and depressive symptoms, with one such mechanism being the development of neural cognitive control.
Present study
Childhood maltreatment represents a deviation from the expected environment during a time in which learning is critical for the development of cognition, emotion, and behavior (Cicchetti, Reference Cicchetti2013). It follows that maltreatment during childhood can disrupt these learning processes that offer foundations for neurocognitive development. Furthermore, it has been proposed that there may be sensitive periods during childhood and adolescence where the effects of maltreatment are most influential for brain development (Fuhrmann et al., Reference Fuhrmann, Knoll and Blackemore2015).
The main goal of this study is to examine neurocognitive processes linking different characteristics of child maltreatment – type, timing, and chronicity – and risk for negative mental health outcomes including substance use and depressive symptoms. This study considers maltreatment occurring in three developmental periods (during ages 1 to 18) to investigate timing and chronicity of maltreatment. Further, following the dimensional model of adversity and psychopathology that proposes threat (i.e., abuse) and deprivation (i.e., neglect) experiences are associated with distinctive neurobiological processes leading to psychopathology (McLaughlin et al., Reference McLaughlin, Sheridan, Humphreys, Belsky and Ellis2021), independent effects of abuse and neglect are examined.
Studies examining longitudinal changes in brain activation during cognitive control have found developmental changes in regions such as the insula and dACC (Kim-Spoon et al., Reference Kim-Spoon, Herd, Brieant, Peviani, Deater-Deckard, Lauharatanahirun, Lee and King-Casas2021; Ordaz et al., Reference Ordaz, Foran, Velanova and Luna2013). The dACC and insula are two main regions involved in the salience network (SN). They play a role in detecting external salient stimuli and filtering and integrating relevant information, which influences future goal-directed behavior (Menon & Uddin, Reference Menon and Uddin2010; Seely et al., Reference Seeley, Menon, Schatzberg, Keller, Glover, Kenna, Reiss and Greicius2007). Further, literature suggests that these two regions are especially important in multiple cognitive control processes including conflict monitoring, motor planning, and inhibition (Botvinick et al., Reference Botvinick, Cohen and Carter2004; Bush et al., Reference Bush, Shin, Holmes, Rosen and Vogt2003; Molnar-Szakacs & Uddin, Reference Molnar-Szakacs and Uddin2022). Disruption in the salience network is not only important for cognitive control processes but also plays a critical role in the development of psychiatric disorders, including depression and substance use (see McTeague et al., Reference McTeague, Rosenberg, Lopez, Carreon, Huemer, Jiang, Chick, Eickhoff and Etkin2020 for a meta-analysis). Thus, this study focuses on dACC–insula connectivity during cognitive control.
The present study has three specific aims: 1) to examine how the type and timing of maltreatment (i.e., abuse and neglect during early childhood, middle childhood, and adolescence, as well as chronicity) are associated with longitudinal changes in dACC–insula connectivity during cognitive control across adolescence; 2) to examine how these neural connectivity changes during adolescence prospectively predict substance use and depressive symptoms during young adulthood; and 3) to examine if changes in dACC–insula connectivity during cognitive control mediate the links between the type and timing of maltreatment in childhood and adolescence with substance use and depressive symptoms during young adulthood.
Method
Participants
The sample included 167 adolescents (53% male) from a southeastern state in the United States. Adolescents completed annual assessments across seven years and were 13 to 14 years of age at Time 1 (M = 14.07, SD = 0.54 for Time 1, M = 15.05, SD = 0.54 for Time 2, M = 16.07, SD = 0.56 for Time 3, M = 17.01, SD = 0.55 for Time 4, M = 18.89, SD = 0.62 for Time 5, M = 20.17, SD = 0.63 for Time 6, M = 21.26, SD = 0.64 for Time 7). About 78% of adolescents identified as White, 14% as Black, 2% as other, and 6% as more than one race. The median annual family income was in the $35,000–$50,000 range. Inclusion criteria included being age 13 or 14 at Time 1. Exclusion criteria were claustrophobia, history of head injury resulting in loss of consciousness for > 10 minutes, orthodontia impairing image acquisition, and other contraindications to magnetic resonance imaging (MRI). At Time 1, 157 families participated, and at Time 2, 10 families were added for a final sample of 167 parent–adolescent dyads. At Time 2 data from 150 participants, at Time 3 data from 147 participants, at Time 4 data from 150 participants, at Time 5 data from 126 participants, and at Time 6 124 participants were collected. Participants did not always participate in all possible assessments for reasons including ineligibility for tasks (i.e., brain abnormality, not meeting MRI safety criteria), declined participant, and lost contact. Rate of participation was not significantly predicted by demographic backgrounds including sex, race, and family income (p = .083).
Procedures
Data included in the current study were collected as part of a larger project. Adolescent participants were recruited via flyers, email announcements, and snowball sampling (word-of-mouth). Data collection was administered at university offices where participants completed self-report questionnaires, behavioral and neuroimaging tasks, and were interviewed by trained research assistants. The study duration was, on average, five hours long and participants were compensated monetarily for their time. All procedures were approved by the institutional review board of the university, and written informed consent or assent was received from all participants.
Measures
Maltreatment
The Maltreatment and Abuse Chronology of Exposure (MACE; Teicher & Parigger, Reference Teicher and Parigger2015), was used to evaluate severity of exposure to different types of maltreatment during each year of childhood and adolescence (ages 1–18 years). Adolescents completed this questionnaire twice at ages 18 and 19–20 years and were asked to retrospectively indicate ages at which they experienced events from 52 items each time. Teicher and Parigger (Reference Teicher and Parigger2015) reported acceptable test–retest reliability (across a range of 5–441 days) for all subtypes (r = .63–.90). The test–retest reliability across time points (over one year) was acceptable for the current sample (r = .56–.85). A maximum score was calculated between both reports across ages and subtypes. To examine the presence of maltreatment during three sensitive developmental periods, early childhood is defined as ages 1–5, middle childhood is 6–12 and adolescence is 13–18. Neglect included two subscales of physical neglect (5 items) and emotional neglect (5 items). Abuse included four subscales of physical abuse (6 items), sexual abuse (7 items), and verbal abuse (4 items), and emotional abuse (6 items). A sample item of physical abuse includes “hit you so hard it left marks for more than a few minutes” and a sample item of physical neglect, reverse coded, includes “were there to take care of you and protect you”. Severity scores at each age were calculated using an algorithm provided by Teicher & Parigger (Reference Teicher and Parigger2015). Chronicity scores were calculated so that a value of 3 represents maltreatment occurring in all three developmental periods, a value of 2 represents maltreatment occurring in 2 developmental periods, a value of 1 represents maltreatment occurring during 1 developmental period, while a value of 0 represents maltreatment occurring during no developmental periods.
Depressive symptoms
Depressive symptoms were assessed using the Adult Self-Report (ASR; Achenbach & Rescorla, Reference Achenbach and Rescorla2001) at ages 20 and 21. The ASR is a 126-item questionnaire that assess the participant’s behavior problems. The DSM oriented scale for depressive symptoms was used. The items in this scale were rated on a 3-point scale with response options ranging from 0 (not true) to 2 (very often or often true). Scores from both time points were averaged to create a depression composite capturing symptomatology during young adulthood. Reliability in the current sample (α = .82–.86) was acceptable.
Substance use
Substance use was assessed using the Adult Self-Report, substance use subscale at ages 20 and 21 (Achenbach & Rescorla, Reference Achenbach and Rescorla2001). Problem behaviors are reported on a 3-point scale range from 0 (not true) to 2 (very true or often true). The substance use subscale assessed alcohol, tobacco, and drug use. Scores from both time points were averaged to create a substance use composite for young adulthood.
Cognitive control
Adolescents completed the Multi-Source Interference Task (MSIT; Bush et al., Reference Bush, Shin, Holmes, Rosen and Vogt2003; See Figure 1) while completing a functional MRI at six time points across ages 14 to 20. Adolescents were presented with three digits in each trial and were asked to report the identity of the different digit (unlike the other two) by pressing a button. In neutral trials, the target number’s identity matched the digit’s presented location, whereas in interference trials, the target number’s identity was not congruent with the digit’s presented location.
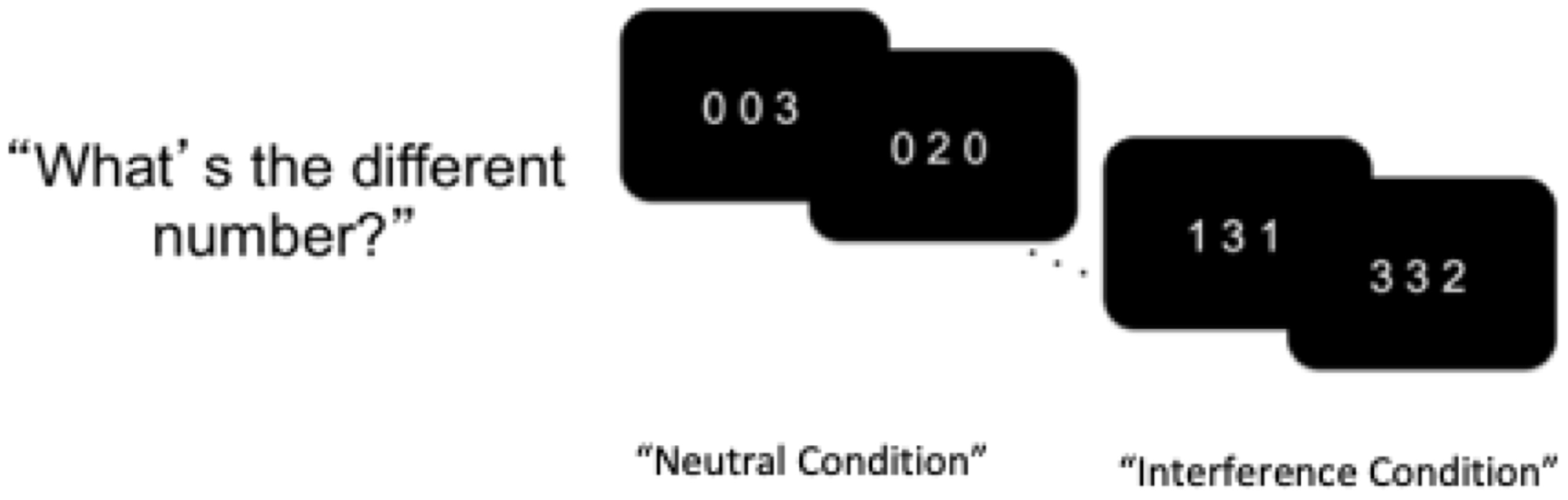
Figure 1. Schematic display of the multi-source interference task (MSIT). Adolescents were instructed to identify the different digit while ignoring its position.
Imaging acquisition and analysis
Neuroimaging data were obtained on a 3T Siemens Tim Trio scanner using a 12-channel head matrix coil. Functional images were obtained with repetition time (TR) = 2 s, slice thickness = 4 mm, 34 axial slices, field of view (FoV) = 220x220 mm, echo time (TE) = 30 ms, flip angle = 90 degrees, voxel size = 3.4x3.4x4 mm, 64x64 grid, and slices were hyperangulated at 30 degrees from anterior–posterior commissure. Anatomical images were acquired with TR = 1.2 s, slice thickness = 1 mm, FoV = 245x245 mm, TE = 2.66 ms, flip angle = 8 degrees, and an isotropic 1mm3 voxel size across 192 slices. SPM8 (Wellcome Trust Neuroimaging Center) was used to preprocess the MRI data at all time points. Using a six-parameter rigid-body transformation, functional scans were corrected for motion. Then, the mean functional was co-registered to the corresponding anatomical image using a rigid-body transformation estimated to maximize the normalized mutual information between the anatomical and mean functional image. Next, the anatomical image was segmented to produce spatial normalization parameters which were then used to normalize the functional images to MNI-152 template. Normalization produced images resliced to an isotropic voxel size of 3 mm3. Finally, the normalized functional images were smoothed using a 6 mm full-width-half-maximum Gaussian kernel. Six rigid-body realignment parameters were included to account for the effect of in-scanner motion, and low-frequency signal was removed using a high- pass filter with cutoff of 0.006 Hz (168 s) for cognitive control data to better capture the expected signal (see Henson, Reference Henson, Penny, Firston, Ashburner, Keibel and Nichols2007, pp 200 – 203).
Neural cognitive control
Preprocessed MRI data were analyzed by entering them into a first-level analysis general linear model (GLM) in SPM8. Interference and neutral blocks were modeled using boxcars convolved with the canonical hemodynamic response function (HRF) with six motion regressors. Framewise displacement (FD) was calculated from the realignment parameters, with rotational displacement converted to millimeters using the surface of a sphere of radius 50 mm (Power et al., Reference Power, Barnes, Snyder, Schlaggar and Petersen2012; Siegel et al., Reference Siegel, Power, Dubis, Vogel, Church, Schlaggar and Petersen2014). Volumes with FD > 0.9 mm were censored by adding a volume-specific regressor for each scrubbed volume in the GLM. This frame censoring approach was used because it appeared to be beneficial to analyzing the repeated measures data simultaneously. An interference greater than neutral contrast map was generated for each GLM by subtracting the neutral beta map from the positive beta map.
gPPI
A generalized psychophysiological interactions (gPPI) toolbox in SPM 8 was used to examine task-based functional connectivity (McLaren et al., Reference McLaren, Ries, Xu and Johnson2012). A whole-brain analysis was run using the dACC as a seed region. Masks were made using WFU PickAtlas Tool in SPM to create 5 mm spheres, centered at the peak coordinates from Time 1 reported in Kim-Spoon et al., Reference Kim-Spoon, Herd, Brieant, Peviani, Deater-Deckard, Lauharatanahirun, Lee and King-Casas2021 during the interference minus neutral condition of the MSIT task. The center of the dACC seed was located at [MNI: −3, 8, 25] and the center of the bilateral insula ROI was located at [MNI: −30, 14, 13] and [MNI: 33, 20, 7]. Extracted time-series data were entered into a first-level statistical model and include the dACC physiological time series, a psychological regressor corresponding to task condition (interference versus neutral for the cognitive control task) and the psychophysiological interaction term. A second-level random effects model was conducted and included individual beta images that correspond to the interaction. Values of connectivity strength with the insula were extracted for longitudinal analyses.
Data analytic plan
Statistical analysis
For all study variables, descriptive statistics were examined to determine normality of distributions. All variable distributions were used to examine skewness, with acceptable levels less than 3, and kurtosis, with acceptable levels less than 10 (Kline, Reference Kline2011). A multivariate GLM analyses was conducted to determine if demographic variables such as sex, race, and family income need to be added as covariates.
The hypothesized models were tested via structural equation modeling (SEM) using in Mplus (Muthén & Muthén, Reference Muthén and Muthén1998-2021). The overall model fit was assessed by χ2 value, degrees of freedom, corresponding p-value, root mean square error of approximation (RMSEA), and confirmatory fit index (CFI). RMSEA values less than .08 and CFI values greater than .90 were considered an acceptable fit (Little, Reference Little2013). Full information maximum likelihood (FIML; Arbuckle, Reference Arbuckle, Marcoulides and Shumacker1996; Little & Rubin, Reference Little and Rubin2003) estimation procedure was used to handle missing data, given the superiority of FIML estimation to those obtained with listwise deletion or other ad hoc methods (Schafer & Graham, Reference Schafer and Graham2002).
We ran four separate path analysis models to assess direct and indirect effects from abuse and neglect during different developmental periods (ages 1 to 5, ages 6 to 12, ages 13–18, or chronicity of maltreatment) to substance use and depressive symptoms through changes in functional connectivity in the dACC and insula during cognitive control. We averaged Time 1 and Time 2 (ages 14–15) dACC–insula connectivity to create an early adolescent composite and averaged Time 5 and Time 6 (ages 18–20) dACC–insula connectivity to create a late adolescent composite. To assess average functional connectivity change from early adolescence to late adolescence, we included early adolescent dACC–insula connectivity as a predictor in the analysis models. To test significant levels of mediated effects, a resampling strategy was used with bootstrapping, with 10,000 iterations with bias-corrected bootstrap estimates of 95% confidence interval (Preacher & Hayes, Reference Preacher and Hayes2008).
Results
Bivariate correlations and descriptive statistics for study variables are presented in Table 1. Skewness and Kurtosis values for the three connectivity variables were within range (i.e., skewness < 3, and kurtosis < 10). Multivariate GLM analyses were conducted and indicated that sex (p = .540) and race (p = .729) were not significant predictors of substance use and depressive symptoms, and thus were not included in subsequent models. Family income was a significant predictor of substance use (p < .001) in the GLM. Subsequent models were tested with family income as a covariate, but the findings were consistent, and income was not a significant predictor of substance use in the models. Therefore, we report the models below without income as a covariate. Additionally, descriptive analyses suggest that dACC–insula connectivity increased from ages 14–15 (M = −.037) to ages 18–20 (M = .002), suggesting that dACC–insula connectivity became stronger across adolescence, although these increases were not statistically significant, t (114) = −1.11, p = .269.
Table 1. Descriptive statistics and correlations of maltreatment, neural cognitive control, substance use, and depressive symptoms
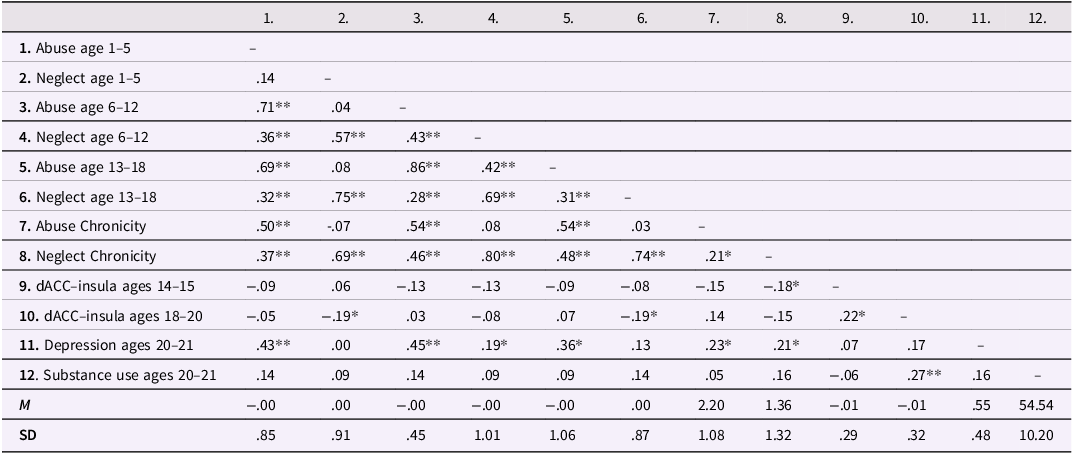
Note. dACC = dorsal anterior cingulate cortex. * p < .05; ** p < .01.
Confirmatory factor analysis for maltreatment subtypes and developmental periods
A confirmatory factor analysis (CFA) was conducted to create latent factors of neglect and abuse at each developmental period. For neglect at ages 1 to 5, the model was fully saturated (χ2 = 0.00, df = 0, p < .001, RMSEA = .00, and CFI = 1.00) and both physical neglect and emotional neglect significantly loaded onto one factor (ps < .001). For abuse at ages 1 to 5, the model based on four indicators (sexual abuse, physical abuse, emotional abuse, verbal abuse) had a model fit that was not acceptable (χ2 = 15.37, df = 2, p < .001, RMSEA = .22, and CFI = 1.86). Skewness (9.65) and kurtosis (98.67) for the sexual abuse factor was high, and the prevalence (19.2% endorsed) was notably low, therefore this subscale was removed for the following model. The next model was fully saturated (χ2 = 0.00, df = 0, p < .001, RMSEA = .00, and CFI = 1.00) and all three indicators significantly loaded onto one factor (ps < .001). Next, for neglect at ages 6 to 12, the model was fully saturated (χ2 = 0.00, df = 0, p < .001, RMSEA = .00, and CFI = 1.00) and both indicators significantly loaded onto one factor (ps < .001). For abuse at ages 6 to 12, model fit was acceptable (χ2 = 0.03, df = 2, p = .986, RMSEA = .00, and CFI = 1.00), and all four indicators significantly loaded onto one factor (ps < .001). Finally, for neglect at ages 13 to 18, the model was fully saturated (χ2 = 0.00, df = 0, p < .001, RMSEA = .00, and CFI = 1.00) and both indicators significantly loaded onto one factor (ps < .001). For abuse ages at 13 to 18, model fit was acceptable (χ2 = 1.45, df = 2, p = .474, RMSEA = .00, and CFI = 1.00), and all four indicators significantly loaded onto one factor (ps < .001).
Path analysis models examining abuse and neglect effects on substance use and depressive symptoms through changes in dACC–insula connectivity during cognitive control
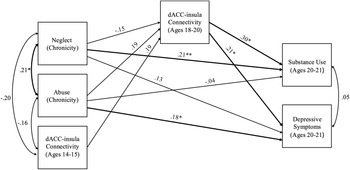
The model including chronic abuse and neglect (occurring in more than one developmental period) had acceptable fit (χ2 = 1.50, df = 2, p = .471, RMSEA = .00 and CFI = 1.00). Chronic abuse marginally predicted stronger dACC–insula connectivity during late adolescence (b = 0.06, SE = .03, p = .055). Chronic neglect did not predict changes in dACC–insula connectivity (b = -0.04, SE = .02, p = .102). Stronger dACC–insula connectivity significantly predicted substance use (b = 9.62, SE = 4.33, p = .026) and depressive symptoms (b = 3.28, SE = 1.43, p = .021) in young adulthood. Additionally, chronic abuse directly predicted higher levels of depressive symptoms (b = 0.85, SE = .41, p = .039), but not substance use (b = -0.34, SE = .68, p = .621). Chronic neglect directly predicted higher levels of substance use (b = 1.62, SE = .73, p = .027), but not depressive symptoms (b = 0.50, SE = .34, p = .138). Indirect effects of chronic abuse were significant such that chronic abuse predicted higher depressive symptoms (95% CI [0.008; 0.561]) as well as higher substance use (95% CI [0.041;1.545]) through stronger dACC–insula connectivity. See Figure 2 for a path analysis model with standardized estimates.
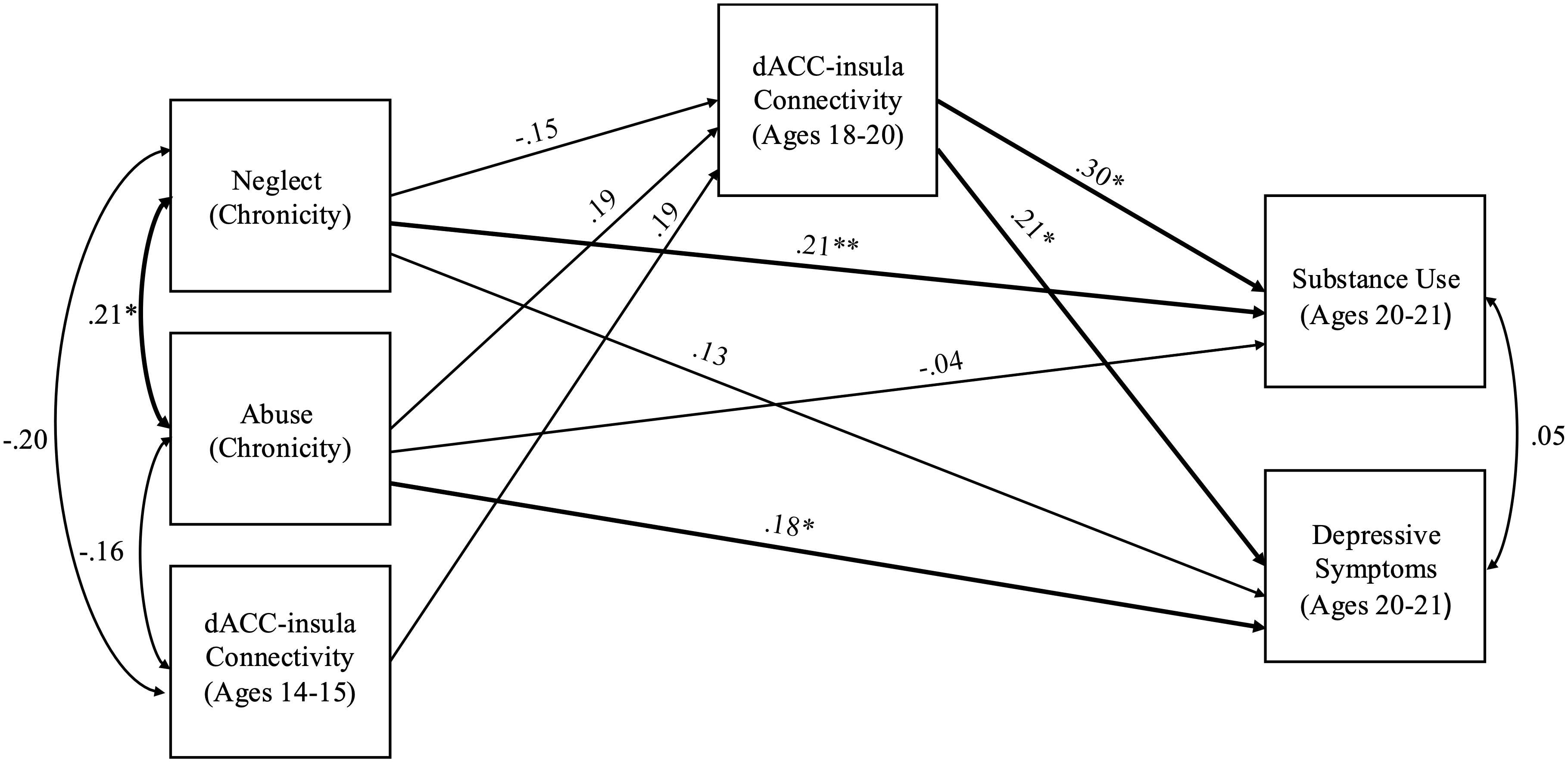
Figure 2. Path model of chronic abuse and neglect on substance use and depressive symptoms through dACC–insula changes from early to late adolescence. Standardized Estimates are presented. dACC = dorsal anterior cingulate cortex. Significant paths are boldface. Significant indirect effects from chronic abuse to higher depressive symptoms and substance use through stronger dACC-insula connectivity. *p < .05, **p < .01.
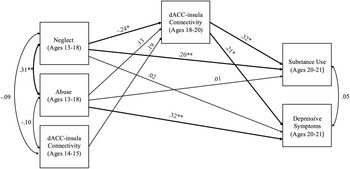
The model including abuse and neglect occurring during adolescence (ages 13 to 18) had acceptable fit (χ2 = 1.48, df = 2, p = .478, RMSEA = .00 and CFI = 1.00). Neglect during adolescence significantly predicted weaker dACC–insula connectivity (b = −0.09, SE = .04, p = .022). Abuse during adolescence did not predict changes in dACC–insula connectivity (b = 0.04, SE = .03, p = .171). Higher dACC–insula connectivity significantly predicted higher levels of substance use (b = 10.04 SE = 4.49, p = .026) and higher depressive symptoms (b = 3.19, SE = 1.36, p = .019). In addition, neglect during adolescence directly predicted higher substance use (b = 2.38, SE = .99, p = .016), but not depressive symptoms (b = 0.11, SE = .53, p = .840). In contrast, abuse during adolescence predicted higher depressive symptoms during young adulthood (b = 1.49, SE = .48, p = .002), but not substance use (b = 0.13, SE = .73, p = .857). Significant indirect effects revealed that higher levels of neglect during adolescence predicted lower substance use (95% CI [−2.478 to −0.097]) and lower depressive symptoms through weaker dACC–insula connectivity (95% CI [−0.822 to −0.035]). See Figure 3 for a path analysis model with standardized estimates.
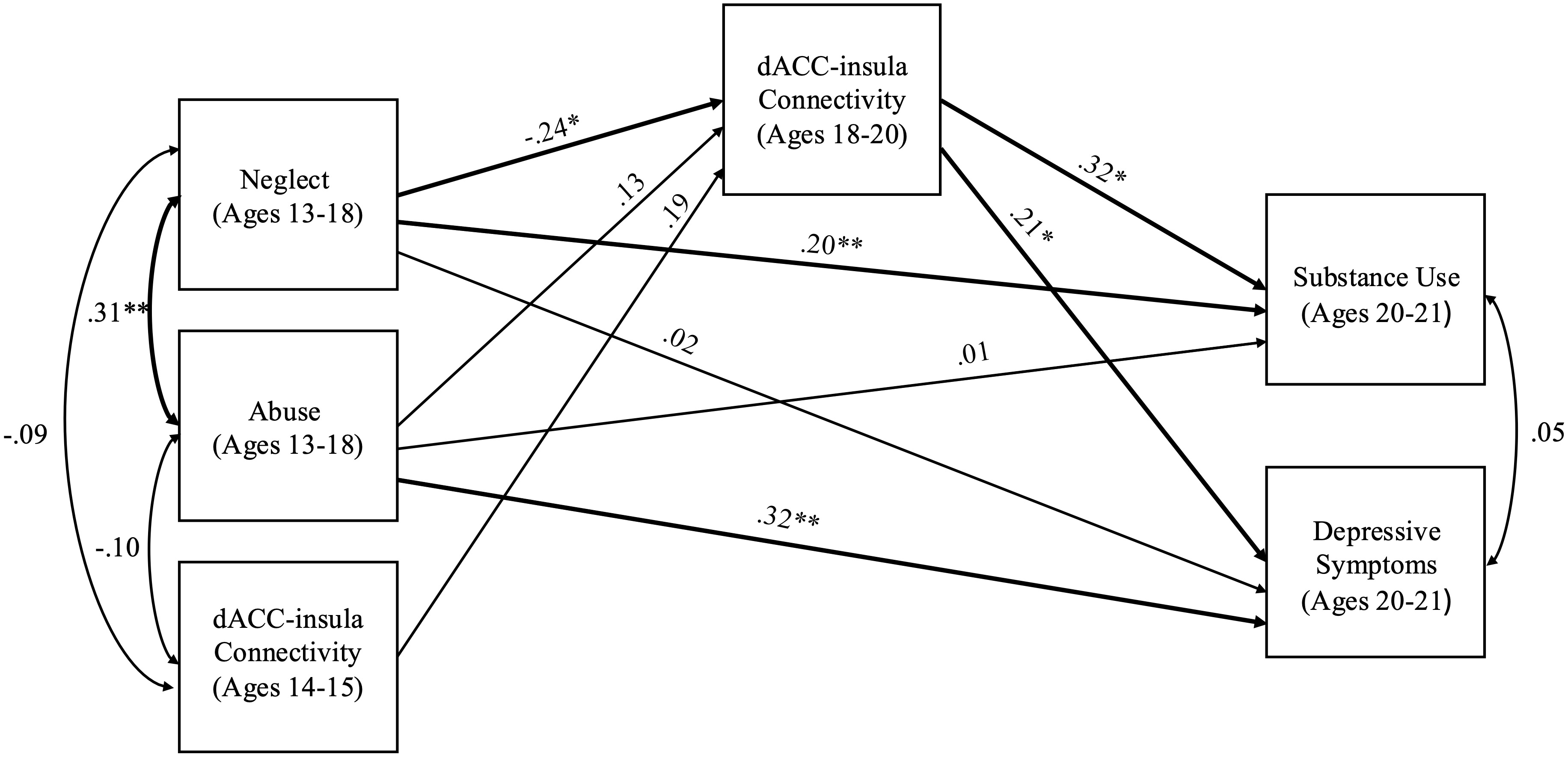
Figure 3. Path model of abuse and neglect during adolescence on substance use and depressive symptoms through dACC–insula changes from early to late adolescence. Standardized Estimates are presented. dACC = dorsal anterior cingulate cortex. Significant paths are boldface. Significant indirect effects from neglect during adolescence to lower depressive symptoms and lower substance use through weaker dACC-insula connectivity. *p < .05, **p < .01.
The model including abuse and neglect occurring during middle childhood (ages 6 to 12) had acceptable fit (χ2 = 1.51, df = 2, p = .471, RMSEA = .00 and CFI = 1.00). Abuse occurring during middle childhood significantly predicted depressive symptoms during young adulthood (b = 4.54, SE = 1.28, p < .001), but not substance use (b = 2.12 SE = 1.71, p = .217). Neglect during middle childhood did not significantly predict depressive symptoms (b = −0.14 SE = .54, p = .800) or substance use (b = 0.76, SE = .82, p = .359). Stronger dACC–insula connectivity significantly predicted higher depressive symptoms (b = 3.01, SE = 1.31, p = .022) and substance use (b = 9.10, SE = 4.39, p = .038). There were no significant associations of abuse (b = 0.08, SE = .07, p = .244) or neglect (b = −0.04, SE = .03, p = .207) with dACC–insula connectivity. There were no significant indirect effects from abuse to substance use (95% CI [−0.235 to 2.683]) or depression (95% CI [−0.095 to 0.943]) and from neglect to substance use (95% CI [−1.543 to 0.080]) or depression (95% CI [−0.482 to 0.029]). See Figure 4 for a path analysis model with standardized estimates.
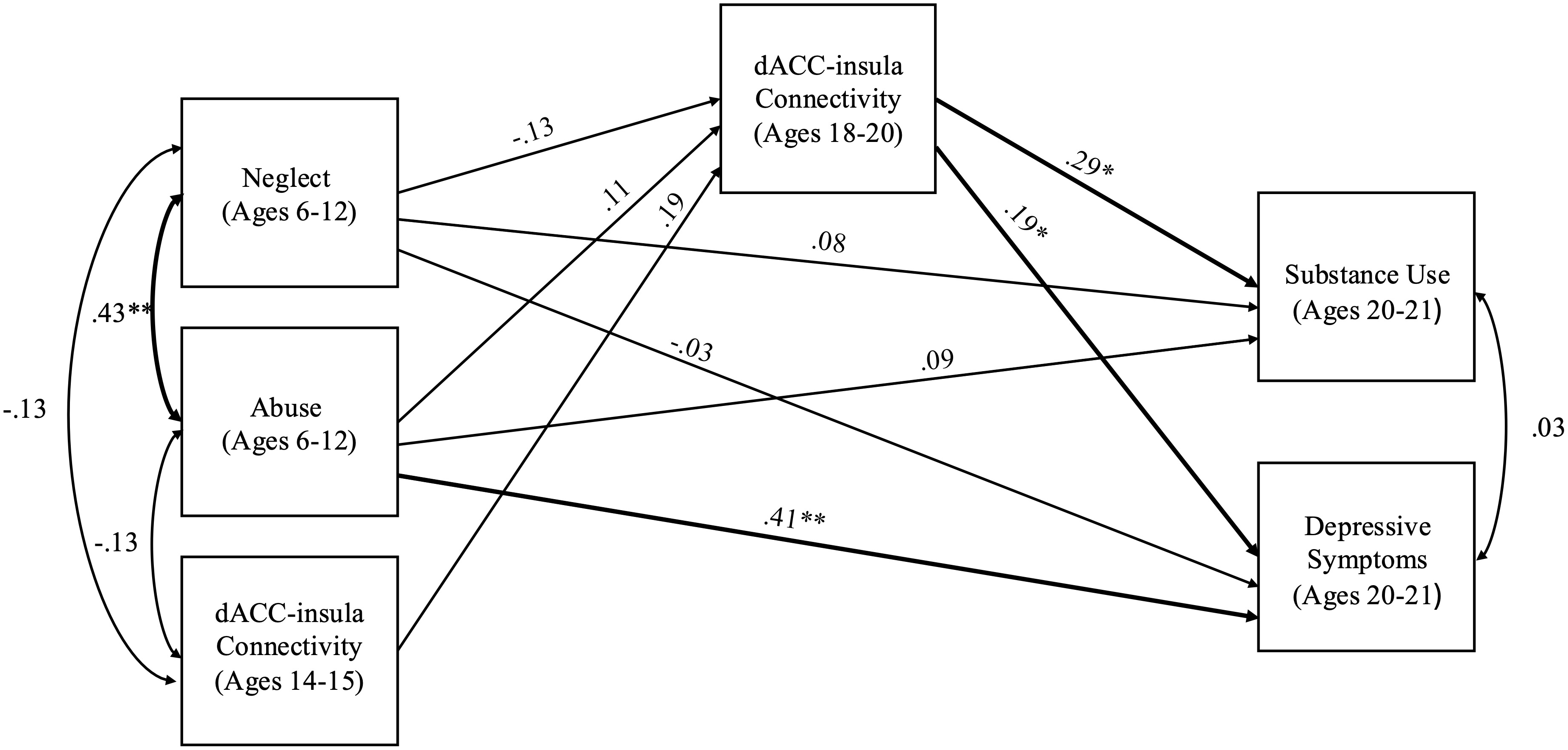
Figure 4. Path model of abuse and neglect during middle childhood on substance use and depressive symptoms through dACC–insula changes from early to late adolescence. Standardized Estimates are presented. dACC = dorsal anterior cingulate cortex. Significant paths are boldface. No significant indirect effects.*p < .05, **p < .01.
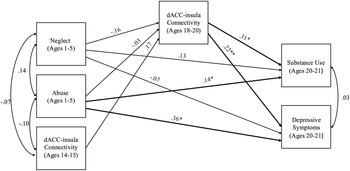
The model including abuse and neglect occurring during early childhood (ages 1 to 5) had acceptable fit (χ2 = 0.92, df = 2, p = .632, RMSEA = .00 and CFI = 1.00). Abuse occurring during childhood significantly predicted depressive symptoms (b = 2.12, SE = .63, p = .001) and substance use (b = 1.70, SE = .87, p = .052) during young adulthood. However, neglect during early childhood did not predict depressive symptoms (b = −0.39, SE = .51, p = .437) or substance use (b = 1.48, SE = .86, p = .087). Stronger dACC–insula connectivity significantly predicted higher depressive symptoms (b = 3.37, SE = 1.32, p = .011) and substance use (b = 9.86, SE = 4.27, p = .021). There were no significant associations of abuse (b = −0.01, SE = .04, p = .780) or neglect (b = −0.06, SE = .04 p = .152) with dACC–insula connectivity. There were no significant indirect effects from abuse to substance use (95% CI [−0.994 to 0.562]) or depression (95% CI [−0.331 to 0.232]) and from neglect to substance use (95% CI [−1.891 to 0.090]) or depression (95% CI [−0.621 to 0.041]). See Figure 5 for a path analysis model with standardized estimates.
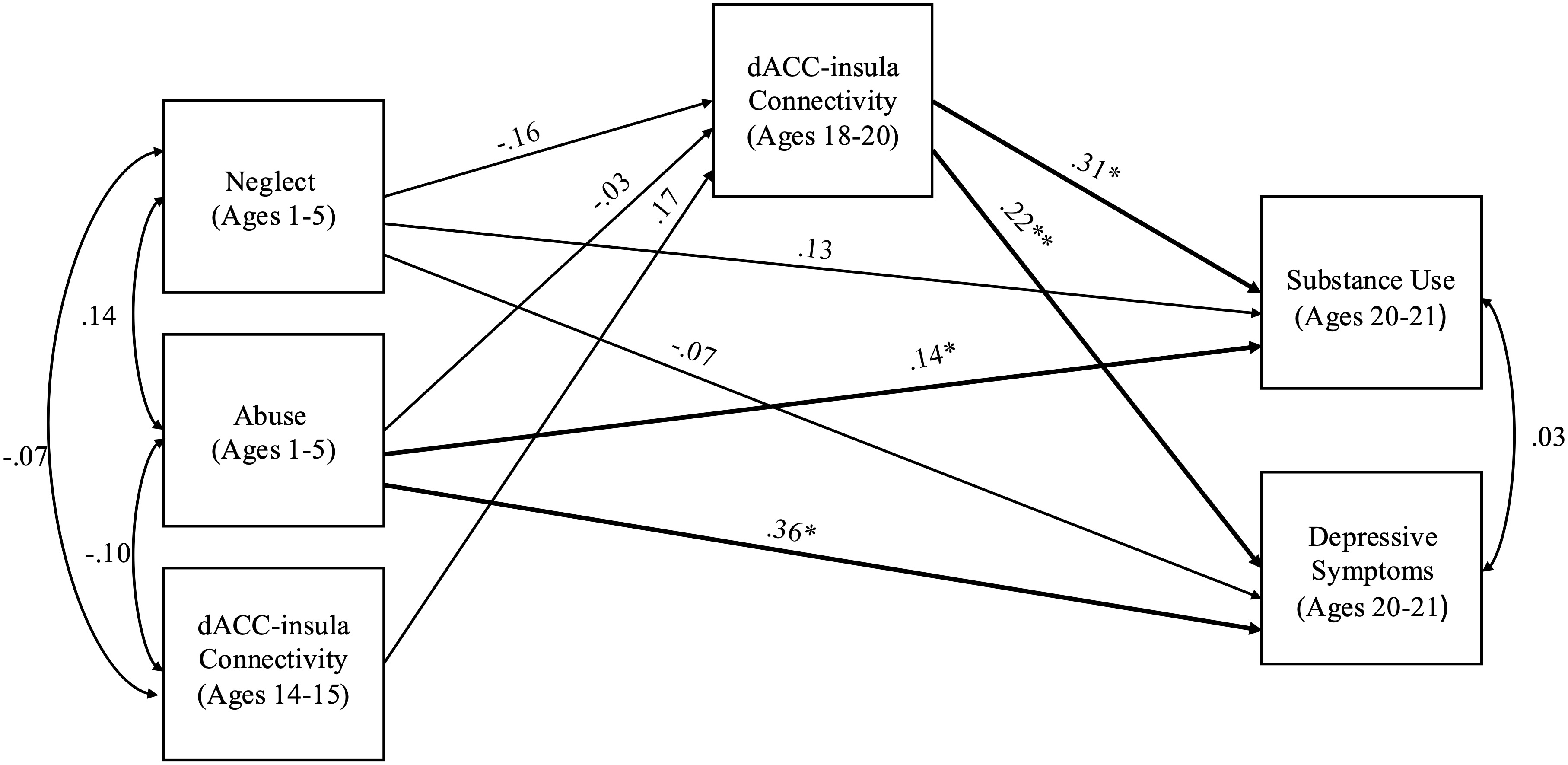
Figure 5. Path model of abuse and neglect during early childhood on substance use and depressive symptoms through dACC–insula changes from early to late adolescence. Standardized Estimates are presented. dACC = dorsal anterior cingulate cortex. Significant paths are boldface. No significant indirect effects. *p < .05, **p < .01.
Supplemental analyses
To understand if maltreatment and cognitive control predict changes in substance use or depressive symptoms from early adolescence to young adulthood, we conducted supplemental analyses to include the baseline levels of substance use and depressive symptoms as covariates. As reported in the Supplemental Materials, all the significant indirect pathways remained. This finding suggests that the predictive effects of maltreatment and dACC–insula connectivity were robust even after controlling for baseline substance use and depressive symptoms.
Additionally, we provide results of using growth curve modeling approach based on brain connectivity data in the Supplemental Materials. Unconditional growth curve models (latent basis growth models) for dACC–insula connectivity based on all six time points (Times 1–6) and on three time points (Times 1–2, Times 3–4, and Times 5–6) as well as conditional growth curve models with maltreatment predictors and mental health outcomes are reported. Given the non-significant mean and variance of the slope factor, along with model convergence issues in the conditional growth curve models, path analysis examining changes in dACC–insula connectivity from early adolescence (ages 14–15) to late adolescence (ages 18–20) was deemed an appropriate modeling approach.
Discussion
Childhood maltreatment is associated with an increased risk for multiple negative mental health outcomes such as depression and early substance use initiation (Green et al., Reference Green, McLaughlin, Berglund, Gruber, Sampson, Zaslavsky and Kessler2010). Adolescence is a peak period for the onset of mental illness (Lee et al., Reference Lee, Heimer, Giedd, Lein, Sestan, Weinberger and Casey2014) and a vulnerable period for the development of brain regions related to cognitive processing (Casey et al., Reference Casey, Jones and Somerville2011). Theoretical work, including the dimensional model of adversity and psychopathology, suggests that early adversity may alter the development of these regions, which are associated with an increased risk for the development of psychopathology and health risk behaviors (Duffy et al., Reference Duffy, Mclaughlin and Green2018; McLaughlin et al., Reference McLaughlin, Weissman and Bitrán2019), and that threat (i.e., abuse) and deprivation (i.e., neglect) experiences may influence distinct neurobiological processes that link adverse experiences and psychopathology (McLaughlin et al., Reference McLaughlin, Sheridan, Humphreys, Belsky and Ellis2021). However, the majority of prior studies on adversity’s effects on brain development have used cross-sectional data (i.e., age differences across different age-cohorts) and examined broadly defined adversity (i.e., low parental education, exposure to violence, negative life events), falling short of identifying when and what types of maltreatment may affect neurocognitive development associated with later psychopathology over time. This prospective longitudinal study aimed to investigate how different characteristics of maltreatment – such as type, timing, and chronicity – are associated with cognitive control functional connectivity changes and predict future depressive symptoms and substance use. Our analyses contribute to the current literature by using prospective data across seven time points to elucidate how abuse and neglect in childhood and adolescence affect not only longitudinal changes in functional connectivity in adolescence, but also the presence of psychopathology in young adulthood. Specifically, our findings provide evidence supporting the dimensional model of adversity and psychopathology by demonstrating how abuse and neglect may differentially predict substance use and depressive symptoms through altered functional connectivity between the dACC and insula.
In the current neuroscience literature, longitudinal examination between functional connectivity and developmental outcomes is lacking. Functional connectivity allows evaluating statistical dependence between brain regions. Here, we examined the “within-person association” between the dACC and insula, regions of the salience network that are known to work together to influence goal-directed behavior (Menon & Uddin, Reference Menon and Uddin2010). We further investigated whether developmental changes in these within-person associations may account for differential vulnerability to young adult mental health problems. To our knowledge, no study has tested whether task-based functional connectivity during cognitive control predicts later psychopathology. Available research examined frontostriatal connectivity during a risk-taking task and found that longitudinal declines in frontostriatal connectivity were associated with decreases in self-reported risk-taking behaviors among adolescents (Qu et al., Reference Qu, Galvan, Fuligni, Lieberman and Telzer2015). Our findings are complementary to this prior research in demonstrating that weaker dACC–insula connectivity during cognitive control was associated with lower substance use and depression among young adults.
Results revealed that chronic abuse (abuse occurring across multiple developmental periods) is associated with higher levels of substance use and depressive symptoms through heightened cognitive control connectivity strength across adolescence. This finding suggests that chronic abuse may facilitate the development of substance use and depression through neural mechanisms that reflect accelerated maturation of cognitive processing within the salience network. These findings are consistent with the theoretical framework posited by Callaghan and Tottenham (Reference Callaghan and Tottenham2016) that there may be accelerated neural development after adversity. Further, our findings based on task-based connectivity during cognitive control converges with a previous longitudinal study examining task-based activation during cognitive control, which reported that abuse was associated with steeper developmental decreases in frontoparietal activation (indicating accelerated maturation) during adolescence (Kim-Spoon et al., Reference Kim-Spoon, Herd, Brieant, Peviani, Deater-Deckard, Lauharatanahirun, Lee and King-Casas2021). Evidence from structural imaging studies indicate accelerated maturation suggested by the association between interpersonal adversity (e.g., family-based maltreatment) and larger fronto-limbic volumes; this accelerated maturation was pronounced earlier in development (Vannucci et al., Reference Vannucci, Fields, Hansen, Katz, Kerwin, Tachida, Martin and Tottenham2023). Our findings based on cognitive control functional connectivity complement such structural findings by specifying that cumulative abuse experience is manifested in accelerated maturation in brain connectivity. According to the stress acceleration model, altered neurodevelopment may occur in response to interpersonal adversities, reflecting adaptations to signals from proximal environments that indicate safety from a caregiver is unreliable (Callaghan & Tottenham, Reference Callaghan and Tottenham2016).
Importantly, findings from the current study present developmental consequences of stress-related accelerated brain maturation. In particular, experiences of threat (i.e., abuse), not deprivation (i.e., neglect), occurring across multiple developmental periods may alter neural functioning of the salience network during cognitive control, which then increases vulnerability to depressive symptoms and substance use later in life. That is, while this faster maturation may be adaptive in adolescence, this acceleration may come with a developmental trade-off that leads to vulnerability to mental health risk in young adulthood. During adolescence, the ability to navigate complex environments and contexts becomes increasingly important, and this ability relies on successful brain functioning that supports goal-directed behaviors, such as salience network functioning during cognitive control. Disruptions in this functioning may increase vulnerability to depressive symptoms and substance use later in life. Adolescents that have experienced abuse and show accelerated brain maturation may have been robbed of the crucial developmental window during which the prefrontal cortex can be profoundly shaped by environment and experiences (e.g., Sapolsky, Reference Sapolsky2017).
We tested whether there may be sensitive periods of maltreatment effects such that maltreatment effects on functional connectivity are particularly pronounced depending on the developmental period during which maltreatment occurred. Our analyses revealed that neglect occurring between ages 13 and 18 (i.e., adolescence) may be more evident in predicting both depression and substance use through developmental changes of dACC–insula connectivity across adolescence, compared to neglect in earlier developmental periods or abuse experienced in any developmental period. This result indicates that adolescence may be a sensitive period for neglect effects on cognitive control related functional connectivity within the salience network. Generally, previous research shows significant effects of deprivation on changes in brain development related to lower cognitive control, in alignment with the dimensional model of adversity and psychopathology (McLaughlin et al., Reference McLaughlin, Sheridan, Humphreys, Belsky and Ellis2021). For example, socioeconomic disadvantage was associated with altered task-based activation in frontoparietal regions during cognitive control among children (Sheridan et al., Reference Sheridan, Sarsour, Jutte, D’Esposito and Boyce2012). Our finding supports theoretical perspectives suggesting that adolescent brains are particularly sensitive to socio-environmental contexts (Blakemore, Reference Blakemore2008). Furthermore, theoretical papers posit that the impacts of specific dimensions of adversity on brain development may be particularly pronounced when it is experienced during specific periods of development (Cohodes et al., Reference Cohodes, Kitt, Baskin-Sommers and Gee2021). Given that cortical areas typically have a protracted development during adolescence (Casey & Jones, Reference Casey and Jones2010), there are increasing opportunities for frontal cortex development to be altered by concurrent environmental input (e.g., Sapolsky, Reference Sapolsky2017).
Significant indirect effects revealed that neglect occurring during adolescence significantly predicted less substance use and depressive symptoms through weaker dACC–insula connectivity (after controlling for the baseline connectivity) in late adolescence. Importantly, combined with the positive link from dACC–insula connectivity to substance use and depressive symptoms, our results suggest that the negative effect of neglect on brain connectivity may indicate a delayed brain functioning that slows down the emergence and progression of substance use and depression. This finding of neglect effects on delayed maturation in cognitive control connectivity is consistent with prior longitudinal resting-state connectivity work showing that maltreatment is associated with weaker resting-state connectivity strength between circuits (such as the default mode network and salience network), implying delayed maturation across two years of adolescence (Rakesh et al., Reference Rakesh, Kelly, Vijayakumar, Zalesky, Allen and Whittle2021b). Compared to abuse, neglect may delay cognitive control related maturation in the brain that is rather protective within this developmental time frame. Regarding the indirect effects, this pattern of neurocognitive functioning may reflect resilience and compensatory development after adolescent-era adversity that acts as a neuroprotective factor against the development of substance use and depressive symptoms.
These findings advance our understanding of the role of brain functioning in linking early adverse experiences to the development of psychopathology, going beyond what was established in two prior studies on brain activation and resting-state connectivity. In one study examining abuse and neglect effects on resting-state connectivity within salience network and mental health outcomes from mid-to late adolescence, significant indirect effects indicated that both abuse and neglect were associated with increases in within salience network connectivity, which in turn was associated with lower problematic substance use (Rakesh et al., Reference Rakesh, Allen and Whittle2021a). Although this study utilized resting-state connectivity whereas the current study examined task-based cognitive control connectivity, both findings are aligned with these results that suggest altered salience network processing may function as an adaptive mechanism protecting against problematic substance use after adolescent maltreatment. Furthermore, another study found that childhood maltreatment (i.e., combined between abuse and neglect) was associated with greater decreases in frontoparietal activation during cognitive control which predicted lower depressive symptoms over time (Kim-Spoon et al., Reference Kim-Spoon, Brieant, Folker, Lindenmuth, Lee, Casas and Deater-Deckard2024). Collectively, these results suggest that adolescence may be a developmental window during which the cognitive control system may be involved in experience-dependent neural plasticity following maltreatment. The current findings further clarify that these neuroprotective effects may be specific to experiences of neglect, not abuse, and specific to the adolescent developmental period. More work, however, is needed to understand long-term effects of adolescent neglect on psychopathology through altered neural functioning to elucidate whether these seemingly neuroprotective effects may be limited to adolescent brains or extended beyond adolescence.
Within the salience network, the insula and the dACC work together to detect external stimuli, filter and integrate relevant information processing, and influence goal-directed behavior (Menon & Uddin, Reference Menon and Uddin2010; Seeley et al., Reference Seeley, Menon, Schatzberg, Keller, Glover, Kenna, Reiss and Greicius2007). The current study examined connectivity between these two critical regions of the salience network in the context of cognitive processing, given that alterations in both the salience network and neural cognitive control play a role in the development of depression and problematic substance use (Droutman et al., Reference Droutman, Read and Bechara2015; Elsayed et al., Reference Elsayed, Rappaport, Luby and Barch2021; Jacobs et al., Reference Jacobs, Barba, Gowins, Klumpp, Jenkins, Mickey, Ajilore, Peciña, Sikora, Ryan, Hsu, Welsh, Zubieta, Phan and Langenecker2016; Tervo-Clemmens et al., Reference Tervo-Clemmens, Simmonds, Calabro, Montez, Lekht, Day, Richardson and Luna2018). Insula and dACC are known to be critically important in integrating external sensory information with internal, emotional, and bodily state signals (Uddin, Reference Uddin2015). In particular, the insula represents interoceptive cues to influence the subjective experience of emotional states (e.g., physical and emotional pain and craving), which can further contribute to drug addiction (Naqvi et al., Reference Naqvi, Gaznick, Tranel and Bechara2014) and depression (Harshaw, Reference Harshaw2015). In our data, altered dACC–insula connectivity underlying cognitive control may signify altered interoceptive processing following threat (abuse) or deprivation (neglect) experiences. Although there is a scarcity of literature examining the effects of adversity on task-based salience network connectivity during cognitive control, a systematic review of prior findings – most of which were cross-sectional and focused on resting-state connectivity or threat processing – suggests that threat-related exposures may have the strongest influence on salience network function (McLaughlin et al., Reference McLaughlin, Weissman and Bitrán2019). Extending this review, our results provide longitudinal evidence of the effects of abuse on salience network disruption during a cognitive control task. The findings suggest that heightened interoceptive processing following chronic abuse – likely elicited by increased threat detection in the environment – may serve as a risk factor for the development of psychopathology in young adulthood. For example, physically abused children often become hypervigilant and overly attuned to negative cues in social contexts (Shackman & Pollak, Reference Shackman and Pollak2014).
Further, our findings contribute to the literature by highlighting the importance of the salience network processing following experiences of neglect. Specifically, our results suggest that attenuated dACC–insula connectivity may reflect a form of resilience to psychopathology following experiences of neglect during adolescence. Deprivation experiences, characterized by a lack of cognitive stimulation and psychosocial input, were associated with attenuated interoceptive processing, which in turn appeared to be protective against substance use and depressive symptoms in young adulthood. Together, findings from the current study highlight that salience network functioning underlying cognitive control functions as a contributor to the etiology of psychopathology, a mechanism linking adversity and psychopathology, and a function of both maladaptation and resilience depending on the timing and type of adversity.
The current study should be interpreted in light of a few limitations. First, although data were longitudinal, these are correlational and prevent us from inferring causality. Second, adolescents reported retrospectively on experiences of maltreatment across 18 years. Research suggests that retrospective self-reports of maltreatment are verifiable (Chu et al., Reference Chu, Frey, Ganzel and Matthews1999) and are associated with poor mental and physical health outcomes regardless of concordance with official records (Danese & Widom, Reference Danese and Widom2022; Negriff et al., Reference Negriff, Schneiderman and Trickett2017). It has also been shown that subjective self-reports of adversity are the strongest predictor of psychopathology, compared to objective measures (Baldwin et al., Reference Baldwin, Coleman, Francis and Danese2024). Although we used the MACE with 18- to 19-year-olds whose ages were close enough to childhood to provide as reliable recall as possible, retrospective self-reports could have been affected by later experiences and recall biases, including those influenced by later psychopathology (Francis et al., Reference Francis, Tsaligopoulou, Stock, Pingault and Baldwin2023). In particular, retrospective reporting on maltreatment occurring during the early childhood developmental period (e.g., ages 1 to 5 years) could have been influenced by later experiences in middle childhood and adolescence (Francis et al., Reference Francis, Tsaligopoulou, Stock, Pingault and Baldwin2023). Past research suggests that memories from early childhood – particularly those from before age 3 – are often inaccurate, with many recollections reflecting later experiences misattributed to that time, memories of being told about events, or false memories (Howe, Reference Howe2013, Reference Howe2022). Even memories from the subsequent early childhood years, while more likely to be retained, remain vulnerable to distortion by later experiences and information. As such, findings involving maltreatment during ages 1 to 5 should be interpreted with caution. Additionally, our longitudinal functional connectivity analyses focused on specific regions of interest selected based on prior research examining brain regions involved in task-based activation during cognitive control. Other research suggests that abuse and neglect may have more broad effects on other neural circuitry (Herzberg et al., Reference Herzberg, McKenzie, Hodel, Hunt, Mueller, Gunnar and Thomas2021). Approaches such as whole-brain and resting-state functional connectivity may offer additional advantages of evaluating between and within-network connectivity which may add clarification on delayed versus accelerated development during adolescence (Chahal et al., Reference Chahal, Miller, Yuan, Buthmann and Gotlib2022; Rakesh et al., Reference Rakesh, Kelly, Vijayakumar, Zalesky, Allen and Whittle2021b). Furthermore, due to issues with model identification, we were not able to model developmental trajectories of dACC–insula connectivity. Rather, we examined changes in dACC–insula connectivity from early adolescence (ages 14–15) to late adolescence (ages 18–20). Lastly, there has been a caution regarding using latent variables to capture categories of adversity, as these approaches tend to measure co-occurring experiences rather than exposures to these experiences (McLaughlin et al, Reference McLaughlin, Weissman and Flournoy2023). However, we clarify that we tested latent factors of abuse and neglect separately and used indicators that were formed according to clearly defined and theoretically informed dimensions of maltreatment and empirically tested for content validity, convergent validity, predictive validity, and test–retest reliability (Teicher & Parigger, Reference Teicher and Parigger2015).
Despite some of these limitations, there are significant strengths in the present study. For instance, we used six time points of neuroimaging data to examine developmental changes in the functional connectivity of brain regions involved in cognitive control. Additionally, we leveraged our measure of maltreatment by examining potential sensitive periods for the effects of maltreatment on brain development. By examining maltreatment subtypes during ages 1 to 18, we were also able to investigate abuse and neglect chronicity across developmental periods such as early childhood (ages 1 to 5), middle childhood (ages 6 to 12) and adolescence (ages 13 to 18). Within each sensitive period model, we included both abuse and neglect predictors, so that we could understand threat vs. deprivation effects relative to the other. This approach was suitable as the goal of the current study was to examine if deprivation and threat experiences might have differential effects on psychopathology, as proposed by the dimensional model of adversity and psychopathology (McLaughlin & Sheridan, Reference McLaughlin and Sheridan2016), through changes in salience network functional connectivity related to cognitive control. Lastly, we conducted supplemental analyses to examine if observed associations apply to changes overtime as well as general levels of psychopathology in young adulthood. The results suggested that changes in dACC–insula connectivity mediated the associations between maltreatment and mental health outcomes, after controlling for baseline symptomatology (see Supplemental Materials).
Overall, our findings suggest that the timing of abuse and neglect may exert differential effects on neurobiological mechanisms underlying cognitive processing and subsequent psychopathology. Both abuse and neglect were associated with higher levels of substance use and depressive symptoms; however, these experiences differentially predicted the emergence of psychopathology through opposite patterns of neural mechanisms. Based on the current findings, which implies that the neural cognitive control system is involved in experience-dependent neural plasticity following maltreatment, future research on neuroplasticity may deepen our understanding of how to target these mechanisms to promote adaptive functioning after adversity.
Supplementary material
The supplementary material for this article can be found at http://doi.org/10.1017/S0954579425100643.
Data availability statement
The dataset used for the final analyses will be deposited in OpenNeuro, an NIH-supported, domain-specific data repository, upon publication.
Acknowledgements
We thank the former and current members of the JK Lifespan Development Lab at Virginia Tech for their help with data collection. We are grateful to the adolescents and parents who participated in our study.
Funding statement
This work was supported by the National Institute on Drug Abuse of the National Institutes of Health under Award Numbers R01DA036017 and R01DA061024 to Jungmeen Kim-Spoon and Brooks Casas.
Competing interests
None